Abstract
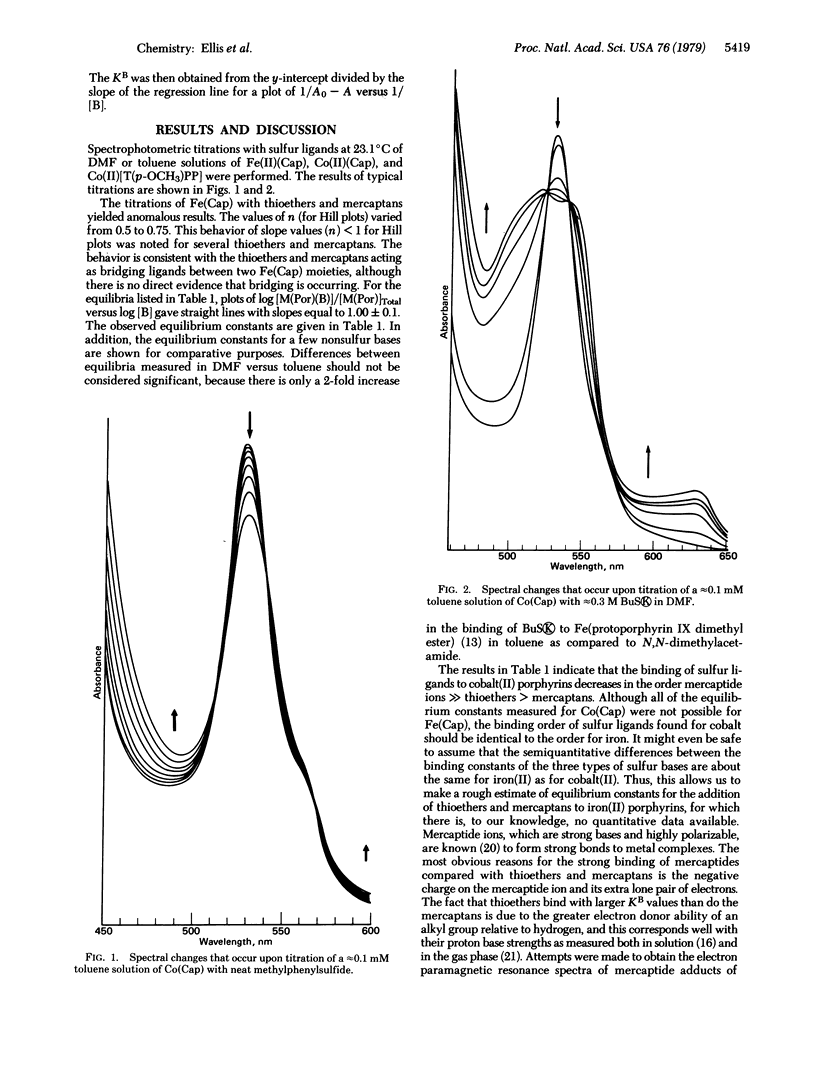
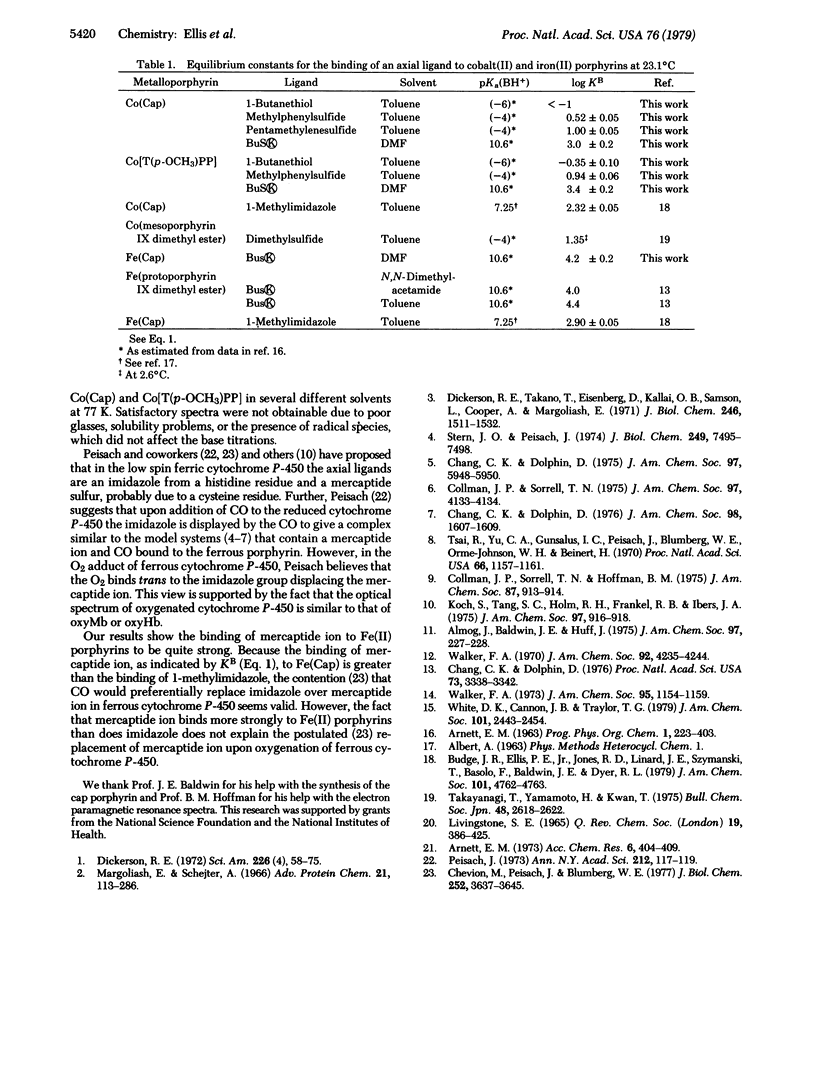
Equilibrium constants for the binding of sulfur bases to cobalt(II) porphyrins were measured in toluene solution by a spectrophotometric method. The order of decreasing binding strength of sulfur ligands to cobalt(II) porphyrins was found to be mercaptide ions » thioethers > mercaptans. It is suggested that a similar stability order of these sulfur ligands should exist towards iron(II) porphyrins, but formation constants could be obtained only for the mercaptide ions.
Keywords: equilibrium constants, cytochrome P-450 mechanism
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang C. K., Dolphin D. Carbon monoxide binding to pentacoordinate mercaptide-heme complexes: kinetic study on models for cytochrome P-450. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3338–3342. doi: 10.1073/pnas.73.10.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. K., Dolphin D. Letter: Ferrous porphyrin-mercaptide complexes. Models for reduced cytochrome P-450. J Am Chem Soc. 1975 Oct 1;97(20):5948–5950. doi: 10.1021/ja00853a069. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Dolphin D. Letter: Oxygen binding to mercaptide-heme complexes. Models for reduced cytochrome P-450. J Am Chem Soc. 1976 Mar 17;98(6):1607–1609. doi: 10.1021/ja00422a069. [DOI] [PubMed] [Google Scholar]
- Chevion M., Peisach J., Blumberg W. E. Imidazole, the ligand trans to mercaptide in ferric cytochrome P-450. An EPR study of proteins and model compounds. J Biol Chem. 1977 Jun 10;252(11):3637–3645. [PubMed] [Google Scholar]
- Collman J. P., Sorrell T. N., Hoffman B. M. Letter: Models for cytochrome P-450. J Am Chem Soc. 1975 Feb 19;97(4):913–914. doi: 10.1021/ja00837a050. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Sorrell T. N. Letter: A model for the carbonyl adduct of ferrous cytochrome P450. J Am Chem Soc. 1975 Jul 9;97(14):4133–4134. doi: 10.1021/ja00847a046. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Dickerson R. E. The structure and history of an ancient protein. Sci Am. 1972 Apr;226(4):58–passim. doi: 10.1038/scientificamerican0472-58. [DOI] [PubMed] [Google Scholar]
- Koch S., Tang S. C., Holm R. H., Frankel R. H., Ibers J. A. Letter: Ferric porphyrin thiolates. Possible relationship to cytochrome P-450 enzymes and the structure of (p-nitrobenzenethiolato)iron(III) protoporphyrin IX dimethyl ester. J Am Chem Soc. 1975 Feb 19;97(4):916–918. doi: 10.1021/ja00837a052. [DOI] [PubMed] [Google Scholar]
- Margoliash E., Schejter A. Cytochrome c. Adv Protein Chem. 1966;21:113–286. doi: 10.1016/s0065-3233(08)60128-x. [DOI] [PubMed] [Google Scholar]
- Stern J. O., Peisach J. A model compound study of the CO-adduct of cytochrome P-450. J Biol Chem. 1974 Dec 10;249(23):7495–7498. [PubMed] [Google Scholar]
- Tsai R., Yu C. A., Gunsalus I. C., Peisach J., Blumberg W., Orme-Johnson W. H., Beinert H. Spin-state changes in cytochrome P-450cam on binding of specific substrates. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1157–1163. doi: 10.1073/pnas.66.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F. A. Reactions of monomeric cobalt-oxygen complexes. I. Thermodynamics of reaction of molecular oxygen with five- and six-coordinate amine complexes of a cobalt porphyrin. J Am Chem Soc. 1973 Feb 21;95(4):1154–1159. doi: 10.1021/ja00785a026. [DOI] [PubMed] [Google Scholar]


