Abstract
Background:
Parenteral nutrition (PN) is a valuable life saving intervention, which can improve the nutritional status of hospitalized malnourished patients. PN is associated with complications including hyperglycemia. This study was conducted to compare two methods of blood glucose control in traumatic brain injury patients on PN.
Materials and Methods:
A randomized, open-label, controlled trial with blinded end point assessment was designed. Traumatic brain injury patients (GCS = 4-9) on PN, without diabetes, pancreatitis, liver disease, kidney complication, were participated. Patients were randomly assigned to receive continuous insulin infusion to maintain glucose levels between 4.4 mmol/l (80 mg/dl) and 6.6 mmol/l (120 mg/dl) (n = 13) or conventional treatment (n = 13). Patients in the conventional group were not received insulin unless glucose levels were greater than 10 mmol/l (>180 mg/dl). These methods were done to maintain normoglycemia in ICU. The primary outcome was hypo/hyperglycemic episodes. Other factors such as C-reactive protein, blood electrolytes, liver function tests, lipid profile and mid-arm circumference were compared.
Results:
Mean glucose concentration were significantly lower in IIT group (118 ± 28 mg/dl) vs conventional group (210 ± 31 mg/dl) (P < 0.01). No hypoglycemic episode occurred in two groups. Triglyceride (P = 0.02) and C-reactive protein (P = 0.001) was decreased in the IIT group, significantly. There were also significant differences in the electrolytes, with magnesium and phosphorus being lower in the IIT group (P = 0.05).
Conclusion:
In this pilot study, blood glucose level, CRP and TG were lower in IIT group. Further data collection is warranted to reach definitive conclusions.
Keywords: Hyperglycemia, hypoglycemia, intensive insulin therapy, parenteral nutrition
INTRODUCTION
Parenteral nutrition (PN) is a form of intravenous nutritional support, originally developed at the Pennsylvania University of Medical School in 1968 to support malnourished surgical patients.[1] PN may cause metabolic changes such as hyperglycemia, hypertriglyceridemia, electrolyte imbalances and steatosis.[2] Hyperglycemia has been shown to exacerbate secondary brain injury and independently predict poor neurologic outcomes in patients with severe traumatic brain injury.[3,4] A previous comparison of 1548 critically ill-patients randomly assigned to either intensive insulin therapy (IIT) or conventional glucose treatment (CGC) showed that the first group whose blood glucose (BG) was maintained at 4.4-6.1 mmol/l had significantly lower morbidity and mortality rates.[5] The results of other studies, however, have been inconsistent since they resulted in reductions in morbidity but not mortality, increased mortality, due to a higher incidence of hypoglycemia in patients on tight glycemic control.[6,7,8] In the largest randomized control trial conducted, which included 6104 patients, the IIT group not only had more severe hypoglycemia but also had increased mortality with no benefits in terms of intensive care unit (ICU) stay or infection rates.[6] The risk of mortality and the consequences of hypoglycemia in critically ill-patients under IIT and especially in patients with brain trauma, are a serious concern, since hypoglycemia is associated with worsening in the Glascow Coma Scale score (GCS).
Due to the fact that previous studies were inconsistent and did not assess PN complications or traumatic brain injury-ICU patients, the present study was designed to compare hypo/hyperglycemic episodes and PN complications in IIT and CGC in traumatic brain injury ICU patients who received PN. It was hypothesized that an IIT would result in fewer hyperglycemic episodes in these patients without any hypoglycemia and improvements in outcome assessments using mid-arm circumference, liver function tests, lipid profiles and inflammatory status.
MATERIALS AND METHODS
Study design
This was a randomized, open-label, controlled trial with blinded end assessment. Figure 1 shows the flow diagram of the study. A total of 26 patients, all male (IIT group: n = 13, age: 31 ± 11 yrs, CGC group: n = 13 age: 36.6 ± 13 yrs) were randomly assigned to receive intensive insulin therapy to maintain BG between 4.4 mmol/l (80 mg/dl) and 6.6 mmol/l (120 mg/dl) or conventional treatment. Patients in the conventional group were not received insulin unless glucose levels were greater than 10 mmol/l (>180 mg/dl).
Figure 1.
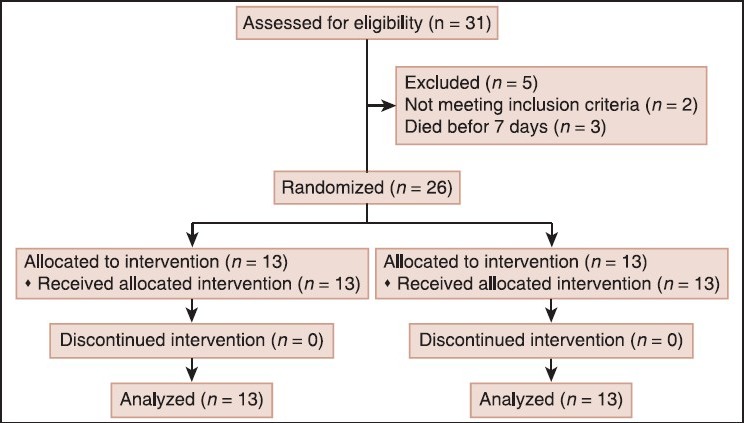
Study flow diagram
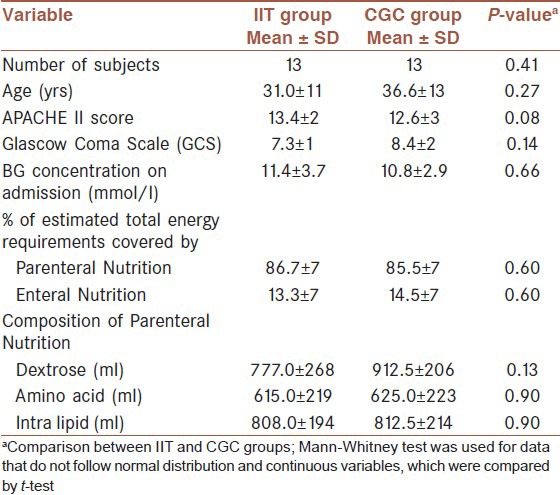
As shown in Table 1, there were no differences in the baseline characteristics of the two groups, including the severity of injury as well as the composition of PN. The dosage/kg body weight of hyperglycemia-associated medication (e. g. corticosteroids, phenytoin, lasix, manitol) provided to the patients did not differ between the two groups (P = 0.72). The study was approved by the Bioethics Committee of Mashhad Medical University and registered in the Iranian Randomized Control Trial Studies Registry (Registration number: IRCT201111158108N1) [Table 1].
Table 1.
Baseline characteristics of the intensive insulin treatment (IIT) group and the conventional glucose control (CGC) group
Subjects
ICU, traumatic brain injury patients receiving PN and aged 18 years or older with a GCS 4-9 were eligible to participate. Patients with liver, kidney, heart or pancreatic failure, Type I or Type II Diabetes Mellitus were excluded from participation. Since the participants were unable to provide consent, written informed assent was provided by a close family member before participation and the study was immediately discontinued if the assent was withdrawn for any reason. Randomization was computer generated with permuted blocks of 4. Each patient was randomly assigned to receive either IIT or CGC method.
Parenteral nutrition
The patients’ energy requirements were estimated as 25 kcal/kg ideal body weight as proposed by the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines.[2] The requirements of macro- and micro-nutrients were also calculated based on ESPEN guidelines and this was used to estimate the composition of PN for each individual patient.[2] Dextrose (dextrose 10%, Shahid Ghazi pharmaceutical co. Tabriz-Iran) was infused for 8-10 hours, lipid emulsion (intralipid 10%, B. Braun melsungen AG, Germany) for 6 hours and amino acids emulsion (aminoacid 10%, Fresenius Kabi, Austria) for 4 hours (as per routine PN prescription in Iran, which is based on the multi-bottle system). Vitamins and minerals (INFUVITE adult. Boucherville, QC, Canada: Sab-Pharma Inc) were injected through normal saline solution. Fluid load was assessed (by recording the fluid output minus input) and controlled for each patient. To preserve gut function, a minimal diet from hospital Gavage providing up to a maximum of 15% of total energy was also prescribed. The most common indications for PN in the present study were gastro-intestinal tract obstruction, poor enteral feeding tolerance including high volume enteral feeding residue and diarrhea. The intakes of PN and enteral feeds were recorded.
Glycemic control protocol
Intensive treatment (18)
Patients in the intensive treatment group received a continuous intravenous insulin infusion (50 IU of regular insulin [Novo Nordisk, Copenhagen, Denmark] in 50 ml of 0.9 percent sodium chloride). In this group, BG level was maintained between 4.4 mmol/l (80 mg/dl) and 6.6 mmol/l (120 mg/dl). We made safety features into our infusion protocol to minimize hypoglycemia. We discontinued the infusion when glucose levels were less than 4.2 mmol/l (75 mg/dl) and initiated dextrose infusion. If glucose levels decreased to less than 3.3 mmol/l (60 mg/dl), we decided to treat hypoglycemia according to a standardized hypoglycemia protocol. BG was checked every 2 hours in this group.
Conventional treatment (18)
Patients in this group did not receive insulin during ICU stay unless their glucose exceeds 10 mmol/l (>180 mg/dl). If BG concentration was between 11.1 (200 mg/dl) and 13.9 mmol/L (250 mg/dl), patients received an intravenous bolus of 4 units insulin every hour until the glucose concentration was less than 11.1 mmol/L (<200 mg/dl). In this group, BG was measured every 4 hours.
In both groups, BG concentration was measured in capillary blood using the IME-DC glucometer (Germany). To check the reliability of the capillary BG concentration, a venous blood sample was assessed once every 24 hours by the glucose-oxidase method and the coefficient of variation between the two methods was found to be 3.1%. The IIT and CGC protocols were maintained until ICU discharge.
Outcome measures
Comparison of hypoglycemic episodes and BG concentration between intensive insulin therapy and conventional group were the primary outcome of this study. The following factors were also assessed as the secondary complications of PN: Mid-arm circumference, C-reactive protein, lipid profile, blood electrolytes and liver function enzymes.
Data collection
The patients were hospitalized in ICU for at least 7 days before enrollment in the study. At baseline (admission to the study), demographic and clinical characteristics were obtained, including the age and gender of the patient, BG concentration, GCS and the Acute Physiology and Chronic Health Evaluation II (APACHE-II) scores, (ranging from 0-71, with the higher values indicating more severe illness) score results and medication type and dose (including hypo/hyperglycemic agents). Any concomitant diseases such as pneumonia or acute renal failure were also recorded and assessed using the following criteria: Pneumonia: Temperature >38.5°C, white blood count >12 × 10000 and positive blood culture; acute renal failure: Serum creatinine was twice than present on admission to the ICU or a peak level of creatinine of >221 μmol/L. Blood samples (non-fasting) were collected to assess C-reactive protein, lipid profile (Total cholesterol, low density lipoprotein (LDL)-cholesterol, high density lipoprotein (HDL)-cholesterol, triglyceride (TG), blood electrolytes (sodium, potassium, magnesium, phosphorus) and liver function tests (alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin, lactate dehydrogenase). Severe hypoglycemic episodes, defined as BG ≤ 2.2 mmol/l, were recorded. Non-fasting blood samples were also collected on days 7, 10 and 14.
Statistical analysis
This was a pilot investigation. A power calculation based on hypoglycemic episodes (as the most important reason of mortality in IIT method) in a previous study,[9] showed that 13 patients were needed in each group to show a statistically significant difference between treatments at a power of 80% and α = 0.05. Data were analyzed according to intention-to-treat and are presented as mean ± standard deviation (SD). Data normality was assessed by the Shapiro-Wilks test and by examining the normality plots. To assess differences between groups at baseline, continuous variables were analyzed using the unpaired student's t-test, if normally distributed or by the Mann-Whitney rank-sum test, if not normally distributed. To assess differences between groups in the variables over the 14-day study period, repeated measures Analysis of Variance (ANOVA) tests were employed. The Statistical Package for the Social Sciences software (SPSS version 14.0) was used for all analyses.
RESULTS
A total of 26 patients enrolled in the study. All of them were male. The average age of men taking part in this study was 31.0 ± 11 (years) in the IIT and 36.6 ± 13 (years) in the CGC group. Mean BG concentration were significantly lower in IIT group (118 ± 28 mg/dl) vs conventional group (210 ± 31 mg/dl) (P < 0.01). Then, hyperglycemic episodes in CGC were more than IIT group. None of the patients suffered from pneumonia or acute renal failure and none of the patients suffered from a severe hypoglycemic episode defined as BG ≤2.2 mmol/l or experienced typical signs and symptoms of hypoglycemia including seizures and hemodynamic instability during the ICU stay.
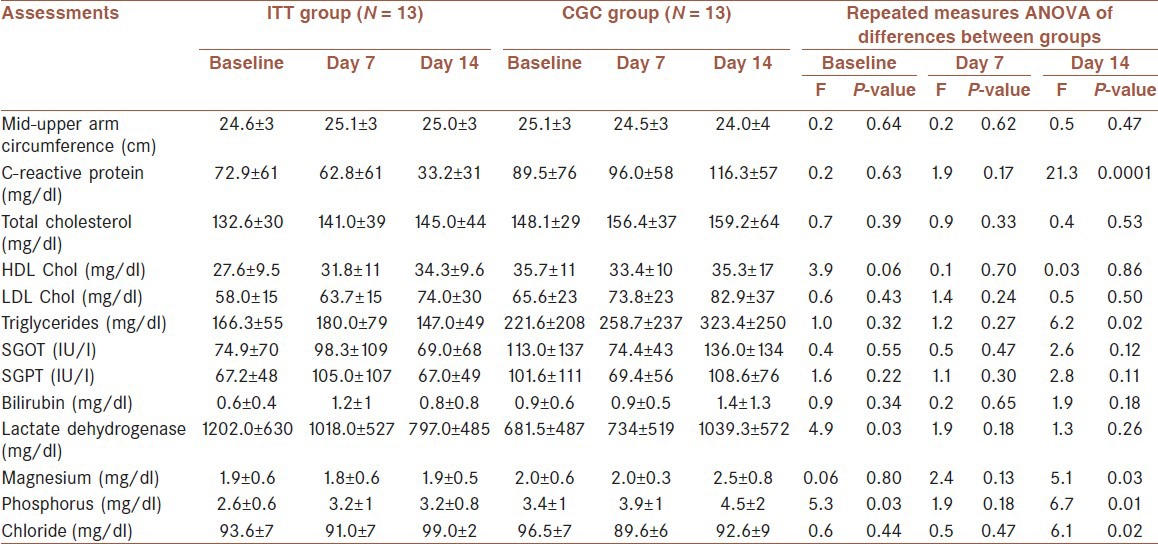
There were no differences between the groups in any of the variables measured on day 7 [Table 2]. On day 14, the IIT group had a significantly lower concentration of C-reactive protein (IIT group: 33.2 ± 31 mg/dl vs CGC group: 116.3 ± 57 mg/dl, P < 0.001) and a significantly lower TG concentration (IIT group: 147 ± 49 mg/dl vs CGC group: 323.4 ± 250 mg/dl, P = 0.02). There were also differences in the electrolytes over the study period. Phosphorus and magnesium were lower in the IIT group compared to the CGC group (P < 0.05). There was an insignificant increase in mid arm circumference in the IIT group. LDL. C, HDL. C, total cholesterol and liver function tests were not different between two groups (P > 0.05). [Table 2]
Table 2.
Comparison of the outcome measures over the study period in each group
DISCUSSION
To our knowledge, this is the first randomized, controlled trial to assess the effect of strict glycemic control on PN complications during ICU hospitalization of brain trauma patients, in Iran that has a homogenous population. When intensive intravenous insulin therapy was administered in a controlled setting by using standardized protocols, it maintained glucose concentrations close to normal during ICU stay in patients on PN without appreciably increasing the risk for hypoglycemia.
In contrast to previous studies[10] that showed that hyperglycemia induced by PN strongly predicted adverse PN outcomes, our study showed that lowering glucose concentrations to near normal levels by intravenous insulin infusion did not lead to PN complications such as hyperglycemia, hypertriglyceridemia and electrolyte imbalances.
There was an insignificant increase in mid-arm circumference in the IIT group. This may be due to insulin's anabolic function and its role in fat and protein synthesis.[11]
Inflammation usually increases during ICU stay as shown by an increase in C-reactive protein.[12] In this study, we showed that intensive insulin therapy leads to a reduction in C-reactive protein in the ITT group although this marker increased in the CGC group, possibly due to hyperglycemia. Liver function tests were not different between the two groups.
The concentration of electrolytes such as magnesium and phosphorus decreased in the IIT group. Insulin is a hormone that shifts the electrolytes from blood into the cells. Then electrolytes are reduced in the blood.
As previously noted, the TG concentration of the IIT group was significantly lower than that of the CGC group. This may be due to the insulin infusion, which leads to an increase in lipoprotein lipase activity that the destruction in triglyceride enriched particles and an increase in HDL. C.[13] It should be noted that HDL. C and TG are negatively correlated. Our results are similar to Liop J et al. showing that hypertriglyceridemia was associated with a high BG concentration.[14] In this context, hyperglycemia could be a marker of disease severity and this severity was improved with IIT.
According to a descriptive systematic review[10] of the four available retrospective studies examining hyperglycemia in hospitalized patients receiving PN, one consistent finding was observed; mortality was increased significantly if blood sugars were above 10 mmol/L (180 mg/dl). Unfortunately, published studies, which examined the hyperglycemia in PN patients, had different glycemic targets, patient population and protocols for monitoring blood sugars. These heterogeneous methods likely account for the variations in results regarding complications and morbidity associated with hyperglycemia. Three of these studies included both critically ill and non-critically ill patients and assessed outcomes in a homogeneous manner, not accounting for potential confounding factors in their analysis, such as the indication for PN. Furthermore, to our knowledge, only a few studies on glycemic control in trauma patients who received PN have been published. Several studies with heterogeneous designs and outcome measures have examined the relationship between strict glycemic control and outcomes in critically ill patients. Griesdale and colleagues report results from a meta-analysis of 26 studies involving over 13,500 patients.[15] The original landmark study conducted by Van den Berge et al.,[5] compared IIT versus conventional treatment among surgical intensive care patients, predominantly PN fed. Fasting BG targets were 4.4-6.1 mmol/L and 10-11.1 mmol/L in the intensive and conventional groups, respectively. They demonstrated a 34% decrease in mortality with IIT. Nevertheless, patients who underwent IIT had lower levels of intracranial pressure, less seizures, and a better prognosis after 6 and 12 months following hospital discharge. Another study of 48 patients with several types of primary brain injury (only 7 patients with severe traumatic brain injury) found no neurologic benefits from IIT.[16] In another randomized controlled trial of 97 patients with sever traumatic brain injury (STBI), Bilotta et al. compared routine management of BG and insulin injection if BG was more than 12.2 mmol/l versus IIT to maintain BG at 4.4-6.7 mmol/l, a significantly reduction in ICU stay was observed in IIT group.[17]
The Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) is currently the largest randomized controlled study comparing intensive versus conventional glucose control among both surgical and medical intensive care patients, who were predominantly enterally fed. The NICE-SUGAR study defined intensive glucose control with a target BG range of 4.5-6.0 mmol/L and conventional control as a target of 10.0 mmol/L or less. The authors found that intensive glucose control increased the absolute risk of death at 90 days by 2.6% compared with conventional glucose control. There was also a 6-fold increase in the rate of occurrence of hypoglycemia with use of intensive therapy in all ICU patients.[6]
In the present study, with a minimal change in insulin sliding scale protocol (discontinue the infusion when glucose levels were less than 4.2 mmol/L (75 mg/dl) and initiate dextrose infusion), hypoglycemic episodes were not observed in the IIT group but complications of PN reduced. This study is limited in that it has a small sample and thus further data collection is warranted before concluding on any of the outcome measures including patient survival or mortality.
CONCLUSION
As already mentioned this is a pilot investigation; and therefore, before drawing any definite conclusions further data collection is warranted. In this study, IIT improved some of PN complications compared to the CGC group.
ACKNOWLEDGMENT
We are very grateful to the physicians and nurses of the Intensive Care Unit of Shahid Kamyab hospital for their corporation. 89341.
Footnotes
Source of Support: Nil
Conflict of Interest: No conflict of interest.
REFERENCES
- 1.Brokenshire E, Plank LD, Gillanders LK, McIlroy K, Parry BR. Adult total parenteral nutrition at Auckland City Hospital: A 6-year review. N Z Med J. 2009;122:17–24. [PubMed] [Google Scholar]
- 2.Singer P, Berger MM, van den Berghe G, Biolo G, Calder P, Forbes A, et al. ESPEN. ESPEN guidelines on parenteral nutrition: Intensive care. Clin Nutr. 2009;28:387–400. doi: 10.1016/j.clnu.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Schirmer-Mikalsen K, Vik A, Gisvold SE, Skandsen T, Hynne H, Klepstad P. Severe head injury: Control of physiological variables, organ failure and complications in the intensive care unit. Acta Anaesthesiol Scand. 2007;51:1194–201. doi: 10.1111/j.1399-6576.2007.01372.x. [DOI] [PubMed] [Google Scholar]
- 4.Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J Trauma. 2005;58:47–50. doi: 10.1097/01.ta.0000135158.42242.b1. [DOI] [PubMed] [Google Scholar]
- 5.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 6.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 7.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 8.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: A meta-analysis. JAMA. 2008;300:933–44. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 9.Coester A, Neumann CR, Schmidt MI. Intensive insulin therapy in severe traumatic brain injury: A randomized trial. J Trauma. 2010;68:904–11. doi: 10.1097/TA.0b013e3181c9afc2. [DOI] [PubMed] [Google Scholar]
- 10.Kumar PR, Crotty P, Raman M. Hyperglycemia in hospitalized patients receiving parental nutrition is associated with increased morbidity and mortality: A review. Gastroenterol Res Pract 2011. 2011 doi: 10.1155/2011/760720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green DM, O’ Phelan KH, Bassin SL, Chang CW, Stern TS, Asai SM. Intensive versus conventional insulin therapy in critically ill neurologic patients. Neurocrit Care. 2010;13:299–306. doi: 10.1007/s12028-010-9417-3. [DOI] [PubMed] [Google Scholar]
- 12.Jeschke MG, Barrow RE, Mlcak RP, Herndon DN. Endogenous anabolic hormones and hypermetabolism: Effect of trauma and gender differences. Ann Surg. 2005;241:759–67. doi: 10.1097/01.sla.0000161028.43338.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, et al. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest. 2005;115:2277–86. doi: 10.1172/JCI25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–8. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 15.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, et al. Intensive insulin therapy and mortality among critically ill patients: A meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–7. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aldawood AS, Tamim HM, Alsultan MA, Rishu AH, Arabi YM. Intensive insulin therapy versus conventional insulin therapy for critically ill trauma patients admitted to ICU. Middle East J Anesthesiol. 2010;20:659–66. [PubMed] [Google Scholar]
- 17.Bilotta F, Rosa G. Glucose management in the neurosurgical patient: Are we yet any closer? Curr Opin Anaesthesiol. 2010;23:539–43. doi: 10.1097/ACO.0b013e32833e150a. [DOI] [PubMed] [Google Scholar]


