Abstract
Nowadays, scientific findings in the field of regeneration of nervous system have revealed the possibility of stem cell based therapies for damaged brain tissue related disorders like stroke. Furthermore, to achieve desirable outcomes from cellular therapies, one needs to monitor the migration, engraftment, viability, and also functional fate of transplanted stem cells. Magnetic resonance imaging is an extremely versatile technique for this purpose, which has been broadly used to study stroke and assessment of therapeutic role of stem cells. In this review we searched in PubMed search engine by using following keywords; “Stem Cells”, “Cell Tracking”, “Stroke”, “Stem Cell Transplantation”, “Nanoparticles”, and “Magnetic Resonance Imaging” as entry terms and based on the mentioned key words, the search period was set from 1976 to 2012. The main purpose of this article is describing various advantages of molecular and magnetic resonance imaging of stem cells, with focus on translation of stem cell research to clinical research.
Keywords: Cell fate, cell tracking, magnetic resonance imaging, nanoparticle, stem cells, stroke, tracking
INTRODUCTION
Recent scientific findings about the possibility of regeneration of the nervous system have revealed the differentiation of stem cells into neural cells and stem cell-based brain tissue regeneration.[1,2] Although, several basic mechanisms were defined for stem cell journey and functions in the body and their therapeutic roles, it needs to be fully clarified.[3] This clarification can be performed by experimental and clinical research projects to promote stem cell transplantation.[4] Therefore, the migration, engraftment, long term viability, and also functional fate of transplanted stem cells should be assessed by noninvasive imaging modalities particularly, in clinical cell transplantation trials.[5,6,7] Magnetic resonance imaging (MRI) is an extremely versatile technique for this purpose,[1] which has been successfully contributed to study stroke and evaluation of therapeutic role of stem cells. Therefore, MRI as a “Magnetic Imaging” technique has taken its place among the other imaging modalities.[8] In this review, we will describe MRI for evaluating the migration, engraftment, and fate of transplanted stem cells in stroke by using nanotechnologies, with focus on translation of stem cell research to clinical research.
MATERIALS AND METHODS
In this review to introduce MRI as a clinical compatible imaging technique for evaluating the fate, engraftment and migration of transplanted stem cells in Stroke, “Stem Cells”, “Cell Tracking”, “Stroke”, “Stem Cell Transplantation”, “Nanoparticles”, and “Magnetic Resonance Imaging” were used as search terms by using PubMed search engine. Subsequently, the search period was set from 1976 to 2012.
Stroke
Stroke is defined by the World Health Organization (WHO), as “rapidly developing signs of focal or global disturbance of cerebral function lasting more than 24 hours (unless interrupted by surgery or death) with no apparent cause other than a vascular origin”.[9] There are two types; ischemic stroke (caused by an occlusion) and hemorrhagic stroke (initiated by a rupture) of a blood vessel in the brain. Among them, ischemic stroke is the most common type.[10] Stroke is the main cause of adult disability and one of the leading causes of irreversible neurological damages and death worldwide.[11,12,13,14,15,16] It is also the 6th leading cause of diseases burden, while around one third of deaths from stroke occur in developing countries.[17] According to the estimation of the WHO, in 2004 the stroke mortality rate was reported around 5.7 million, that was equal to 9.7% of the deaths worldwide. Of them, more than 85%, happened in low and middle-income countries.[18] There are some considerable geographic and regional variations and differences in stroke types, incidence, mortality, and distribution around the world.[19]
Incidence pattern of stroke in Iran
The epidemiologic data of stroke in the Middle East regions are limited and unreliable.[19] Stroke is becoming a serious health problem in this region, with the mortality rate expected to double by 2030. There is a lack of data on the burden of stroke in Iran.[17] Tran et al., reported stroke incidence rate as 10.4 per 1,00,000 in Iran, with a higher rate in women.[18] Azarpazhooh et al., demonstrated that, the incidence of stroke in Iran is significantly greater than most of the western countries, with an occurrence in younger ages. They also indicated the history of hypertension as a main risk factor[16] followed by smoking and diabetes.[17] Ischemic stroke is the most common type in Iran, similar to previous reports from other regions of the world.[17] As stroke is an important health problem, with a huge burden on health economy, it specifically requires effective and novel treatments to regenerate damaged brain tissue.
Stem cell transplantation in stroke
In spite of recent considerable advances in stroke management, current acute treatment methods, have only mild effects and moderately restore its lost function.[11,15] Therefore stroke remains a major cause of disability and needs much more efficient management methods, such as cell replacement in the ischemic region to prevent further disability.[11,20] Cell transplantation is a novel treatment method in many fields of medicine, including stroke as a nervous system disorder.[21] In the last decade, evidence of neurogenesis probability in the human adult brain has provided the basic scientific hypothesis of (stem) cell transplantation therapy in various neurological disorders including; Parkinson's disease, multiple sclerosis, and stroke, to improve neurological defects and relieve disability.[11,22,23,24] Initial animal and experimental studies explored the significant benefit of stem cell transplantation in neuroregeneration and improvement in neural functioning.[11,13,25,26] Stroke is one of the neurological disorders, which has been selected as a pioneering trial in the clinical application of stem cell.[27] Furthermore several studies demonstrated the feasibility of stem cell-based therapy for the restoration of lost brain function[13,14,20] and improvement of the clinical outcome in stroke patients.[10,28,29,30] Several experimental and clinical researches have introduced different types of stem cell for transplantation in stroke.[11,14,15,16,20,22,27,28,29,30,31,32,33,34,35,36,37] In recent years, different experimental and clinical cell transplantation studies have been started in Iran, as a leading country in the Middle East[38] for central nervous system (CNS) disorders including; spinal cord injury (SCI)[21,39,40,41] and stroke.[42] Also, based on our suggestion, the Iranian Food and Drug Organization (FDO) started working on a policy to regulate and harmonize human cell and tissue manufacturing activities specially for improving clinical (stem) cell transplantation safety and efficacy as a national standard. This national standard can be a basic reference for cell therapy facilities, to address minimal safety considerations.[43] According to such scientific and organizational advances, in the field of stem cell therapy in the country, it sounds crucially important to clarify the shady corners of cell transplantation therapy.[34] Moreover clarification of optimal cell dosing, route of transplantation, cell delivery methods, and in vivo cell imaging techniques is needed to ensure safety, efficacy, expected outcome, and more success of potential stem cell transplantation trials.[30]
Stem cell imaging and tracking modalities in stroke
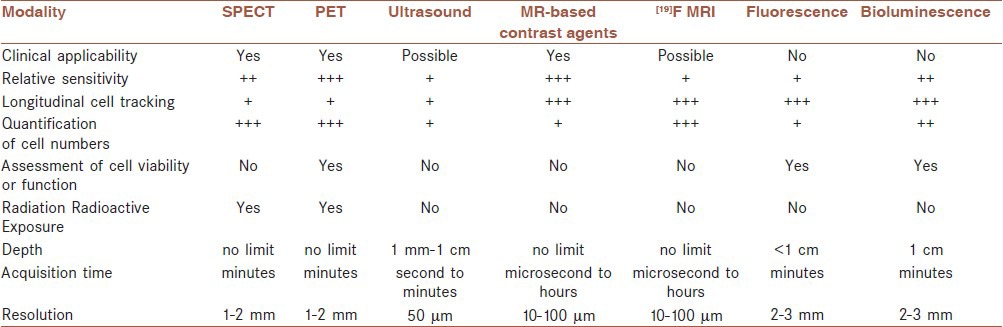
Stem cell's therapeutic effects in the treatment of various neurological disorders have been experimentally demonstrated. Well-guided stem cell migration, differentiation, survival, and engraftment to the site of injury, may reinforce these beneficial effects on the treatment of human damaged brain.[44,45] Therefore, stem cell therapy needs to be assessed and monitored by imaging and tracking the transplanted cells,[46] for developing the brain restorative treatment strategies.[4,47,48,49] Hence, to achieve this aim, stem cell imaging techniques are performed as pioneering investigation to monitor, control, and treat biological systems, in particular, the brain.[50] Moreover, stem cell imaging by non-invasive modalities allows their monitoring overtime. Therefore, finding a non-invasive method to track stem cells in the human body is an essential step before translating stem cell research into clinical research. Several studies in animal models and humans have demonstrated that, clinical translation of imaging modalities from the basic to the clinical research is feasible.[51] During the last few years, various imaging techniques, particularly magnetic resonance imaging, equipped with different contrast agents, have been applied for this purpose.[52,53,54,55] It is elucidated that, noninvasive imaging modalities facilitates accomplishing suitable therapeutic effects in clinical stem cell trials[47,56,57,58] while understanding stem cell migration and differentiation mechanisms.[59] Ideally, in vivo stem cell tracking and imaging depict stem cell migration, viability, and also their functions,[3,60] considering the preferential engraftment of stem cells on to the site of the injured brain.[61] These techniques are crucial to guide and promote significant advances in stem cell transplantation research and its clinical application for neurological diseases in the future.[62] There are various cell imaging modalities including optical imaging, bioluminescence imaging (BLI), ultrasound, computed tomography (CT), positron emission tomography (PET), single photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI) that clinical stem cell trials will benefit from[29,49,56,63] real-time depiction of cell migration and journey in the body, and promotes optimizing preclinical and clinical stem cell transplantation studies. Advantages and disadvantages of several imaging modalities are demonstrated in Table 1.[53,64,65] Nowadays, PET, SPECT, and MRI are suitable candidates for human nervous system cellular imaging. Among them, PET is more sensitive to low concentration of contrast agents. However, it has some limitations as low spatial resolution, radiation exposure, and short-term signal production.[50] Another technique is optical imaging, which is a sensitive method with some distinct advantages in small animal models but, it is not feasible for human whole-body visualization because of the limited penetration depth and low spatial resolution. Although, a high spatial resolution can be provided by other methods like micro-CT, this technique is not always suitable for in vivo human studies and it needs to be optimized for a better cell detection in the whole body.[66] With respect to the full commitment of clinical studies and trials to patients’ safety, radiation and radioactive exposures are important limitations of CT, PET, SPECT, and Scintigraphy. Therefore, MRI can be preferred as a superior method for cell tracking and imaging particularly, in clinical trials.[3,51] Several stem cell tracking studies have been performed by using MRI. A main clinical field in which, MRI has been used for stem cell tracking is neurological diseases.[56,67]
Table 1.
Several in vivo cell imaging modalities
MR Imaging of transplanted stem cells in stroke
Nowadays, in vivo stem cell imaging introduces a novel view on stem cell research in stroke.[68] Although, various clinical trials have depicted the stem cell transplantation safety in stroke, revealing probable mechanisms of cell delivery is crucially important as a main subdivision of stem cell therapy[11,36] in different types of disease such as neurological disorders. Recently, MRI stem cell tracking has become an important method for real-time, noninvasive imaging and following cell migration, engraftment, survival, differentiation, and subsequently the efficacy of clinical cell therapy trials.[25,56,69,70] MRI is a well-defined noninvasive cell imaging technique, which has some valuable advantages,[71] for instance, it is able to provide an excellent image quality, high spatial 3D resolution, superior sensitivity, identifying labeled cells in their anatomical context, additional information about the surrounding milieu, and clinical applicability with no exposure to ionizing radiation.[3,5,26,54,56,63,66,68,72,73,74,75] The MR tracking of transplanted progenitor cells in the CNS has been performed by several investigators. The first relevant studies were reported in 1992, in which superparamagnetic contrast agents were used for cell imaging in rat brain.[26] Today, imaging of Superparamagnetic iron oxide (SPIO) labeled (stem) cells is already routinely used in animal models of neurological diseases[52,56] including, SCI and stroke.[26] In summary, MRI provides many requirements of a noninvasive cell imaging and monitoring in vivo,[4] while it can be equipped with nanotechnology.[76] It can be also used serially to follow and identify the distribution scenery of transplanted stem cells in stroke.[61] Hence, its application in human trials needs safety controls. Gadolinium chelates and iron oxide particles are currently the best contrast agent candidates to label cells for MRI, because they are well tolerated when directly injected in the blood stream.[50,77,78] Several contrast agents which are used in MR cell imaging are compared in [Table 2].[71] Some of them such as Gadolinium may be coupled with the fluorescent compound allowing their detection by histology in experimental studies.[61,77,79]
Table 2.
MRI contrast agents for cell imaging
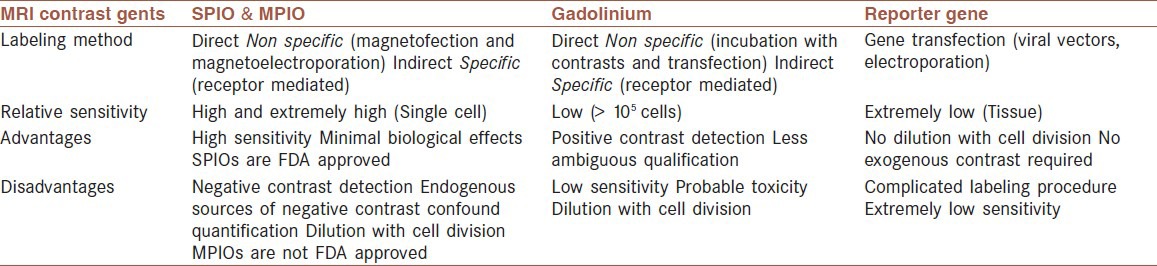
Gadolinium as a T1-weighted contrast agent increases T1 relaxation time thus resulting in bright contrast image. On the other hand, SPIO nanoparticles as negative contrast agents reduce T2 relaxation and consequently, produce hypointense negative (black) signals.[3] Furthermore, in susceptibility weighted image (SWI) and diffusion-weighted image (DWI) of MRI, labeled stem cells with SPIO nanoparticles produce dark spots.[80]
Limitation of MR imaging of stem cells
MRI has some limitations which restrict its unique advantages in some cases particularly, long-term cell imaging. For instance, contrast agent may be diluted due to cell division, especially when the cells are rapidly dividing.[45,56,81,82,83] Sometimes, certain endogenous conditions can introduce hypointense MR signals, which can be confused with the MRI contrast agents. Another limitation can be induced by macrophages, while are loaded with hemosiderin from hemorrhage or contrast agents, and shown as hypointense signals similar to the labeled cells.[56,84] Also, discrimination between live and dead cells is not possible by MRI, because magnetic contrast agents could remain in the site of injured or ischemic brain tissue and produce detectable signals.[45,56,72,81] Furthermore, clinical imaging has more limitation compared with experimental animal studies, for instance, animal MRI scanners can reach16T or higher, whereas high field in human studies is around 7T, as most clinical MRI scanners being less than 3T in the country.[2,8] Another concern is the probable negative effect of MRI contrast agents such as SPIO or USPIO, on the differentiation and metabolism of labeled cells, which were reported in a few studies, while several researches stated no negative or harmful effect.[77,85,86] In spite of MRI single cell detection in some experimental studies, in most cases it requires clusters of labeled cells to detect.[74] Of course, some novel techniques of transfecting agents and new methods for producing contrast labels try to overcome the limitations of MRI stem cell tracking in neurological diseases, which introduce promising results for future clinical stem cell tracking trials using these novel methods[5] in different disorders including neurological diseases.
CONCLUSION AND FUTURE PERSPECTIVES
Neuro-transplantation by using (stem) cells has introduced some promising aspects for the treatment of several CNS disorders such as stroke.[34] Monitoring of transplanted cells is an interesting field to achieve desirable therapeutic effect after transplantation. Although this modality is still in its infancy of development, several experimental and further clinical studies have demonstrated that molecular imaging methods as in vivo monitoring modalities can potentially depict the manner of cell migration and journey in the body.[72] In vivo cell imaging and tracking can provide special purposes, for instance, cell engraftment, migration, and survival.[3,58] It has also been performed based on extensive longitudinal and histopathological studies, which are conducted by sacrificing animal subjects in different intervals after transplantation, whereas molecular imaging modalities can track transplanted (stem) cells in real time.[58,64,87] Various studies have revealed that tracking transplanted (stem) cells in SCI and stroke is perfectly feasible. Such modalities can be linked to stem cell transplantation methods for facilitating translation of stem cell transplantation from the basic research into the clinic.[58,88] As it has been described previously, MRI cell imaging has several advantages over other techniques such as PET, SPECT, CT, and ultrasound that make it more compatible to clinical grade cell monitoring purpose. On the other hand, MRI has an established role in different preclinical and clinical studies on stroke.[6] Therefore, it is becoming a fundamental part of clinical cell transplantation trials to periodically monitor the distribution of transplanted (stem) cells[25,89] in various diseases including, ischemic brain disorders.[68] For instance, successful cell tracking in 19 human trials, have been reported by McColgan et al., which revealed increasing progress in imaging techniques as a crucial part of various medical disciplines.[47] Stem cell imaging can also guide cell delivery, optimize transplantation protocols, and subsequently increase desirable therapeutic effects of (stem) cell therapy trials. Ideally, various imaging technologies should be combined to make it a noninvasive, safe and highly efficient multimodal manner which will be able to perform as a qualitative and also quantitative technique[48,53] to play its cardinal role in the field of advanced stem cell therapy[54] and transfer stem cell-based therapies from the bench to the bedside.[71] Thus, application of these methods to neurological diseases can increase (stem) cell therapeutic effects and improve the patient's outcome.[81] Furthermore, more clinical grade studies are needed to overcome some limitations of existing MR cell imaging methods.[79,90] For instance, recently, transgenic cell lines with inbuilt contrast agents are proposed for transplantation.[8,55,91] Also MR reporter genes were introduced for reporting the survival of implanted (stem) cells and overcoming agent dilution following cell division that were two major limitations of the present MR imaging techniques by using routine contrast agents.[56,57] In spite of performing such advanced instances of researches, for contributing novel cell imaging techniques,[3] further investigations are needed to elucidate different points of view towards clinical stem cell imaging in the human body.[66] This review suggests that equipping various (stem) cell therapy modalities with noninvasive MR imaging techniques particularly, in neurological disorders such as stroke, will strongly improve cellular therapy protocols, subsequent therapeutic effects, and patients’ outcome.
ACKNOWLEDGMENT
The authors would like to acknowledge Mehrnaz Sahebjam, Firoozeh Ghaderi, Fereshteh Mohamadi-Jahani, Maryam Kavousi, Faezeh Nouraei, and Mahrokh Nikmohamadi for their assistance in literature collection.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jendelova P, Herynek V, Urdzikova L, Glogarova K, Kroupova J, Andersson B, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232–43. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 2.Shyu WC, Chen CP, Lin SZ, Lee YJ, Li H. Efficient tracking of non-iron-labeled mesenchymal stem cells with serial MRI in chronic stroke rats. Stroke. 2007;38:367–74. doi: 10.1161/01.STR.0000254463.24655.14. [DOI] [PubMed] [Google Scholar]
- 3.Long CM, Bulte JW. In vivo tracking of cellular therapeutics using magnetic resonance imaging. Expert Opin Biol Ther. 2009;9:293–306. doi: 10.1517/14712590802715723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Focke A, Schwarz S, Foerschler A, Scheibe J, Milosevic J, Zimmer C, et al. Labeling of human neural precursor cells using ferromagnetic nanoparticles. Magn Reson Med. 2008;60:1321–8. doi: 10.1002/mrm.21745. [DOI] [PubMed] [Google Scholar]
- 5.Rogers WJ, Meyer CH, Kramer CM. Technology insight: In vivo cell tracking by use of MRI. Nat Clin Pract Cardiovasc Med. 2006;3:554–62. doi: 10.1038/ncpcardio0659. [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Cabrer P, Hoehn M. MRI stem cell tracking for therapy in experimental cerebral ischemia. Transl Stroke Res. 2012;3:22–35. doi: 10.1007/s12975-011-0111-3. [DOI] [PubMed] [Google Scholar]
- 7.Kraitchman DL, Bulte JW. Imaging of stem cells using MRI. Basic Res Cardiol. 2008;103:105–13. doi: 10.1007/s00395-008-0704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber R, Ramos-Cabrer P, Hoehn M. Present status of magnetic resonance imaging and spectroscopy in animal stroke models. J Cereb Blood Flow Metab. 2006;26:591–604. doi: 10.1038/sj.jcbfm.9600241. [DOI] [PubMed] [Google Scholar]
- 9.Hatano S. Experience from a multicentre stroke register: A preliminary report. Bull World Health Organ. 1976;54:541–53. [PMC free article] [PubMed] [Google Scholar]
- 10.Luo Y. Cell-based therapy for stroke. J Neural Transm. 2011;118:61–74. doi: 10.1007/s00702-010-0478-4. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee S, Williamson D, Habib N, Gordon M, Chataway J. Human stem cell therapy in ischemic stroke: A review. Age Ageing. 2011;40:7–13. doi: 10.1093/ageing/afq133. [DOI] [PubMed] [Google Scholar]
- 12.Vandeputte C, Thomas D, Dresselaers T, Crabbe A, Verfaillie C, Baekelandt V, et al. Characterization of the inflammatory response in a photothrombotic stroke model by MRI: Implications for stem cell transplantation. Mol Imaging Biol. 2011;13:663–71. doi: 10.1007/s11307-010-0395-9. [DOI] [PubMed] [Google Scholar]
- 13.Bersano A, Ballabio E, Lanfranconi S, Boncoraglio GB, Corti S, Locatelli F, et al. Clinical studies in stem cells transplantation for stroke: A review. Curr Vasc Pharmacol. 2010;8:29–34. doi: 10.2174/157016110790226570. [DOI] [PubMed] [Google Scholar]
- 14.Roh JK, Jung KH, Chu K. Adult stem cell transplantation in stroke: Its limitations and prospects. Curr Stem Cell Res Ther. 2008;3:185–96. doi: 10.2174/157488808785740352. [DOI] [PubMed] [Google Scholar]
- 15.Jiang M, Lv L, Ji H, Yang X, Zhu W, Cai L, et al. Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol Cell Biochem. 2011;354:67–75. doi: 10.1007/s11010-011-0806-5. [DOI] [PubMed] [Google Scholar]
- 16.Jang KS, Lee KS, Yang SH, Jeun SS. In vivo tracking of transplanted bone marrow-derived mesenchymal stem cells in a murine model of stroke by bioluminescence imaging. J Korean Neurosurg Soc. 2010;48:391–8. doi: 10.3340/jkns.2010.48.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosseini AA, Sobhani-Rad D, Ghandehari K, Benamer HT. Frequency and clinical patterns of stroke in Iran - Systematic and critical review. BMC Neurol. 2010;10:72. doi: 10.1186/1471-2377-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran J, Mirzaei M, Anderson L, Leeder SR. The epidemiology of stroke in the Middle East and North Africa. J Neurol Sci. 2010;295:38–40. doi: 10.1016/j.jns.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Azarpazhooh MR, Etemadi MM, Donnan GA, Mokhber N, Majdi MR, Ghayour-Mobarhan M, et al. Excessive incidence of stroke in Iran: Evidence from the Mashhad stroke incidence study (MSIS), a population-based study of stroke in the middle east. Stroke. 2010;41:e3–e10. doi: 10.1161/STROKEAHA.109.559708. [DOI] [PubMed] [Google Scholar]
- 20.Haas S, Weidner N, Winkler J. Adult stem cell therapy in stroke. Curr Opin Neurol. 2005;18:59–64. doi: 10.1097/00019052-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Aghayan HR, Arjmand B, Norouzi-Javidan A, Saberi H, Soleimani M, Tavakoli SA, et al. Clinical grade cultivation of human Schwann cell, by using human autologous serum instead of fetal bovine serum and without growth factors. Cell Tissue Bank. 2012;13:281–5. doi: 10.1007/s10561-011-9250-8. [DOI] [PubMed] [Google Scholar]
- 22.Savitz SI, Rosenbaum DM. Stroke recovery with cellular therapies. In: Tarsy D, editor. Current Clinical Neurology. 2nd edn. Totowa, New Jersy: Humana Press; 2008. pp. vii–xii. [Google Scholar]
- 23.Kim DE, Schellingerhout D, Ishii K, Shah K, Weissleder R. Imaging of stem cell recruitment to ischemic infarcts in a murine model. Stroke. 2004;35:952–7. doi: 10.1161/01.STR.0000120308.21946.5D. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Xu F, Zhang C, Lei D, Tang Y, Xu H, et al. High MR sensitive fluorescent magnetite nanocluster for stem cell tracking in ischemic mouse brain. Nanomedicine. 2011;7:1009–19. doi: 10.1016/j.nano.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002;22:899–907. doi: 10.1097/00004647-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367–83. doi: 10.1016/S0079-6123(06)61026-1. [DOI] [PubMed] [Google Scholar]
- 27.Onteniente B, Polentes J. Regenerative medicine for stroke — Are we there yet? Cerebrovasc Dis. 2011;31:544–51. doi: 10.1159/000324325. [DOI] [PubMed] [Google Scholar]
- 28.Hicks A, Jolkkonen J. Challenges and possibilities of intravascular cell therapy in stroke. Acta Neurobiol Exp (Wars) 2009;69:1–11. doi: 10.55782/ane-2009-1724. [DOI] [PubMed] [Google Scholar]
- 29.Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. 2010;37:275–83. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker PA, Harting MT, Shah SK, Day MC, El Khoury R, Savitz SI, et al. Progenitor cell therapy for the treatment of central nervous system injury: A review of the state of current clinical trials. Stem Cells Int 2010. 2010:369578. doi: 10.4061/2010/369578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shyu WC, Lin SZ, Chiang MF, Su CY, Li H. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing betal integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26:3444–53. doi: 10.1523/JNEUROSCI.5165-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikegame Y, Yamashita K, Hayashi SI, Mizuno H, Tawada M, You F, et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. 2011;13:675–85. doi: 10.3109/14653249.2010.549122. [DOI] [PubMed] [Google Scholar]
- 33.Peterson DA. Umbilical cord blood cells and brain stroke injury: Bringing in fresh blood to address an old problem. J Clin Invest. 2004;114:312–4. doi: 10.1172/JCI22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke. 2007;38:817–26. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- 35.Copeland N, Harris D, Gaballa MA. Human umbilical cord blood stem cells, myocardial infarction and stroke. Clin Med. 2009;9:342–5. doi: 10.7861/clinmedicine.9-4-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auriat AM, Rosenblum S, Smith TN, Guzman R. Intravascular stem cell transplantation for stroke. Transl Stroke Res. 2011;2:250–65. doi: 10.1007/s12975-011-0093-1. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguesa MC, Voltarelli J, Sanberga PR, Allicksonc JG, Kuzmin-Nicholsd N, Garbuzova-Davisa S, et al. Recent progress in cell therapy for basal ganglia disorders with emphasis on menstrual blood transplantation in stroke. Neurosci Biobehav Rev. 2012;36:177–90. doi: 10.1016/j.neubiorev.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Arjmand B, Emami-Razavi SH, Larijani B, Norouzi-Javidan A, Aghayan HR. The implementation of tissue banking experiences for setting up a cGMP cell manufacturing facility. Cell Tissue Bank. 2012;13:587–96. doi: 10.1007/s10561-011-9276-y. [DOI] [PubMed] [Google Scholar]
- 39.Saberi H, Moshayedi P, Aghayan HR, Arjmand B, Hosseini SK, Emami-Razavi SH, et al. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: An interim report on safety considerations and possible outcomes. Neurosci Lett. 2008;443:46–50. doi: 10.1016/j.neulet.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 40.Saberi H, Firouzi M, Habibi Z, Moshayedi P, Aghayan HR, Arjmand B, et al. Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases. J Neurosurg Spine. 2011;15:515–25. doi: 10.3171/2011.6.SPINE10917. [DOI] [PubMed] [Google Scholar]
- 41.Salehi M, Pasbakhsh P, Soleimani M, Abbasi M, Hasanzadeh G, Modaresi MH, et al. Repair of spinal cord injury by co-transplantation of embryonic stem cell-derived motor neuron and olfactory ensheathing cell. Iran Biomed J. 2009;13:125–35. [PubMed] [Google Scholar]
- 42.Seyed-Jafari SS, Ali-Aghaei A, Asadi-Shekaari M, Nematollahi-mahani SN, Sheibani V. Investigating the effects of adult neural stem cell transplantation by lumbar puncture in transient cerebral ischemia. Neurosci Lett. 2011;495:1–5. doi: 10.1016/j.neulet.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 43.Aghayan HR, Mahdavi-Mazdeh M, Goodarzi P, Arjmand B, Emami-Razavi SH. Coding and traceability in Iran. Cell Tissue Bank. 2010;11:397–400. doi: 10.1007/s10561-010-9224-2. [DOI] [PubMed] [Google Scholar]
- 44.Carney BJ, Shah K. Migration and fate of therapeutic stem cells in different brain disease models. Neuroscience. 2011;197:37–47. doi: 10.1016/j.neuroscience.2011.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen PK, Nag D, Wu JC. Methods to assess stem cell lineage, fate and function. Adv Drug Deliv Rev. 2010;62:1175–86. doi: 10.1016/j.addr.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boersma HH, Tromp SC, Hofstra L, Narula J. Stem cell tracking: Reversing the silence of the lambs. J Nucl Med. 2005;46:200–3. [PubMed] [Google Scholar]
- 47.McColgan P, Sharma P, Bentley P. Stem cell tracking in human trials: A meta-regression. Stem Cell Rev. 2011;7:1031–40. doi: 10.1007/s12015-011-9260-8. [DOI] [PubMed] [Google Scholar]
- 48.Vaccaro DE, Yang M, Weinberg JS, Reinhardt CP, Groman EV. Cell tracking using nanoparticles. J Cardiovasc Trans Res. 2008;1:217–20. doi: 10.1007/s12265-008-9039-8. [DOI] [PubMed] [Google Scholar]
- 49.Villa C, Erratico S, Razini P, Fiori F, Rustichelli F, Torrente Y, et al. Stem cell tracking by nanotechnologies. Int J Mol Sci. 2010;11:1070–81. doi: 10.3390/ijms11031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dousset V, Tourdias T, Brochet B, Boiziau C, Petry KG. How to trace stem cells for MRI evaluation? J Neurol Sci. 2008;265:122–6. doi: 10.1016/j.jns.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 51.Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011;8:677–88. doi: 10.1038/nrclinonc.2011.141. [DOI] [PubMed] [Google Scholar]
- 52.Yan L, Han Y, He Y, Xie H, Liu J, Zhao L, et al. Cell tracing techniques in stem cell transplantation. Stem Cell Rev. 2007;3:265–9. doi: 10.1007/s12015-007-9004-y. [DOI] [PubMed] [Google Scholar]
- 53.Arbab AS, Frank JA. Cellular MRI and its role in stem cell therapy. Regen Med. 2008;3:199–215. doi: 10.2217/17460751.3.2.199. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Porcel M. In vivo imaging and monitoring of transplanted stem cells: Clinical applications. Curr Cardiol Rep. 2010;12:51–8. doi: 10.1007/s11886-009-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoehn M, Wiedermann D, Justicia C, Ramos-Cabrer P, Kruttwig K, Farr T, et al. Cell tracking using magnetic resonance imaging. J Physiol. 2007;584:25–30. doi: 10.1113/jphysiol.2007.139451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cromer Berman SM, Walczak P, Bulte JW. Tracking stem cells using magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:343–55. doi: 10.1002/wnan.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bulte JW. In vivo MRI cell tracking: Clinical studies. AJR Am J Roentgenol. 2009;193:314–25. doi: 10.2214/AJR.09.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guzman R, Uchida N, Bliss TM, He D, Christopherson KK, Stellwagen D, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A. 2007;104:10211–6. doi: 10.1073/pnas.0608519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoehn M, Kustermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, et al. Monitoring of implanted stem cell migration in vivo: A highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A. 2002;99:16267–72. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Massoud TF, Gambhir SS. Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–80. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 61.Modo M, Mellodew K, Cash D, Fraser SE, Meade TJ, Price J, et al. Mapping transplanted stem cell migration after a stroke: A serial, in vivo magnetic resonance imaging study. Neuroimage. 2004;21:311–7. doi: 10.1016/j.neuroimage.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 62.Shichinohea H, Kuroda S, Lee JB, Nishimura G, Yano S, Sekia T, et al. In vivo tracking of bone marrow stromal cells transplanted into mice cerebral infarct by fluorescence optical imaging. Brain Res Brain Res Protoc. 2004;13:166–75. doi: 10.1016/j.brainresprot.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Muja N, Bulte JW. Magnetic resonance imaging of cells in experimental disease models. Prog Nucl Magn Reson Spectrosc. 2009;55:61–77. doi: 10.1016/j.pnmrs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barbosa da Fonseca LM, Gutfilen B, Rosado de Castro PH, Battistella V, Goldenberg RC, Kasai-Brunswick T, et al. Migration and homing of bone-marrow mononuclear cells in chronic ischemic stroke after intra-arterial injection. Exp Neurol. 2010;221:122–8. doi: 10.1016/j.expneurol.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 65.Zhao C, Tian M, Zhang H. In vivo stem cell imaging. Nucl Med J. 2010;2:171–7. [Google Scholar]
- 66.Villa C, Erratico S, Razini P, Farini A, Meregalli M, Belicchi M, et al. In vivo tracking of stem cell by nanotechnologies: Future prospects for mouse to human translation. Tissue Eng Part B Rev. 2011;17:1–11. doi: 10.1089/ten.TEB.2010.0362. [DOI] [PubMed] [Google Scholar]
- 67.Bulte JW, Zhang S, van Gelderen P, Herynek V, Jordan EK, Duncan ID, et al. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: Magnetic Resonance tracking of cell migration and myelination. Proc Natl Acad Sci U S A. 1999;96:15256–61. doi: 10.1073/pnas.96.26.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wegener S, Hoehn M. Novel imaging modalities to monitor implanted embryonic stem cells in stroke. In: Tarsy D, editor. Current Clinical Neurology. 2nd edn. Totowa, New Jersy: Humana Press; 2008. pp. 71–94. [Google Scholar]
- 69.Rice HE, Hsu EW, Sheng H, Evenson DA, Freemerman AJ, Safford KM, et al. Superparamagnetic Iron oxide labeling and transplantation of adipose-derived stem cells in middle cerebral artery occlusion-injured mice. AJR Am J Roentgenol. 2007;188:1101–8. doi: 10.2214/AJR.06.0663. [DOI] [PubMed] [Google Scholar]
- 70.Lee ES, Chan J, Shuter B, Tan LG, Chong MS, Ramachandra DL, et al. Microgel iron oxide nanoparticles for tracking human fetal mesenchymal stem cells through magnetic resonance imaging. Stem Cells. 2009;27:1921–31. doi: 10.1002/stem.112. [DOI] [PubMed] [Google Scholar]
- 71.Politi LS. MR-based imaging of neural stem cells. Neuroradiology. 2007;49:523–34. doi: 10.1007/s00234-007-0219-z. [DOI] [PubMed] [Google Scholar]
- 72.Hoehn M, Himmelreich U, Kruttwig K, Wiedermann D. Molecular and cellular MR Imaging: Potentials and challenges for neurological applications. J Magn Reson Imaging. 2008;27:941–54. doi: 10.1002/jmri.21280. [DOI] [PubMed] [Google Scholar]
- 73.Hoehn M. How do we assess regenerative success after stem cell implantation? An experimental approach. Regen Med. 2011;6:417–9. doi: 10.2217/rme.11.34. [DOI] [PubMed] [Google Scholar]
- 74.Ferreira L, Karp JM, Nobre L, Langer R. New Opportunities: The use of nanotechnologies to manipulate and track stem cells. Cell Stem Cell. 2008;3:136–46. doi: 10.1016/j.stem.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 75.Stroh A, Faber C, Neuberger T, Lorenz P, Sieland K, Jakob PM, et al. In vivo detection limits of magnetically labeled embryonic stem cells in the rat brain using high-field (17.6 T) magnetic resonance imaging. Neuroimage. 2005;24:635–45. doi: 10.1016/j.neuroimage.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 76.Kaur S, Singhal B. When nano meets stem: The impact of nanotechnology in stem cell biology. J Biosci Bioeng. 2012;113:1–4. doi: 10.1016/j.jbiosc.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 77.Dousset V, Brochet B, Deloire MS, Lagoarde L, Barroso B, Caille JM, et al. MR imaging of relapsing multiple sclerosis patients using ultra-small-particle iron oxide and compared with gadolinium. AJNR Am J Neuroradiol. 2006;27:1000–5. [PMC free article] [PubMed] [Google Scholar]
- 78.Petry KG, Boiziau C, Dousset V, Brochet B. Magnetic resonance imaging of human brain macrophage infiltration. Neurotherapeutics. 2007;4:434–42. doi: 10.1016/j.nurt.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bulte JW. Intracellular endosomal magnetic labeling of cells. Methods Mol Med. 2006;124:419–39. doi: 10.1385/1-59745-010-3:419. [DOI] [PubMed] [Google Scholar]
- 80.Reddy AM, Kwak BK, Shim HJ, Ahn C, Lee HS, Suh YJ, et al. In vivo tracking of mesenchymal stem cells labeled with a novel chitosan-coated superparamagnetic iron oxide nanoparticles using 3.0T MRI. J Korean Med Sci. 2010;25:211–9. doi: 10.3346/jkms.2010.25.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu Y, Kedziorek D, Kraitchman DL. Recent developments and future challenges on imaging for stem cell research. J Cardiovasc Transl Res. 2010;3:24–9. doi: 10.1007/s12265-009-9158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slotkin JR, Cahill KS, Tharin SA, Shapiro EM. Cellular magnetic resonance imaging: Nanometer and micrometer size particles for noninvasive cell localization. Neurotherapeutics. 2007;4:428–33. doi: 10.1016/j.nurt.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Modo M, Beech JS, Meade TJ, Williams SC, Price J. A chronic 1 year assessment of MRI contrast agent-labelled neural stem cell transplants in stroke. Neuroimage. 2009;47:T133–42. doi: 10.1016/j.neuroimage.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ward RJ, Wilmet S, Legssyer R, Crichton RR. The influence of iron homoeostasis on macrophage function. Biochem Soc Trans. 2002;30:762–5. doi: 10.1042/bst0300762. [DOI] [PubMed] [Google Scholar]
- 85.Corot C, Robert P, Idee JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58:1471–504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 86.Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–7. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 87.Kraitchman DL, Bulte JW. In vivo imaging of stem cells and beta cells using direct cell labeling and reporter gene methods. Arterioscler Thromb Vasc Biol. 2009;29:1025–30. doi: 10.1161/ATVBAHA.108.165571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Srinivas M, Aarntzen EH, Bulte JW, Oyen WJ, Heerschap A, de Vries IJ, et al. Imaging of cellular therapies. Adv Drug Deliv Rev. 2010;62:1080–93. doi: 10.1016/j.addr.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 89.Weissleder R, Stark DD, Engelstad BL, Bacon BR, Compton CC, White DL, et al. Superparamagnetic iron oxide: Pharmacokinetics and toxicity. AJR Am J Roentgenol. 1989;152:167–73. doi: 10.2214/ajr.152.1.167. [DOI] [PubMed] [Google Scholar]
- 90.Frangioni JV, Hajjar RJ. In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation. 2004;110:3378–83. doi: 10.1161/01.CIR.0000149840.46523.FC. [DOI] [PubMed] [Google Scholar]
- 91.Genove G, De Marco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med. 2005;11:450–4. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]


