Abstract
Background:
This double-blinded, randomized clinical trial was designed to evaluate the comparison of intravenous versus intraarticular (IA) administration of midazolam on postoperative pain after knee arthroscopy.
Materials and Methods:
In this study, 75 patients randomized in three groups to receive 75 mc/kg IA injection of midazolam and 10 ml intravenous injection of isotonic saline (Group I), 75 mc/kg intravenous injection of midazolam and 10 cc IA injection of isotonic saline (Group II) or IA and intravenous injection of isotonic saline (Group III) at the end of knee arthroscopy. Pain scores, time until the first request for analgesics, cumulative analgesic consumption, satisfaction, sedation, and complications as studied outcomes were assessed. Patients were observed for 24-h.
Results:
IA administration of midazolam significantly reduced pain scores in the early postoperative period compared with intravenous injection. Mean of time to first analgesic requirement in Group III (33.6 min) was significantly lower than Group II (288.8 min) and Group I (427.5 min). Cumulative analgesic consumption was increased in Groups II (35.5 mg), and III (70 mg) compared with Group I (16 mg), (P < 0.0001). Complications significantly occurred in 3 of 25 patients in Group I in contrast to 20 of 25 patients in Group III (P < 0.0001). At 2-, 4- and 8-h after arthroscopy pain score significantly decreased in Group I than other groups (P < 0.0001). Patients in Group I were significantly satisfy than other groups (P < 0.0001).
Conclusion:
Results show the greater analgesic effect after IA administration of midazolam than after intravenous injection and hence, IA administration may be is the method of choice for pain relief after knee arthroscopy.
Keywords: Intraarticular administration, knee arthroscopy, midazolam, postoperative pain
INTRODUCTION
In the field of anesthesia postoperative pain management is an important and challenging matter, and at present, as optimal for diminishing postoperative pain, there is no method of preemptive analgesia accepted. For the management of postoperative pain, different methods have been assessed in many studies.[1,2]
In an outpatient setting arthroscopy of the knee under general anesthesia is one of the most common surgical procedures.[3] It seems that the majority of patients prefer the ambulatory arthroscopic surgery of the knee.[4] As this common procedure may cause pain, which has a negative impact on the patient's psychology, causing discomfort and affects the patient's activity level and satisfaction.[3,4,5] A significant number of patients have reported moderate to severe pain 24-h after knee arthroscopy in a particular day.[5,6] In care arthroscopy pain management is essential for early hospital discharge and patient comfort.[7] Previously, studies using different drugs and regimes have been published an effort to provide an effective, safe and long lasting after arthroscopy analgesia, also, to control pain after arthroscopic knee surgery systemic medication, peripheral or central blocks, and intraarticular (IA) drug administration, have been used.[8,9,10,11]
Intraarticular route is one of the analgesic techniques for pain management after knee arthroscopy, and has been used by many orthopedic surgeons during arthroscopic procedures,[12] and previously, the efficacy of this technique has been reported in some studies for midazolam,[13] tramadol,[12,13,14] bupivacaine,[7] ropivacaine,[3] dexmedetomidine,[15] morphine,[16] and etoricoxib.[17] Midazolam as one of the clinically available water soluble benzodiazepines is effective in the pediatric, adult, and obstetric population when administered by the centroneuraxial route and has been reported to have an analgesic effect through neuraxial pathways.[8,9,10,11,12,13,14,15,16,17,18,19] Midazolam has analgesic effect via gamma-aminobutyric acid receptor in spinal cord.[20,21] Both in human and animals benzodiazepines receptor exist in other organs and joints.[22]
After knee arthroscopy IA administration of local anesthetic solutions is used to provide better analgesia and reduce consumption and possible side-effects of intravenous anesthetic. To the best of our knowledge, published studies reporting the comparison of intravenous versus IA administration of midazolam on postoperative pain after knee arthroscopy are limited and the site of midazolam action (systemic absorption versus local peripheral action) is questioned. Thus, this study was aimed to assess the comparison of intravenous versus IA administration of midazolam on postoperative pain after knee arthroscopy in a randomized controlled trial.
MATERIALS AND METHODS
This study was a randomized, parallel-group, double-blind study which was conducted between April, and October, 2013, on 75 patients who scheduled for knee arthroscopy under general anesthesia in Kashani Hospital in Isfahan, Iran. The Ethics Committee of Isfahan University of Medical Sciences investigates and approves this study, and written informed consent was obtained from all studied patients. Eligibility was define as age older than 18 years old in both gender, American Society of Anesthesiologists classification I and II, no history of chronic disease, no use of nonsteroidal antiinflammatory drugs up to 2 weeks before surgery. Furthermore, patients with history use of opioids, patients with postoperative complications that increased postoperative pain, and those whose pain evaluation was judged unreliable because of neurologic disease did not enrolled in the study.
Using random-maker software “random allocation” eligible patients were randomly divided into three 25-member groups. Group I include patients who received 75 mc/kg IA injection of midazolam and 10 cc intravenous injection of isotonic saline. Group II include patients who received 75 mc/kg intravenous injection of midazolam and 10 cc IA injection of isotonic saline.[23] Patients in Group III received IA and intravenous injection of isotonic saline (10 cc).
In all groups, standardized general anesthesia was selected, and in the entire cases anesthesia was induced intravenously with fentanyl (2 mcg/kg), sodium thiopental (5 mg/kg) and atracurium (0.5 mg/kg). Endotracheal intubation was performed with a single-lumen tube. Balanced anesthesia was maintained using with inhalation of isoflurane (1.2%) and morphine (0.1 mg/kg). Patients’ lungs were mechanically ventilated with the same setting during ventilation (10 mL/kg and respiratory rate, 10 min). The patients were monitored and observed using an electrocardiogram, noninvasive arterial blood pressure device, end tidal CO2, and pulse oximeter. Studied drugs were prepared in syringes in a double-blind fashion by a team member who was not involved in data recording. Furthermore, to maintain blinding, patients were unaware of the treatment allocation.
Collected data included age, and sex combination, weight, duration of anesthesia (from induction of anesthesia until disconnecting of anesthesia drug), duration of surgery (from the beginning of incision until last suture), extubation time (from disconnecting of anesthesia drugs until extubation of trachea), duration of recovery (from extubation until achieving modified Alderet score 9/10)[24] Time to first analgesic requirement, 24-h analgesic consumption, number of patients requiring analgesics, sedation, complications (desaturation, bradycardia, allergy, hypotension, local infection) satisfaction, pain and mean arterial pressure (MAP) which were assessed in all patients. Degree of sedation 30 min after extubation evaluated and recorded in the postanesthesia care unit (PACU); (scale 1-5, 1 = completely awake, 2 = awake but drowsy, 3 = asleep but responsive to verbal commands, 4 = asleep but responsive to tactile stimulus, 5 = asleep and not responsive to any stimulus time to first analgesic requirement assessment of pain was done by a 10-score visual analogue scale; 0, no pain; 10, worst imaginable pain, after arrival in the recovery room and 2-, 4-, 5-, 12- and 24-h after surgery by an independent nurse blinded to group allocation. Also, patients were asked to indicate the degree of overall satisfaction on a five-point satisfaction scale: 0 = Unsatisfactory, 1 = somewhat satisfactory, 2 = satisfactory, 3 = very good, 4 = excellent.[30]
The sample size was calculated using the comparison of means formula with two-sided log-rank test, α = 0.05, and 80% power. All statistical analyses were performed using (Statistical Package for Social Sciences) SPSS software for Windows, version 20. Descriptive data are reported as mean ± standard deviation, median (interquartile range) or number (percent) as appropriate. One-way ANOVA, Chi-square test, and Kruskal–Wallis were used to comparing all studied variables between groups as appropriate. Trend pain at time points were compared between groups by repeated measurements of ANOVA. The level of significance is considered to be <0.05.
RESULTS
Of 84 reviewed patients, nine patients did not enter to the study (six patients were not eligible and three patients refused informed consent). Seventy-five patients were eligible and randomly assigned in to three intervention groups. Patients were followed for 24-h; finally, 75 patients (each group 25 patients) completed the study and analyzed [Figure 1].
Figure 1.
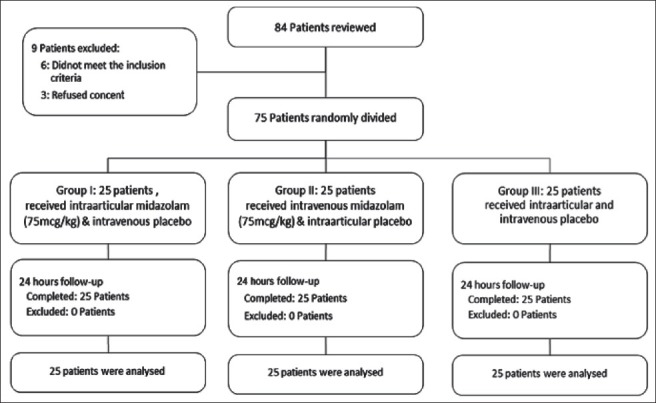
Patients who entered to the study, divided into the study groups and analyzed
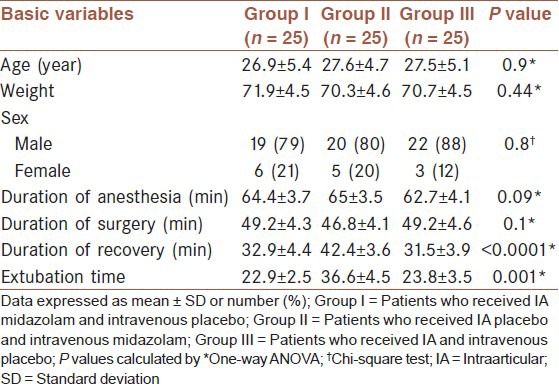
The mean age of the studied patients was 27.3 ± 5 years, 61 patients (88%) were male and 14 patients (22%) were female. Table 1 shows baseline characteristics of studied patients. No significant differences were noted between intervention groups for the mean of age and sex combination, weight, duration of anesthesia, and duration of surgery (P ≥ 0.5). Duration of recovery and extubation time in Group II was significantly higher than groups (P < 0.05).
Table 1.
Baseline characteristics in 75 studies patients by groups
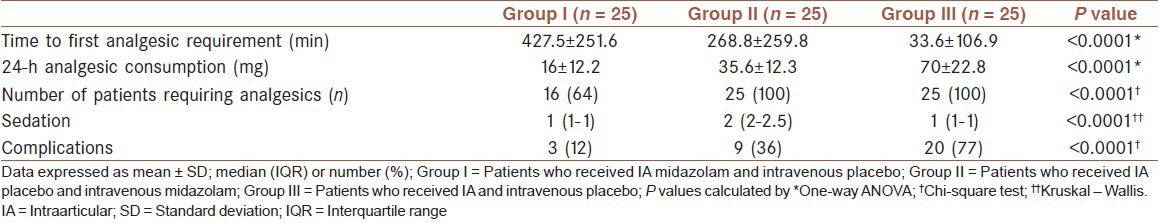
Mean of time to first analgesic requirement in Group III (33.6 min) was significantly lower than Group II (288.8 min) and Group I (427.5 min), and in Group II was significantly lower than Group I. All patients in Group II and III requiring analgesics, whereas only 16 of 25 patients in Group I requiring analgesics. Furthermore, mean of 24-h analgesic consumption was increased in Group II compared with Group I and in Group III compare with Groups I and II (1635.5 and 70 mg, respectively, P < 0.0001). Differences in sedation score among groups were statistically significant, patients in Group II had higher sedation score than other groups in the PACU (P < 0.0001). Frequency of complications desaturation and apnea in PACU, bradycardia, allergy, hypotension, local infection) among groups was significantly different (P < 0.0001) and occurred only in 3 of 25 patients in Group I in contrast to 20 of 25 patients in Group III [Table 2].
Table 2.
Comparison of analgesic requirement, sedation and complications among study groups
Pain score among groups was compared at time points [Table 3] and as a trend in the follow-up period time [Figure 2]. At baseline and hours 12 and 24 median of pain score was not significantly different among groups (P > 0.05). At hours 2, 4 and 8 pain score decreased in all groups, however decrease in Group I was significantly more than other groups (P < 0.0001). Also, as shown in Figure 2, the difference in the trend of pain in time points during the follow-up period was statistically significant among groups (P < 0.0001).
Table 3.
Comparison of pain score among study groups at time points
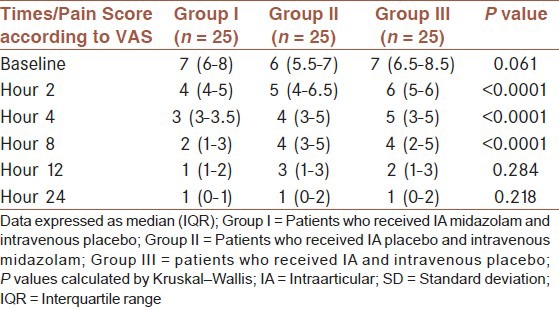
Figure 2.
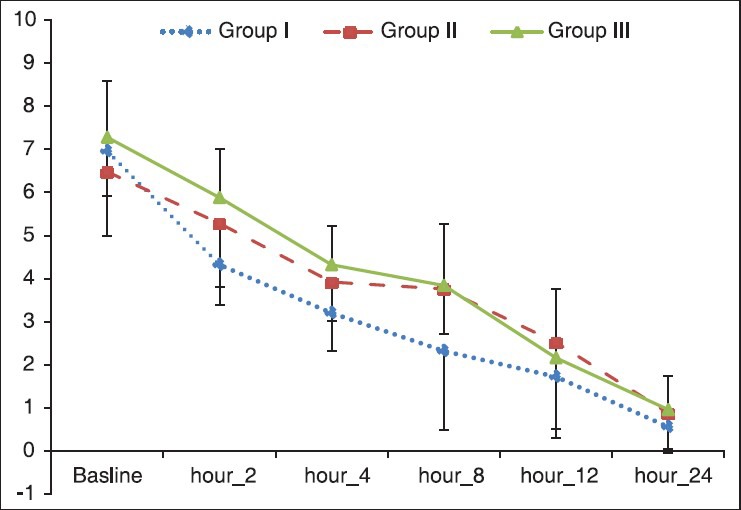
Comparison of pain among study groups by repeated measurements of ANOVA. Group I, included 25 patients who received intraarticular (IA) midazolam and intravenous placebo; Group II, included 25 patients who received IA placebo and intravenous midazolam; Group III, included 25 patients who received IA and intravenous placebo. The difference of the trend of pain was statistically significant among groups (P < 0.0001)
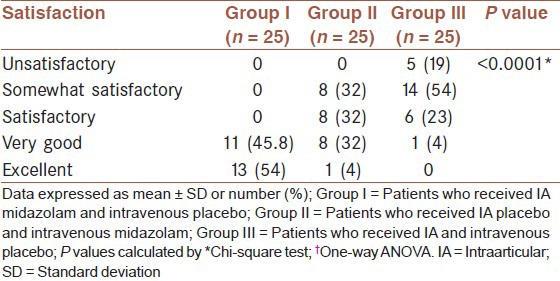
Table 4 shows the comparison of patients’ satisfaction and MAP between study groups. All patients in Group I reported very good and excellent satisfy, most of the patients in Group II reported somewhat satisfactory and satisfactory and in Group III no patient reported excellent and one patient reported very good. Patients’ satisfaction was significantly different among groups (P < 0.0001). MAP in Group I was significantly lowers than other groups at baseline and hour 1 (P < 0.0001), but at hour 2 the difference in the mean of MAP was not statistically significant (P = 0.057).
Table 4.
Comparison of patients satisfaction between study groups
DISCUSSION
Arthroscopic surgery is associated with a variable amount of postoperative pain, but it may be quite considerable. The pain is caused by an irritation of free nerve endings of the synovial tissue, anterior fat pad, and joint capsule due to surgical excision and resection.[25] In this randomized study, we found that the administration of IA midazolam improved postoperative pain, and patient satisfaction. It was also associated with lower sedation scores following administration, decreased total postoperative analgesic consumption, and delayed the time of first analgesic administration compared with intravenous midazolam and saline controls group. This is supported by a significant decrease in pain scores during the first eight postoperative hours and by a prolonged delay between the IA administration of midazolam and additional analgesic requirement. These beneficial effects of midazolam were not as marked when the drug was given intravenous and it produced a limited improvement in postoperative pain and less effect on postoperative analgesic requirement and the time of first analgesic administration compared with saline controls group. Based on our results, the analgesic effect of IA midazolam appears to be mainly act at a peripheral site in the joint, as this effect was less pronounced after systemic administration, however, a central analgesic effect resulting from systemic absorption cannot be excluded. Opioid receptors are existed on peripheral nerve endings and opioid peptides are produced in various immune cells of synovial tissue after knee trauma. Corticotropin-releasing hormone (CRH) acts through its receptors on endorphin-containing immune cells,[26] Likar et al. investigated whether the IA injection of CRH reduces postoperative pain intensity and supplemental analgesic consumption in patients undergoing arthroscopic knee surgery. They found evidence for a short analgesic effect of a single dose of IA CRH in patients undergoing arthroscopic knee surgery.[26]
In a double-blinded, randomized study by Batra et al.,[13] postoperative pain, the time to first analgesic consumption and the total dose of analgesics used over 48-h after IA administration of midazolam 50 μg/kg, 75 μg/kg, or isotonic saline were assessed in 60 patients undergoing knee arthroscopy. Authors in this study reported that both doses of midazolam decreases postoperative pain after arthroscopic knee surgery in compared with placebo. Also, a prolonged delay between the IA administration of midazolam and additional analgesic requirement, and during the first four postoperative hours a significant decrease in pain scores were reported in this study in both doses of midazolam compared with the placebo group. They did not demonstrate a dose dependent effect with administration of midazolam 50 μg/kg or 75 μg/kg doses. Like Batra et al.[13] we found greater analgesic effect after IA administration than placebo, and lower additional analgesic requirement. Results of these studies show the effect of IA administration of midazolam as an analgesic technique for postoperative pain management after knee arthroscopy.
To the best of our knowledge, there are no published studies reporting the comparison of intravenous versus IA administration of midazolam on postoperative pain after knee arthroscopy and other studies were done using different drugs. Alagol et al.[14] in a double-blinded, randomized study assessed the effect of intravenous versus IA administration of tramadol on postoperative pain after knee arthroscopy. In this study, different doses of tramadol were used and results are shown that IA tramadol provide longest duration of analgesia, lower pain scores and minimal analgesic consumption during 24-h with few side-effects in compare with intravenous administration of tramadol. Effect of dexmedetomidine after IA and intravenous administration was evaluated in Al-Metwalli et al. study,[15] and reported that IA dexmedetomidine enhanced postoperative analgesia after arthroscopic knee surgery, with an increased time to first analgesic request and a decreased need for postoperative analgesia compared with intravenous administration. In another randomized study, Ho et al.[27] assessed the effect of intravenous versus IA administration of morphine on postoperative pain after knee arthroscopy and reported that patients who received IA morphine consumed less rescue analgesia than those who received intravenous morphine with fewer side-effects. Arti and Arti studied the analgesic effects of different opioids in the early postoperative period in comparison to control group. They found that morphine in comparison to meperidine or methadone is more useful in reducing pain or analgesic need when is added to bupivacaine injection following arthroscopic menisectomy.[28] Isik et al. studied IA injection of ketamine or ketamine plus levobupivacaine on postoperative analgesia in patients undergoing arthroscopic meniscectomy. They found that IA ketamine provides effective postoperative analgesia, and addition of IA levobupivacaine to ketamine may provide better pain relief after outpatient arthroscopic meniscectomy.[29]
In agreement with these three studies, our results show that greater analgesic effect and lower side-effects of midazolam after IA administration than after intravenous injection. Although, studied drugs in these studies is different but their findings display that the mechanism of the analgesic effect of intraarticularly administered of these drugs is not due to the systemic effects and there is limited absorption of the drug.
Strength of our study is that to determine the site of midazolam action and the utility of IA midazolam, patients were assessed in three comparing groups receiving IA midazolam with intravenous saline, IA saline with intravenous midazolam, and IA saline with intravenous saline. Furthermore, the low number of sample size is the possible main limitation of our study. Therefore, future studies with appropriate sample size are necessary to specifically assess the effect of IA administration of midazolam compare to intravenous injection. For the purpose of ethical considerations and study limitations, because we used IA midazolam, then before starting of administration we discussed the procedure for every patient and received written informed consent, Also we coordinated about the study with the surgical team as study coworker before starting of the study, therefore no ethical barrier or limitation has been determined.
CONCLUSION
The results of our study show the greater analgesic effect after IA administration of midazolam than after intravenous injection, with better patient satisfaction score, lower sedation scores following administration, decreased total postoperative analgesic consumption, and delayed the time of first analgesic administration and lower side-effects. Thus, IA administration of midazolam may be the method of choice for pain relief after arthroscopic knee surgery.
ACKNOWLEDGMENTS
This article has been originated from a medical thesis, which has been conducted in Anesthesia Department of Isfahan University of Medical Sciences and approved by the University Department of Research, with the research code 291211. The researchers would like to thank the Vice-Chancellor for Research at Isfahan University of Medical Sciences, contributors who help in accomplishment of this project, nurse and operating room technicians who helped with this work. We are also grateful to those patients who enthusiastically helped us with conducting the present study by their participation.
Footnotes
Source of Support: Financial support by Research Department, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Cagla Ozbakis Akkurt B, Inanoglu K, Kalaci A, Turhanoglu S, Asfuroglu Z, Tumkaya F. Effects of intravenous small dose ketamine and midazolam on postoperative pain following knee arthroscopy. Pain Pract. 2009;9:289–95. doi: 10.1111/j.1533-2500.2009.00278.x. [DOI] [PubMed] [Google Scholar]
- 2.Dahl MR, Dasta JF, Zuelzer W, McSweeney TD. Lidocaine local anesthesia for arthroscopic knee surgery. Anesth Analg. 1990;71:670–4. doi: 10.1213/00000539-199012000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Samoladas EP, Chalidis B, Fotiadis H, Terzidis I, Ntobas T, Koimtzis M. The intra-articular use of ropivacaine for the control of post knee arthroscopy pain. J Orthop Surg Res. 2006;1:17. doi: 10.1186/1749-799X-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weale AE, Ackroyd CE, Mani GV, Winson IG. Day-case or short-stay admission for arthroscopic knee surgery: A randomised controlled trial. Ann R Coll Surg Engl. 1998;80:146–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Pavlin DJ, Chen C, Penaloza DA, Buckley FP. A survey of pain and other symptoms that affect the recovery process after discharge from an ambulatory surgery unit. J Clin Anesth. 2004;16:200–6. doi: 10.1016/j.jclinane.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 6.McGrath B, Elgendy H, Chung F, Kamming D, Curti B, King S. Thirty percent of patients have moderate to severe pain 24 hr after ambulatory surgery: A survey of 5,703 patients. Can J Anaesth. 2004;51:886–91. doi: 10.1007/BF03018885. [DOI] [PubMed] [Google Scholar]
- 7.Campo MM, Kerkhoffs GM, Sierevelt IN, Weeseman RR, Van der Vis HM, Albers GH. A randomised controlled trial for the effectiveness of intra-articular ropivacaine and bupivacaine on pain after knee arthroscopy: The DUPRA (DUtch pain relief after arthroscopy)-trial. Knee Surg Sports Traumatol Arthrosc. 2012;20:239–44. doi: 10.1007/s00167-011-1562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aasbø V, Raeder JC, Grøgaard B, Røise O. No additional analgesic effect of intra-articular morphine or bupivacaine compared with placebo after elective knee arthroscopy. Acta Anaesthesiol Scand. 1996;40:585–8. doi: 10.1111/j.1399-6576.1996.tb04492.x. [DOI] [PubMed] [Google Scholar]
- 9.Franceschi F, Rizzello G, Cataldo R, Denaro V. Comparison of morphine and ropivacaine following knee arthroscopy. Arthroscopy. 2001;17:477–80. doi: 10.1053/jars.2001.19684. [DOI] [PubMed] [Google Scholar]
- 10.Møiniche S, Mikkelsen S, Wetterslev J, Dahl JB. A systematic review of intra-articular local anesthesia for postoperative pain relief after arthroscopic knee surgery. Reg Anesth Pain Med. 1999;24:430–7. doi: 10.1016/s1098-7339(99)90010-x. [DOI] [PubMed] [Google Scholar]
- 11.Rautoma P, Santanen U, Avela R, Luurila H, Perhoniemi V, Erkola O. Diclofenac premedication but not intra-articular ropivacaine alleviates pain following day-case knee arthroscopy. Can J Anaesth. 2000;47:220–4. doi: 10.1007/BF03018916. [DOI] [PubMed] [Google Scholar]
- 12.Tuncer B, Babacan A, Arslan M. Preemptive intraarticular tramadol for pain control after arthroscopic knee surgery. Agri. 2007;19:42–9. [PubMed] [Google Scholar]
- 13.Batra YK, Mahajan R, Kumar S, Rajeev S, Singh Dhillon M. A dose-ranging study of intraarticular midazolam for pain relief after knee arthroscopy. Anesth Analg. 2008;107:669–72. doi: 10.1213/ane.0b013e3181770f95. [DOI] [PubMed] [Google Scholar]
- 14.Alagöl A, Calpur OU, Kaya G, Pamukçu Z, Turan FN. The use of intraarticular tramadol for postoperative analgesia after arthroscopic knee surgery: A comparison of different intraarticular and intravenous doses. Knee Surg Sports Traumatol Arthrosc. 2004;12:184–8. doi: 10.1007/s00167-003-0454-8. [DOI] [PubMed] [Google Scholar]
- 15.Al-Metwalli RR, Mowafi HA, Ismail SA, Siddiqui AK, Al-Ghamdi AM, Shafi MA, et al. Effect of intra-articular dexmedetomidine on postoperative analgesia after arthroscopic knee surgery. Br J Anaesth. 2008;101:395–9. doi: 10.1093/bja/aen184. [DOI] [PubMed] [Google Scholar]
- 16.Drosos GI, Stavropoulos NI, Katsis A, Kesidis K, Kazakos K, Verettas DA. Post-operative pain after knee arthroscopy and related factors. Open Orthop J. 2008;2:110–4. doi: 10.2174/1874325000802010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lierz P, Losch H, Felleiter P. Evaluation of a single preoperative dose of etoricoxib for postoperative pain relief in therapeutic knee arthroscopy: A randomized trial. Acta Orthop. 2012;83:642–7. doi: 10.3109/17453674.2012.747053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tucker AP, Mezzatesta J, Nadeson R, Goodchild CS. Intrathecal midazolam II: Combination with intrathecal fentanyl for labor pain. Anesth Analg. 2004;98:1521–7. doi: 10.1213/01.ANE.0000112434.68702.E4. [DOI] [PubMed] [Google Scholar]
- 19.Iida R, Iwasaki K, Kato J, Saeki S, Ogawa S. Reflex sympathetic activity after intravenous administration of midazolam in anesthetized cats. Anesth Analg. 2007;105:832–7. doi: 10.1213/01.ane.0000275201.64587.1f. [DOI] [PubMed] [Google Scholar]
- 20.Nishiyama T, Tamai H, Hanaoka K. Serum and cerebrospinal fluid concentrations of midazolam after epidural administration in dogs. Anesth Analg. 2003;96:159–62. doi: 10.1097/00000539-200301000-00032. [DOI] [PubMed] [Google Scholar]
- 21.Sajedi P, Islami M. Supplementing epidural lidocaine with midazolam: Effect on sensorymotor block level. Acta Anaesthesiol Taiwan. 2004;42:153–7. [PubMed] [Google Scholar]
- 22.Bazzichi L, Betti L, Giannaccini G, Rossi A, Lucacchini A. Peripheral type benzodiazepine receptors in human mononuclear cells of patients affected by osteoarthritis, rheumatoid arthritis or psoriatic arthritis. Clinic Biochem. 2003;36:57–60. doi: 10.1016/s0009-9120(02)00408-3. [DOI] [PubMed] [Google Scholar]
- 23.Batra YK, Mahajan R, Kumar S, Rajeev S, Singh Dhillon M. A dose-ranging study of intraarticular midazolam for pain relief after knee arthroscopy. Anesth Analg. 2008;107:669–72. doi: 10.1213/ane.0b013e3181770f95. [DOI] [PubMed] [Google Scholar]
- 24.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49:924–34. [PubMed] [Google Scholar]
- 25.Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26:773–7. doi: 10.1177/03635465980260060601. [DOI] [PubMed] [Google Scholar]
- 26.Likar R, Mousa SA, Steinkellner H, Koppert W, Philippitsch G, Stein C, et al. Involvement of intra-articular corticotropin-releasing hormone in postoperative pain modulation. Clin J Pain. 2007;23:136–42. doi: 10.1097/01.ajp.0000210954.93878.0d. [DOI] [PubMed] [Google Scholar]
- 27.Ho ST, Wang TJ, Tang JS, Liaw WJ, Ho CM. Pain relief after arthroscopic knee surgery: Intravenous morphine, epidural morphine, and intra-articular morphine. Clin J Pain. 2000;16:105–9. doi: 10.1097/00002508-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Arti H, Arti S. The Effects of Intraarticular Opioids in pain relief after arthroscopic menisectomy: A Randomized clinical trial study. Pak J Med Sci. 2013;29:625–8. doi: 10.12669/pjms.292.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isik C, Demirhan A, Yetis T, Oktem K, Sarman H, Tekelioglu UY, Duran T. Efficacy of intraarticular application of ketamine or ketamine-levobupivacaine combination on post-operative pain after arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc Mar. 2014 Mar 27; doi: 10.1007/s00167-014-2962-0. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg PH. 1992 ASRA Lecture. Intravenous regional anesthesia: Nerve block by multiple mechanisms. Reg Anesth. 1993;18:1–5. [PubMed] [Google Scholar]


