Abstract
Background:
Radiation-induced discomfort is frequently observed during pelvic radiotherapy. This study was performed to determine the effect of a green tea tablet to reduce the incidence of radiation-induced diarrhea and vomiting in patients with abdomen and pelvic malignancy.
Materials and Methods:
This randomized controlled clinical trial recruited 42 patients with abdomen and pelvic malignancy considered for treatment with 50 Gy radiotherapy, randomly assigned to the green tea tablet 450 mg (n = 21) or placebo group (n = 21) for 5 weeks. Acute gastrointesinal complications (Diarrhea and vomiting) were weekly assessed using Common Toxicity Criteria of the National Cancer Institute version 3.0 and functional living index emesis, respectively. Two-sample t-tests, Pearson's Chi-square, Mann-Whitney U-test, and Friedman were used for analysis.
Results:
There was a significant difference in frequency of reported diarrhea between two groups of study at the end of study (P < 0.002). About 81% of patients in green tea group reported no history of diarrhea at week 5. The treatment group have reported no history of severe diarrhea during radiotherapy. There was no significant difference between two groups of study in frequency of vomiting throughout the study, but 9.5% of cases in placebo group showed severe vomiting.
Conclusion:
Green tea contains a high concentration of catechins could be effective in decreasing the frequency and severity of radiotherapy induced diarrhea. Green tea (450 mg/day) could be considered to be a safe for prevention diarrhea and vomiting in patients undergoing pelvic or abdomen radiotherapy.
Keywords: Abdomen irradiation, cancer, green tea, pelvic irradiation, radiotherapy induced diarrhea
INTRODUCTION
Radiation therapy (RT) is commonly administered for a range of pelvic malignancies. Although the benefits of this treatment have been well-established, the majority of patients experience toxicity (nausea, vomiting, and acute diarrhea) because of the effect of the radiation on the small and large intestine.[1,2,3] Acute emesis is seen most commonly with radiotherapy lasting 30 min to 4 h.[4] Although, the toxicity during radiotherapy is always self-limited but decreases patient compliance, increases the cost of care, and may alter therapeutic results by increasing generally treatment period.[5,6,7,8] Irradiation caused acute small bowel injury characterized by increased apoptosis of crypt epithelial cells and by lymphocyte infiltration of the underlying tissue.[9] Inflammatory changes lead to the acute diarrhea by different mechanisms, including the increase of gastrointestinal motility, reduced bile acid reabsorption, and the impaired maturation and depletion of villi with denudation of the mucosa.[10,11] Secondary to the inflammatory reaction there may be release of histamines, serotonin and other transmitters inducing emesis.[4] Various treatment techniques may decrease small bowel injury associated with pelvic irradiation. Routine medical treatment, based on guideline has included nonspecific agents, such as loperamide, atropine-diphenoxylate, octreotide, and antibiotics.[12,13,14] Aside pharmacological therapy, other elements such as probiotic have been examined in order to decrease postradiation gastrointestinal side-effect.[15,16] In this area, Green tea has attracted significant attention recently in the scientific communities for its health benefits for a variety of disorders, ranging from cancer to weight loss. Green tea contains relatively large amounts of catechins (a subclass of the flavonoid family). It has been shown that tea catechins could have diverse pharmacological activities included antioxidative activities,[17] antibacterial,[18] antiinflammatory[19] and antiintestinal motility.[20] To the best of our knowledge, no clinical trial study has been reported to evaluate the effect of green tea on prevention and treatment of the RT induced diarrhea. It is therefore logical to explore the use of green tea in prophylaxis for radiation-induced diarrhea. This study aimed to assess the efficacy of green tea tablet, 450 mg/day[21,22] administered orally throughout the irradiation period, to reduce acute diarrhea, nausea and vomiting in subjects receiving abdomen and pelvic irradiation.
MATERIALS AND METHODS
Study design
This study is a randomized controlled clinical trials (The registry code IRCT2013052213433N1) designed to determine whether the use of a green tea tablet would decrease the frequency and severity of radiation-induced diarrhea, nausea and vomiting. The Medical Ethics Committee of Isfahan University of Medical Sciences has approved the study design, protocols, and informed consent procedure (Ethical Number: 1957).
Study population
Participants were recruited from research center, St. S. Al-Shohada Hospital, which is the one of the referral center for cancer treatment and rehabilitation in Isfahan, Iran. According to the inclusion and exclusion criteria, a total number of 42 participants (21/group) were enrolled in the study. Patients receiving standardized abdomen and pelvic irradiation 5000 cGy (1000 cGy weekly) for prostate, uterus, cervix, bladder, rectum and colon, willing and able to provide written informed consent for study participation were included. Patients with past history of irradiation, diarrhea before the beginning of pelvic irradiation were ineligible for the study. Exclusion criteria included occurrence of unbearable diarrhea, taking another drug for treatment of diarrhea during the study and unwilling to participate in the study at any time.
Sampling and random allocation
Eligible participants were selected from patients who were candidate for abdomen or pelvic radiotherapy by using simple sampling method. Selected patients were randomly assigned to intervention or placebo groups.
Treatment
Patients in intervention group receive one tablet daily of either 450 mg green tea (Camgreen, Iran Giahessence Pharmacy Co.) or a placebo, which appeared identical for 5 weeks. The placebo tablets were composed of coloring agent, lactose and purified water (Isfahan Farabi Pharmacy Co., Iran).
Sample size and power
The Sample size was based on the frequency of severe radiation-induced diarrhea in the placebo group needing antidiarrheal medication (p1 = 0.32), as against 9% of patients in the study drug group (p2 = 0.09).[23] With α = 0.05 and β = 20%. The calculations yielded 42 subjects for the study (21 subjects/group).
Blinding
Both groups of patient (intervention and control) were blinded to the treatment (Green tea or placebo). Physicians responsible for irradiation and toxicity grading were blinded to the results of randomization. The key of coding concerning group assignment is only known by the programmer of the database that is used during the study.
Assessment
Every week all patients were requested to record daily the number and consistency of their stools and the occurrence of symptoms such as nausea, vomiting, and intestinal cramps, by means of diary cards from 1st day of 2nd week to end of 5th weeks. The mean value of every week was calculated.
Diarrhea was evaluated according to the Common Toxicity Criteria of the National Cancer Institute version 3.0 developed by National Cancer Institute.[24] If the patient were located in group of 0, 1 and 2, 3 and 4, the diarrhea was accepted as grade normal, mild, severe, respectively.
The severity of vomiting was assessed using the functional living index emesis:[25] mild, score ≤6; moderate, score = 7-12; severe, score = 13-15.
Follow-up protocol
For both intervention and control group, all assessments (diarrhea and vomiting scoring) were weekly conducted for 4 weeks (2nd to 5th week).
Sociodemographic data included demographic information (age, gender, receiving chemotherapy, and intestinal resection), collected through a questionnaire.
Analysis
Analyses were performed to estimate and compare the frequency and severity of diarrhea and vomiting in treatment and placebo group. All descriptive statistics are presented as mean ± standard deviation for quantitative variables and number and percentage for categorical variables. Two-sample t-tests were used for the comparison of continuous variables and Pearson's Chi-square tests were used for the comparison of categorical variables. To compare the grade of diarrhea and vomiting between two groups, we used Mann–Whitney U-test. To compare the score of diarrhea and vomiting during 4 weeks, nonparametric analysis (Friedman) was used. Intention-to-treat method was used for statistical analysis. Surgery procedure and receiving chemotherapy would consider as a confounder if the results of the Chi-square test were statistically significant.
All comparisons were carried out on a two-tailed basis. Statistical analysis was carried out with the SPSS (version 20) and P < 0.05 was considered to be statistically significant. 95% confidence intervals for the proportions were calculated.
RESULTS
Between February 2013 and September 2013 a total of 42 participants acquired the inclusion criteria and were randomly allocated to either the treatment with green tea or placebo [Figure 1]. All patients weekly received radiation for 5 weeks and completed the course of study. Patient characteristics (mean age, gender, the number of receiving chemotherapy and undergoing intestinal resection) are presented in Table 1. There were no significant differences in distribution of demographic characteristics and the frequency of receiving chemotherapy and surgery between intervention and control groups.
Figure 1.
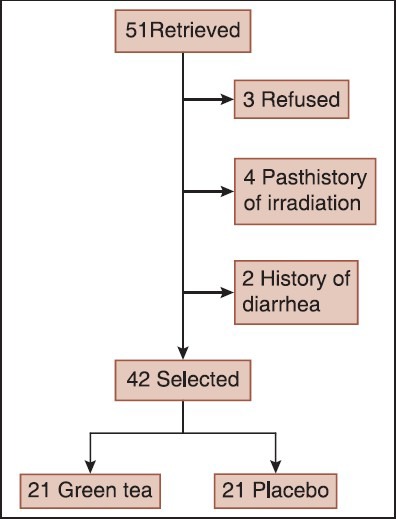
The study design
Table 1.
Patient's characteristics at baseline
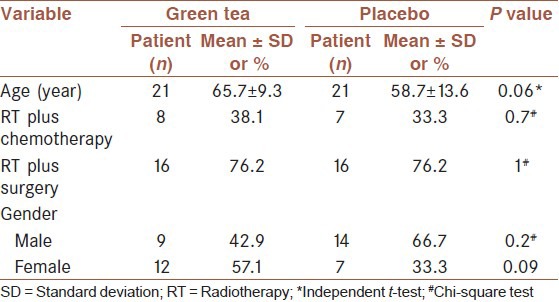
Average patient age was 62.2 years, with no significant difference between groups (P < 0.06).
During 1st week irradiation, diarrhea occurred in 23% and 14% of the green tea and placebo groups, respectively (P < 0.4) as well as, vomiting reported in 23% of green tea group and 19% of the placebo groups (P < 0.2). The average reported diarrhea and vomiting in placebo group were higher than green tea throughout 4 weeks [Tables 2 and 3].
Table 2.
Frequency of diarrhea grade (mild, severe) in different times
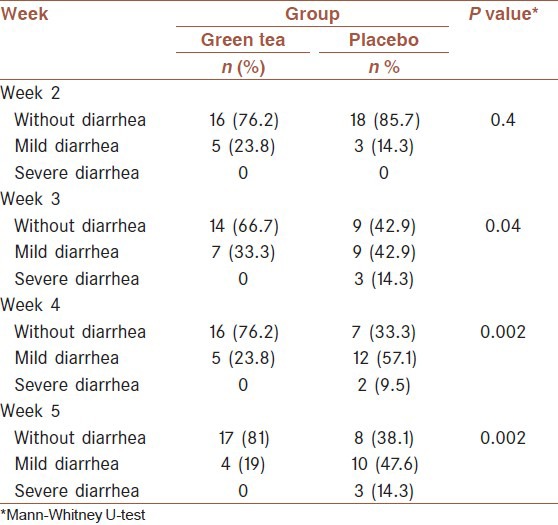
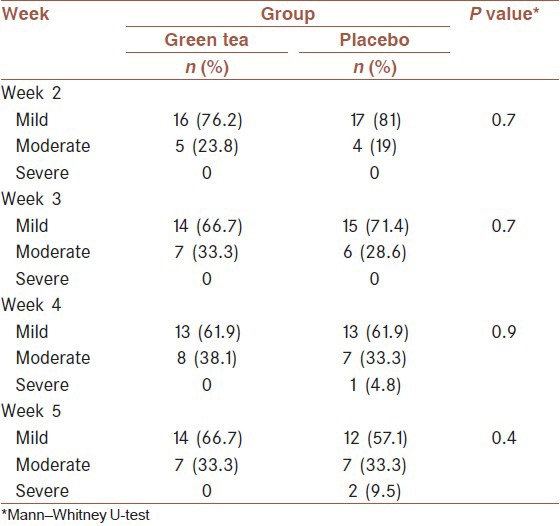
Table 3.
Frequency of grade (mild, moderate, and severe) vomiting by group
As shown in Table 2, Mann-Whitney U-test analyses of participants indicated that treatment with green tea had a significant effect on decreasing diarrhea induced radiotherapy. The majority of patients in the treatment group (81%) had no history of diarrhea at the end of 4th week, while 61.9% of patients received placebo reported having diarrhea at the same time. As shown in Figure 2, curves of severity of diarrhea differ between the two groups.
Figure 2.
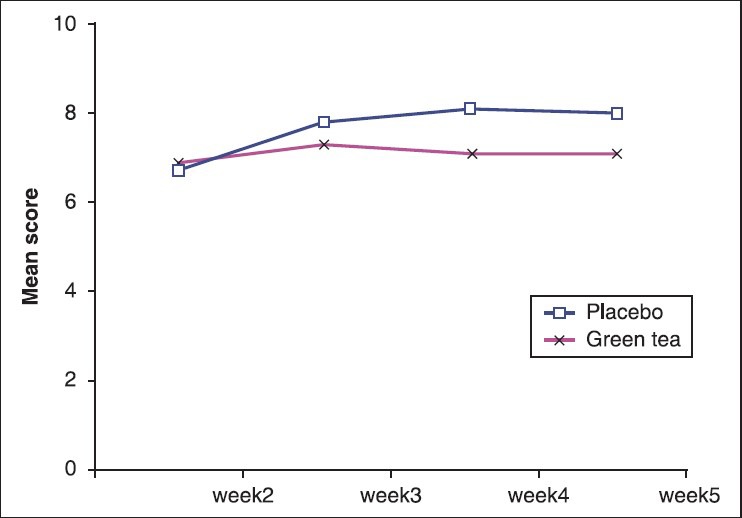
Mean score of diarrhea in 2 groups
Mann-Whitney U-test result for vomiting was presented in Table 3. Although not achieving statistical significance, no severe vomiting reported in treatment group during 3rd and 4th week.
Using Friedman analysis, the score of diarrhea did not significantly increase throughout 4 weeks in patients receiving green tea (P < 0.3). However not surprisingly, there was a significant increase in the score of diarrhea in placebo group (P < 0.005). Friedman analysis indicated that there was no significant increase in vomiting in both groups during irradiation period.
The green tea tablet was well-tolerated, and any adverse events reported.
DISCUSSION
We have reported the effect of treatment with green tea on the radiation induced diarrhea and vomiting of patients with abdomen or pelvic malignancy. This study shows that irradiation procedure induced diarrhea in about 37% of the patients and induced vomiting in 42% of the patients receiving a standardized protocol for pelvic malignancies that this side-effect was rarely severe, and that green tea treatment was successful to decrease diarrhea. Patients who received green tea experienced fewer diarrheas than those who received placebo and reported having any severe diarrhea. Green tea 450 mg daily was found to be effective and well tolerated. As we mentioned, Gilinsky et al. also reported that diarrhea is a problem encountered in patients receiving abdomen and pelvic radiotherapy, occurs in approximately 75% of patients and usually begins in the 2nd or 3rd week of RT.[23] as well as, some studies showed that the majority of patients having mild to moderate diarrhea and vomiting.[5,24] Studies reported radiotherapy has a direct effect on small bowel motility, and this change in motility in turn predisposes to bacterial overgrowth and in some patients episodic pseudo-obstruction.[24]
There is empirical evidence for the effectiveness of green tea in treating diarrhea in Asia since ancient times.[25,26,27] The health effects of green tea are attributed to its epigallocatechin-3-gallate content. Catechin inhibits the stimulation of nitric oxide (NO)-synthase. NO is a bioactive molecule that plays an essential role in inflammation.[22,28] some studies reported Rats and Mice with colitis similar to the condition found in humans improved when treated with a catechin-rich extract of green tea leaves.[29,30] As well as, researchers found that a catechin from green tea could inhibit the bacterial toxic effect on the cells.[29] In their study Ikigai et al. they found that there is a strong antibacterial catechin in green tea.[31]
Stryker et al. examined the effect of cholestyramine on treating diarrhea related radiotherapy and they reported cholestyramine may not be clinically useful because of its side effects.[11] While in our study, there was no reported side effect and all patients can successfully complete the study course. It is logical if we mentioned that an ideal treatment for any complication occurred during radiotherapy or chemotherapy in patients with cancer should have little or no toxicity, high efficacy, capability of oral consumption, a known mechanism for action, low cost, and acceptance.
Our study failed to show a significant effect on radiotherapy induced vomiting when the two groups were compared. When vomiting grade was evaluated, none of the patients in the green tea-treated group had severe vomiting, but in the placebo group, severe vomiting was seen in 14% of the patients. According to the antiemetic guidelines radiotherapy induced vomiting suggested that the 5-HT 3 antagonists had significantly greater protection than other treatment such as non-5-HT 3 antagonists and corticosteroids.[32]
Our study, while having much strength, involved a limitation that should be considered. This study had a small sample size, which reduced the statistical power. Future research with larger sample size should compare the effect of green tea treatment with other traditional treatment on radiotherapy induced diarrhea and vomiting.
CONCLUSION
Our detailed analysis of gastrointestinal toxicity of this randomized clinical trial suggests that green tea administration reduces the frequency and severity of acute radiation-induced diarrhea in patient with pelvic and abdomen malignancy. We feel these results can suggest an evaluation of green tea in comparing other available drugs to conclusively assess its ability to decrease gastrointestinal complication of radiotherapy.
AUTHORS’ CONTRIBUTION
F. Nikoobin was the main investigator, who analyzed the data and wrote the paper. H. Emami helped in designing the study, contributed to the analysis, and helped in writing the final paper. M. Roayaei contributed to the study design, data analysis, and writing of the paper. H. Zia contributed to data analysis and writing of the paper. All authors read and approved the final version of the paper.
ACKNOWLEDGMENT
This study was funded by research chancellor of Isfahan University of Medical Sciences as a dissertation Project no1957. The authors’ heartfelt thanks are extended to all the patients who so graciously agreed to participate in this study.
Footnotes
Source of Support: This study was funded by research chancellor of Isfahan University of Medical Sciences as a dissertation Project no1957
Conflict of Interest: No conflict of interests.
REFERENCES
- 1.Yeoh E, Horowitz M, Russo A, Muecke T, Robb T, Maddox A, et al. Effect of pelvic irradiation on gastrointestinal function: A prospective longitudinal study. Am J Med. 1993;95:397–406. doi: 10.1016/0002-9343(93)90309-d. [DOI] [PubMed] [Google Scholar]
- 2.Kao MS. Intestinal complications of radiotherapy in gynecologic malignancy — Clinical presentation and management. Int J Gynaecol Obstet. 1995;49(Suppl):S69–75. doi: 10.1016/0020-7292(95)02412-6. [DOI] [PubMed] [Google Scholar]
- 3.Prevention of chemotherapy — and radiotherapy-induced emesis: Results of Perugia Consensus Conference. Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC) Ann Oncol. 1998;9:811–9. [PubMed] [Google Scholar]
- 4.Feyer PC, Stewart AL, Titlbach OJ. Aetiology and prevention of emesis induced by radiotherapy. Support Care Cancer. 1998;6:253–60. doi: 10.1007/s005200050163. [DOI] [PubMed] [Google Scholar]
- 5.Miller RC, Martenson JA, Sargent DJ, Kahn MJ, Krook JE. Acute treatment-related diarrhea during postoperative adjuvant therapy for high-risk rectal carcinoma. Int J Radiat Oncol Biol Phys. 1998;41:593–8. doi: 10.1016/s0360-3016(98)00084-4. [DOI] [PubMed] [Google Scholar]
- 6.Fyles AW, Pintilie M, Kirkbride P, Levin W, Manchul LA, Rawlings GA. Prognostic factors in patients with cervix cancer treated by radiation therapy: Results of a multiple regression analysis. Radiother Oncol. 1995;35:107–17. doi: 10.1016/0167-8140(95)01535-o. [DOI] [PubMed] [Google Scholar]
- 7.Rose PG, Ali S, Watkins E, Thigpen JT, Deppe G, Clarke-Pearson DL, et al. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:2804–10. doi: 10.1200/JCO.2006.09.4532. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton AS, Stanford JL, Gilliland FD, Albertsen PC, Stephenson RA, Hoffman RM, et al. Health outcomes after external-beam radiation therapy for clinically localized prostate cancer: Results from the Prostate Cancer Outcomes Study. J Clin Oncol. 2001;19:2517–26. doi: 10.1200/JCO.2001.19.9.2517. [DOI] [PubMed] [Google Scholar]
- 9.Polistena A, Johnson LB, Ohiami-Masseron S, Wittgren L, Bäck S, Thornberg C, et al. Local radiotherapy of exposed murine small bowel: Apoptosis and inflammation. BMC Surg. 2008;8:1. doi: 10.1186/1471-2482-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giralt J, Regadera JP, Verges R, Romero J, de la Fuente I, Biete A, et al. Effects of probiotic Lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhea: Results from multicenter, randomized, placebo-controlled nutritional trial. Int J Radiat Oncol Biol Phys. 2008;71:1213–9. doi: 10.1016/j.ijrobp.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Stryker JA, Chung CK, Layser JD. Colestipol hydrochloride prophylaxis of diarrhea during pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 1983;9:185–90. doi: 10.1016/0360-3016(83)90097-4. [DOI] [PubMed] [Google Scholar]
- 12.Doyle D, Hanks GVC, MacDonald N, editors. UK: Oxford University Press; 1993. Oxford Textbook of Palliative Medicine. [Google Scholar]
- 13.Shah A. BCCA guidelines for management of chemotherapy-induced diarrhea. 2007 [Google Scholar]
- 14.Yavuz MN, Yavuz AA, Aydin F, Can G, Kavgaci H. The efficacy of octreotide in the therapy of acute radiation-induced diarrhea: A randomized controlled study. Int J Radiat Oncol Biol Phys. 2002;54:195–202. doi: 10.1016/s0360-3016(02)02870-5. [DOI] [PubMed] [Google Scholar]
- 15.Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2013 doi: 10.1016/j.clnu.2013.10.015. In press. [DOI] [PubMed] [Google Scholar]
- 16.Hamad A, Fragkos KC, Forbes A. A systematic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353–60. doi: 10.1016/j.clnu.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Zaveri NT. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–80. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Stapleton PD, Shah S, Ehlert K, Hara Y, Taylor PW. The beta-lactam-resistance modifier (-)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology. 2007;153:2093–103. doi: 10.1099/mic.0.2007/007807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donà M, Dell’Aica I, Calabrese F, Benelli R, Morini M, Albini A, et al. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003;170:4335–41. doi: 10.4049/jimmunol.170.8.4335. [DOI] [PubMed] [Google Scholar]
- 20.Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: A literature review. Chin Med. 2010;5:13. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 22.Mukhtar H, Ahmad N. Green tea in chemoprevention of cancer. Toxicol Sci. 1999;52:111–7. doi: 10.1093/toxsci/52.2.111. [DOI] [PubMed] [Google Scholar]
- 23.Gilinsky NH, Burns DG, Barbezat GO, Levin W, Myers HS, Marks IN. The natural history of radiation-induced proctosigmoiditis: An analysis of 88 patients. Q J Med. 1983;52:40–53. [PubMed] [Google Scholar]
- 24.Andreyev HJ, Vlavianos P, Blake P, Dearnaley D, Norman AR, Tait D. Gastrointestinal symptoms after pelvic radiotherapy: Role for the gastroenterologist? Int J Radiat Oncol Biol Phys. 2005;62:1464–71. doi: 10.1016/j.ijrobp.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 25.Wu CH, Lu FH, Chang CS, Chang TC, Wang RH, Chang CJ. Relationship among habitual tea consumption, percent body fat, and body fat distribution. Obes Res. 2003;11:1088–95. doi: 10.1038/oby.2003.149. [DOI] [PubMed] [Google Scholar]
- 26.Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, et al. Glucuronides of tea catechins: Enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003;31:452–61. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]
- 27.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother Res. 2006;20:519–30. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- 28.Dell’Aica I, Niero R, Piazza F, Cabrelle A, Sartor L, Colalto C, et al. Hyperforin blocks neutrophil activation of matrix metalloproteinase-9, motility and recruitment, and restrains inflammation-triggered angiogenesis and lung fibrosis. J Pharmacol Exp Ther. 2007;321:492–500. doi: 10.1124/jpet.106.116459. [DOI] [PubMed] [Google Scholar]
- 29.Chan L. Highways and the digestive tract. Wellness Options. 2009;4:25. [Google Scholar]
- 30.Brückner M, Westphal S, Domschke W, Kucharzik T, Lügering A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J Crohns Colitis. 2012;6:226–35. doi: 10.1016/j.crohns.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta. 1993;1147:132–6. doi: 10.1016/0005-2736(93)90323-r. [DOI] [PubMed] [Google Scholar]
- 32.Feyer PCh, Maranzano E, Molassiotis A, Clark-Snow RA, Roila F, Warr D, et al. Radiotherapy-induced nausea and vomiting (RINV): Antiemetic guidelines. Support Care Cancer. 2005;13:122–8. doi: 10.1007/s00520-004-0705-3. [DOI] [PubMed] [Google Scholar]


