Abstract
Pancreatic β-cells are responsible for insulin production, and loss of functional β-cell mass is now recognized as a critical step in the pathogenesis of both type 1 and type 2 diabetes. However, the factors controlling the life and death of the pancreatic β-cell have only started to be elucidated. Discovered as the top glucose-induced gene in a human islet microarray study 12 years ago, thioredoxin-interacting protein (TXNIP) has now emerged as such a key player in pancreatic β-cell biology. Since then, β-cell expression of TXNIP has been found to be tightly regulated by multiple factors and to be dramatically increased in diabetic islets. Elevated TXNIP levels induce β-cell apoptosis, whereas TXNIP deficiency protects against type 1 and type 2 diabetes by promoting β-cell survival. TXNIP interacts with and inhibits thioredoxin and thereby controls the cellular redox state, but it also belongs to the α-arrestin family of proteins and regulates a variety of metabolic processes. Most recently, TXNIP has been discovered to control β-cell microRNA expression, β-cell function, and insulin production. In this review, the current state of knowledge regarding regulation and function of TXNIP in the pancreatic β-cell and the implications for drug development are discussed.
The gene encoding thioredoxin-interacting protein (TXNIP) was first cloned in 1994 (20 years ago) from a 1,25-dihydroxyvitamin D3-treated HL-60 human promyelocytic cell line (1) and therefore was initially referred to as vitamin D3-up-regulated protein 1 (1–3). However, subsequent TXNIP promoter analyses failed to reveal a consensus vitamin D response element (2), and there are no reports confirming vitamin D-induced TXNIP transcription in other cell systems, suggesting that the effect may have been conferred indirectly by vitamin D-induced differentiation of this promyelocytic cell line.
Four years later, TXNIP was found to be spontaneously mutated in the combined hyperlipidemia (Hyplip1) locus (4) of an inbred congenic C3H mouse strain (HcB-19) (5, 6). The TXNIP gene in these HcB-19 mice has an inactivating nonsense mutation in exon 2 at codon 97, resulting in dramatically reduced TXNIP mRNA and protein levels (7), and the mice are characterized by mild hypoglycemia and elevated plasma insulin, triglycerides, ketone bodies, and free fatty acids (8, 9). Follow-up studies revealed that TXNIP was not associated with familial combined hyperlipidemia in humans (10).
The TXNIP protein was identified in a yeast 2-hybrid system aimed at finding thioredoxin-binding proteins and was therefore designated thioredoxin-binding protein-2 (3). (P40phox had been identified as thioredoxin-binding protein-1 [11].)
To date, the designation TXNIP is being used predominantly, reflecting at least some of the protein's actions. TXNIP binds to and inhibits thioredoxin and thereby can modulate the cellular redox state and induce oxidative stress (3, 11–14) (Figure 1). More specifically, TXNIP interferes with thioredoxin-mediated protein denitrosylation (15). Thioredoxin is a thiol-oxidoreductase and part of a major cellular reducing system protecting cells against oxidative stress (16). The thioredoxin system reduces oxidized proteins, resulting in oxidation of the 2 cysteine residues of thioredoxin. To return to a reduced and active state, thioredoxin has to be reduced back by the NADPH-dependent thioredoxin reductase (11, 17). The thioredoxin system has been shown to be involved in multiple cellular processes including cell proliferation and apoptosis (11, 18–20).
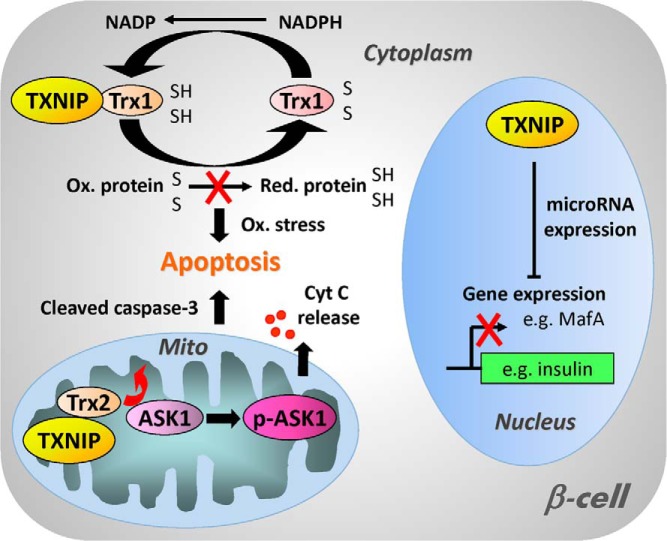
Figure 1.
Schematic diagram of the cellular functions of TXNIP. In the cytoplasm, TXNIP binds to and inhibits thioredoxin 1 (Trx1) and thereby interferes with the ability of Trx1 to reduce oxidized proteins, resulting in oxidative stress and increased susceptibility to apoptosis. In addition, TXNIP can also enter the mitochondria where it interacts with mitochondrial thioredoxin 2 (Trx2), releasing ASK1 from its inhibition by Trx2 and allowing for phosphorylation and activation of ASK1. This in turn leads to cytochrome c (Cyt C) release from the mitochondria, cleavage of caspase-3, and apoptosis. TXNIP has also been found to be localized in the nucleus and to modulate the expression of various microRNAs (eg, miR-204). These microRNAs down-regulate the expression of target genes including important β-cell transcription factors such as MafA, which results in reduced insulin transcription and impaired β-cell function.
Based on this interaction with thioredoxin and function as a cellular redox regulator, TXNIP has been thought to be localized in the cytoplasm (12, 21, 22). However, more recent findings have revealed that TXNIP can also translocate into the mitochondria where it binds to mitochondrial thioredoxin 2, releasing apoptosis signal-regulating kinase 1 (ASK1) from its inhibition by thioredoxin 2 and allowing for phosphorylation and activation of ASK1 (23). This in turn leads to cytochrome c release from the mitochondria, cleavage of caspase-3, and apoptosis. TXNIP has also been found to be localized in the nucleus (23) and by regulating the expression of various microRNAs to control the expression of target genes including transcription factors critical for insulin production such as MafA (24) (Figure 1).
Human TXNIP is a 46-kDa ubiquitously expressed protein that contains 391 amino acid residues and is encoded on chromosome 1q21.1. TXNIP is highly conserved across species and, for example, mouse TXNIP shows 94% homology with the human protein, contains 395 amino acids, and is located in a region of mouse chromosome 3 that is syntenic to human 1q21 (2). TXNIP belongs to the protein family of α-arrestins characterized by SH3 and PPxY domains, but among these proteins only TXNIP is capable of interacting with thioredoxin (25). The TXNIP protein forms an intramolecular disulfide bond conferred by 2 cysteines, Cys247 and Cys63, and Cys247 is also essential for the interaction of TXNIP with Cys32 of thioredoxin (14). Because of problems overexpressing recombinant full-length TXNIP as a soluble protein, only a partial N-terminal domain of TXNIP could be crystallized until recently (26). However, now the crystal structure of the thioredoxin-TXNIP complex has been resolved, and these analyses have further established that the inhibition of thioredoxin by TXNIP is conferred by an intermolecular disulfide bond-switching mechanism (27).
In regard to TXNIP in pancreatic β-cells, TXNIP was discovered in 2002 to be the most strongly up-regulated gene in response to glucose in a human islet oligonucleotide gene expression microarray study (28). This finding suggested that its expression was regulated by glucose and that it may play an important role in β-cell biology and possibly diabetes. Pancreatic β-cells are uniquely susceptible to oxidative stress because of their low expression level of antioxidant enzymes (29), and loss of functional β-cell mass by apoptosis is a crucial component in the pathogenesis of both type 1 and type 2 diabetes (30–35). Indeed, over the last 12 years TXNIP has emerged as a key player in β-cell biology and an attractive target for novel diabetes therapies (36–39). This minireview is therefore focused on the regulation, signaling, and function of TXNIP in the pancreatic β-cell and summarizes the key findings in this regard (Figure 2). Other redox-dependent and independent effects of TXNIP have recently been reviewed (40, 41).
Figure 2.

Regulation and signaling of β-cell TXNIP. β-Cell TXNIP expression is strongly induced by glucose and is increased in diabetes as well as in response to ER stress, and this induction is conferred at the transcriptional level by ChREBP and at the posttranscriptional level by a decrease in miR-17 and is modulated by a number of additional factors. TXNIP in turn inhibits thioredoxin function and promotes oxidative stress and β-cell death. In addition, by modulating the expression of distinct microRNAs (miR-204 and miR-124a) and with that the expression of their target genes (MafA and FoxA2) TXNIP inhibits insulin transcription while inducing IAPP transcription. These detrimental effects are further magnified by the fact that TXNIP promotes its own expression, again via ChREBP.
Factors and Processes Regulating TXNIP
TXNIP is considered an early response gene (42) and is strongly and acutely regulated at the transcriptional level with generally good concordance between changes in mRNA and protein levels (38, 43, 44). However, recent work has also revealed that TXNIP is controlled at the level of mRNA stability (45) as well as protein stability (46–48).
Glucose and diabetes
TXNIP was initially identified to be strongly up-regulated by glucose in a human islet gene expression microarray study (28), and these findings were later confirmed by quantitative real-time RT-PCR and immunoblotting in primary islets as well as in INS-1 β-cells (36, 38, 43, 44). Analyses to identify the cis- and trans-acting factors conferring this glucose-induced TXNIP expression revealed a well conserved E-box repeat in the proximal TXNIP promoter (36). Although in alignment with previously reported carbohydrate response elements consisting of 2 E-boxes, the one in the TXNIP promoter differed by the fact that it was nonpalindromic (36). Nevertheless, it has been shown to serve as the binding site for the major CRE carbohydrate response element–binding protein (ChREBP) known today (49). (Whereas ChREBP is the predominantly expressed form of this transcription factor in pancreatic β-cells [49] and liver [50] and has been shown to play a major role in β-cell glucose toxicity [51], tissues such as skeletal muscle and heart express primarily its paralog MondoA [52]. In analogy to ChREBP, MondoA confers glucose responsiveness of TXNIP in these tissues [52]). Interestingly, “nonmetabolizable” sugars such as 2-deoxyglucose and even 3-O-methylglucose have been found to induce TXNIP transcription (36, 53). Recent studies indicated again that neither phosphorylation nor effects on d-glucose metabolism are required for induction of TXNIP expression in response to 3-O-methylglucose (54). A potential alternate explanation suggests that ChREBP/MondoA enters the nucleus and transcribes TXNIP not only in response to glucose 6-phosphate but also in response to glucosamine 6-phosphate and the hexosamine biosynthetic pathway (55).
Interestingly, glucose-induced TXNIP expression has been found to be inhibited by fatty acids such as palmitate (38, 56). In contrast, glucocorticoids have been shown to induce β-cell TXNIP expression (57), and TXNIP has been suggested to play a potential role in the perturbed glucose homeostasis of Cushing syndrome (58).
Consistent with its induction by glucose, β-cell TXNIP expression is also markedly increased in diabetes, including a variety of rodent models with and without obesity (24, 36, 37, 39, 59).
Insulin and other drugs
In contrast, insulin has been reported to decrease TXNIP expression in vitro in β-cells (59) as well as in muscle and fat tissues (60), suggesting that this effect is not dependent on any changes in glucose. However, in vivo hyperglycemia seems to override any insulin effects, because TXNIP levels are dramatically increased in diabetes even in the context of severe hyperinsulinemia (37, 39).
The glucagon-like peptide 1 (GLP-1) receptor agonist, exenatide, has also been shown to reduce β-cell TXNIP expression (61). Because exenatide is a strong insulin secretagogue, this could be due to the resulting increase in insulin secretion. In addition, proteasome-dependent ubiquitination and degradation of TXNIP have been suggested to be involved in GLP-1-mediated TXNIP down-regulation (47).
Recently, calcium channel blockers such as the antihypertensive drug verapamil were found to effectively reduce β-cell TXNIP expression in vitro as well as in vivo (44). This effect was due to the associated decrease in intracellular calcium and was found with other calcium channel blockers of the L-type and the T-type and in response to calcium chelation. It was further mediated by inhibition of calcineurin signaling and nuclear exclusion of ChREBP (44).
TXNIP and transcription factors
Interestingly, TXNIP itself has recently been shown to stimulate its own expression via a feed-forward loop (62), which means that any TXNIP induction as, for example, by glucose is further amplified. Once again, the effect was mediated by the transcription factor ChREBP.
ChREBP was initially discovered in liver (63) and later shown to also play a role in β-cells (49, 64). ChREBP activity is primarily regulated by its cellular localization, nuclear entry, and posttranslational modification, ie, phosphorylation status (50, 65–67). Glucose and nutrients have been shown to promote dephosphorylation and with that activation of ChREBP via induction of protein phosphatase 2A (68) and/or inhibition of AMP-activated protein kinase (AMPK) (56, 69). Interestingly, TXNIP-induced TXNIP expression was also mediated via inhibition/dephosphorylation of AMPK (62). Once in the nucleus, ChREBP binds as heterodimer with Mlx and in the case of TXNIP recruits the coactivator and histone deacetylase p300, resulting in histone H4 deacetylation and opening up of the chromatin structure to allow for polymerase II progression (49).
Recently, another transcription factor, forkhead box (Fox) O1, has been shown to modulate β-cell TXNIP expression by competing with ChREBP for DNA binding at the TXNIP promoter and to thereby inhibit glucose-induced TXNIP expression (43). Interestingly, FoxO1 also regulates the induction of MafA and NeuroD, both β-cell transcription factors involved in the activation of insulin gene expression (70, 71). FoxO1 is therefore believed to promote normal β-cell function, which is consistent with its inhibitory effects on TXNIP. In contrast, FoxO1 has been suggested to impair compensatory β-cell differentiation and proliferation (72–74) by interfering with the essential transcription factor involved in these processes, Pdx1.
Endoplasmic reticulum (ER) stress and microRNA miR-17
ER stress has recently been found to induce β-cell TXNIP expression (45, 75). These 2 back-to-back articles further revealed that both transcriptional as well as posttranscriptional factors were involved. Accumulation of unfolded proteins in the ER leads to activation of signaling pathways referred to as the unfolded protein response. If the problem is not able to be resolved, this results in ER stress and ultimately apoptosis via 3 pathways involving protein kinase R-like ER kinase (PERK), serine/threonine-protein kinase/endoribonuclease (IRE1α), and activating transcription factor (ATF) 6 (76). Interestingly, PERK and IRE1α, but not ATF6 seemed to mediate ER-stress–induced TXNIP expression (45, 75). PERK signaling led to increased TXNIP transcription again via enhanced ChREBP expression and nuclear translocation as well as via ATF5, a member of the ATF/cAMP response element-binding protein family of transcription factors (75). On the other hand, activated/phosphorylated IRE1α resulted in increased TXNIP mRNA stability. IRE1α functions as a bifunctional kinase/RNase and reduces the levels of a TXNIP-destabilizing microRNA, miR-17 (45). microRNAs are small, noncoding RNAs that bind predominantly to the 3′-UTR of target mRNAs leading to mRNA degradation or translational inhibition of the target mRNA and down-regulation of target gene expression (77–79). miR-17 has been shown to target the 3′-UTR of TXNIP and to inhibit TXNIP expression (45, 80). ER stress–induced TXNIP expression seems therefore to be due to an increase in TXNIP transcription as well as to a release from the inhibitory effects of miR-17 and the resulting increase in TXNIP mRNA stability and translation.
Posttranslational processes
Most factors described so far regulate TXNIP at the mRNA level, be it through transcription or mRNA stability, and the effects are translated accordingly into altered TXNIP protein levels. However, there have also been a few reports suggesting some posttranslational regulation of TXNIP. Interestingly, TXNIP has been found to be phosphorylated by AMPK, which in turn leads to its degradation (46). (On the other hand, TXNIP inhibits AMPK, as mentioned above [62]). In addition, GLP-1/cAMP signaling has been suggested to enhance proteasome-dependent TXNIP degradation in β-cells (47). Insulin has also been reported to promote TXNIP degradation through an ubiquitin/proteasome pathway, but this effect is specific to adipocytes and myotubes and does not occur in pancreatic β-cells (48).
Taken together, these results show that TXNIP expression is tightly regulated at the mRNA level and strongly induced by pathophysiological stressors such as diabetes. Given the critical role TXNIP plays in controlling cellular life vs death decisions, it is not surprising that its expression is further regulated by a number of endogenous and exogenous factors (eg, ER stress, fatty acids, FoxO1, and calcium channel blockers, respectively). Interestingly, all these effects are mediated by or involve the transcription factor ChREBP, which seems to play a key role in the regulation of β-cell TXNIP expression (Figure 2). Nevertheless, additional posttranscriptional and posttranslational TXNIP regulatory mechanisms have been reported and include miR-17-mediated TXNIP down-regulation as well as proteasome-dependent TXNIP degradation.
Roles of TXNIP in β-Cell Biology
TXNIP has been found not only to be highly regulated in β-cells as outlined above, but also to exert a number of functions that have a great impact on the life and death of the pancreatic β-cell as well as the development and progression of diabetes (Figure 2).
β-Cell apoptosis and survival
By inhibiting thioredoxin, TXNIP plays a major role in inducing oxidative stress (3, 11–14), and overexpression of TXNIP in β-cells has been shown to promote apoptosis (36, 81). This is consistent with the particular susceptibility of β-cells to oxidative stress (29) as well as with findings in extrapancreatic tissues (12, 82). The proapoptotic effect is mediated primarily by induction of the mitochondrial death pathway and involves phosphorylation/activation of ASK1, cytochrome c release, and caspase-3 cleavage (23, 38, 39). In contrast, TXNIP deficiency is protective, enhances Akt/Bcl-xL signaling, and promotes β-cell survival even in the context of high glucose (37, 38). In fact, TXNIP was identified as a critical link between glucose toxicity and β-cell apoptosis (39, 83, 84).
β-Cell function and insulin production
Recently, TXNIP has also been discovered to regulate the most crucial aspect of β-cell function, namely insulin production (24). TXNIP induces the expression of a specific microRNA, miR-204, by inhibiting the activity of signal transducer and activator of transcription 3 (24), a transcription factor involved in miR-204 regulation (85, 86). In turn, miR-204 directly targets the 3′-UTR of MafA, a known insulin transcription factor (87), and down-regulates its expression. This results in decreased insulin transcription and insulin production (24). Importantly, this increase in miR-204 and decrease in MafA and insulin production was not only observed in vitro in response to TXNIP overexpression in β-cells and in primary human islets, but also in vivo in several diabetes mouse models associated with increased TXNIP expression (eg, B6-obese, BTBR ob/ob, and A-ZIP/F-1), suggesting that this pathway might play a role in the β-cell dysfunction of diabetes (24, 36, 37, 39). In addition, TXNIP deficiency was associated with an increase in insulin production, and this effect was completely blunted by miR-204 overexpression (24).
Islet amyloid polypeptide (IAPP) is the major component of islet amyloid and tends to aggregate into insoluble amyloid fibrils. IAPP deposits are often colocalized with β-cell apoptosis and are found in islets of most patients with type 2 diabetes (88). IAPP has therefore been strongly associated with the progressive loss of pancreatic β-cell mass in diabetes. Although IAPP is cosecreted with insulin by β-cells (88, 89) and similarities between the IAPP and the insulin promoter exist, TXNIP has recently been found to specifically promote IAPP expression (90). Interestingly, it does so by inhibiting the expression of another microRNA, miR-124a, which in turn stabilizes the mRNA of the FoxA2 transcription factor. FoxA2 then binds to the IAPP promoter and induces IAPP transcription, a process that can be blocked by miR-124a overexpression (90).
Additional processes
Based on its role in cellular redox regulation, TXNIP was previously considered a purely cytosolic protein (12, 21, 22). However, it has now been found to be also localized in β-cell nuclei (23) where it may help exert the observed effects on gene/microRNA expression. TXNIP has also been shown to shuttle into mitochondria, where it interacts with mitochondrial thioredoxin 2, which markedly contributes to TXNIP-induced apoptosis and oxidative stress (23).
TXNIP has also been discovered to induce activation of the NLRP3 inflammasome, a NACHT, LRR, and PYD domain-containing protein (91). NLRP3 inflammasome activation leads to caspase-1 activation and cleavage of pro-IL-1β to mature IL-1β, and both inflammation and IL-1β have been shown to play important roles in the pathogenesis of type 1 and type 2 diabetes (45, 75, 91, 92). However, although some initial studies proposed that IL-1β was being produced in β-cells (93), NLRP3 inflammasome expression and IL-1β production are largely restricted to monocytes and cells of the innate immune system (94, 95), which has been further confirmed in more recent studies (96). Although it is therefore conceivable that TXNIP-induced inflammasome activation and IL-1β production take place in resident macrophages present in the intact islet or in any infiltrating immune cells, they are less likely to occur in the β-cell per se.
In non-β-cells and particularly in skeletal muscle, adipocytes, and hepatocytes, TXNIP was found to inhibit glucose uptake, and this effect is conferred by inhibition of the glucose transporter 1 (GLUT1) (46, 60). TXNIP thereby reduces GLUT1 mRNA expression and also binds to GLUT1, leading to its internalization (46).
TXNIP Deficiency and Diabetes Prevention
Diabetes has become a major health issue worldwide. Although some of the underlying causes and cornerstones of type 1 and type 2 diabetes are distinct, ie, autoimmunity and insulin resistance, respectively, loss of functional β-cell mass represents a crucial common feature in the pathogenesis of both diabetes types (30–35). Moreover, elevated TXNIP levels have emerged as a critical factor involved in β-cell dysfunction, β-cell death, and the resulting development of diabetes and its complications. On the other hand, genetic deletion and pharmacological inhibition of β-cell TXNIP have been shown to protect against type 1 and type 2 diabetes.
Type 1 diabetes
Interestingly, TXNIP-deficient HcB-19 mice demonstrate an increase in their β-cell mass under normal conditions. In addition, they have been found to be protected against multiple low-dose streptozotocin (STZ)-induced β-cell destruction and diabetes (37). As mentioned above, TXNIP deletion also has peripheral effects and promotes glucose uptake in skeletal muscle and adipose tissue (60) and inhibits hepatic glucose production (97), all features that could contribute to improved glucose homeostasis. However, it is important to note that STZ-induced diabetes has also been shown to be completely prevented in bTKO (β-cell-specific TXNIP knockout) mice suggesting that deletion of TXNIP in the β-cell is sufficient to achieve these protective effects (37). Moreover, bTKO mice also exhibit a dramatic reduction in β-cell apoptosis and an increase in β-cell mass compared with those in STZ-treated control lox/lox mice (37). Furthermore, β-cell-specific overexpression of thioredoxin has been shown to protect against STZ-induced diabetes as well as against autoimmune diabetes in the NOD (nonobese) diabetic mouse (98), and graft survival of islets transduced with thioredoxin is prolonged in NOD mice (99). Together these findings seem to indicate that the β-cell–protective effects of TXNIP deficiency are mediated by thioredoxin. In contrast, some of the metabolic and peripheral TXNIP effects such as inhibition of glucose uptake were found to be independent of thioredoxin and were attributed to the arrestin domains of TXNIP (40).
More recently, the calcium channel blocker and antihypertensive drug verapamil has been shown to reduce β-cell TXNIP expression and to protect against STZ-induced diabetes (44). Importantly, even when the oral verapamil administration was started after overt diabetes had fully developed, verapamil was able to rescue mice from diabetes, normalize blood glucose levels, and restore insulin producing β-cells (44).
Aside from the type 1 diabetes models mentioned above, TXNIP deletion has also been found to reduce β-cell death and diabetes caused by proinsulin misfolding and ER stress in the Akita mouse (45).
Type 2 diabetes
Leptin deficiency in BTBR ob/ob mice leads not only to severe obesity and insulin resistance as on the C57BL/6 background but also to severe diabetes, and mice typically die spontaneously at age 14 to 16 weeks, making this one of the most stringent type 2 diabetes models (100). Nevertheless, TXNIP deficiency is still able to protect these BTBR ob/ob mice against diabetes and completely normalize their blood glucose levels for >6 months (44). Again this is associated with a striking reduction in β-cell apoptosis and increase in β-cell mass as well as some improvement in insulin sensitivity. Intriguingly though, these beneficial effects occur in the face of severe obesity, demonstrating that the lack of TXNIP is able to unlink diabetes from obesity (44). Of note, very similar results have been observed in TXNIP-null mice fed a high-fat diet (101). Diabetes progression has also been found to be suppressed in transgenic leptin receptor-deficient db/db mice with β-cell-specific expression of thioredoxin (102). Furthermore, pharmacological inhibition of TXNIP again with verapamil has been shown to improve β-cell survival and diabetes in BTRB ob/ob mice (44). One obvious question is why such potentially beneficial effects of calcium channel blockers in terms of diabetes control and progression have not been recognized previously in humans. In this regard, it is important to remember that calcium channel blockers are antihypertensive drugs, and most clinical studies focus on mortality and cardiovascular events as primary and secondary outcomes, rather than metabolic control. Nevertheless, verapamil has been shown to have beneficial effects in diabetic cardiomyopathy (103) and spinoff studies of the International Verapamil SR/Trandolapril Study (INVEST) revealed that verapamil SR reduced the risk of new-onset diabetes (104, 105).
Implications for Drug Development
Diabetes therapies have significantly improved over the last several decades, but curative approaches are still missing. In addition, even though loss of functional β-cell mass by apoptosis has now been well established as a critical step in the pathogenesis of type 1 as well as type 2 diabetes (30–35), approaches targeting this problem are lacking. Based on the studies summarized above, TXNIP has emerged as an attractive novel therapeutic target for diabetes and for the promotion of endogenous β-cell mass and insulin production (24, 37, 44). In addition, the results provide a genetic as well as pharmacological proof of concept for the beneficial effects of TXNIP inhibition and its ability to not only prevent but also reverse diabetes (44). In fact, large screening efforts for drug discovery have already revealed potential specific TXNIP inhibitors, and ongoing studies are currently testing promising lead compounds.
Obvious concerns regarding therapeutic TXNIP inhibition include possible off-target effects due to the ubiquitous expression pattern of TXNIP and any potential neoplastic risk due to the inhibition of a proapoptotic factor. However, inhibition of TXNIP has also been shown to have beneficial effects in other tissues (60, 97), including the cardiovascular system (106, 107) and the diabetic heart (108, 109), kidney (110), and retina (111) making a tissue-specific approach unnecessary and even undesirable. In addition, even lifelong whole-body TXNIP deficiency, as in the HcB-19 mice, has been found to be associated only with an increased risk of development of hepatocellular carcinomas later in life (112), without any notable increase in leukemias, lymphomas, or other tumors as would be expected in response to inhibition of a classic tumor-suppressor gene. Most importantly, though, TXNIP inhibition targets a disease problem (ie, increased TXNIP), and the apparent goal is therefore to normalize TXNIP to its physiological, nondiabetic levels, thereby limiting any potential side effects due to abnormally low TXNIP.
Concluding Remarks
The knowledge gathered to date about TXNIP has provided major new insights into β-cell biology and diabetes development, but it has also raised some teleological questions such as whether the observed increase in β-cell TXNIP is a cause or just a consequence of diabetes and why such a “detrimental” TXNIP signaling cascade may even exist.
Given the well-defined mechanisms of glucose-induced TXNIP expression, there is no doubt that the observed increase in β-cell TXNIP is a result of diabetic hyperglycemia. However, several lines of evidence also suggest that TXNIP plays a causative role in the pathogenesis of diabetes. Considering that its expression is rapidly and strongly induced by glucose, it is highly conceivable that even short-term postprandial glucose excursions (as often seen in prediabetes) may lead to a gradual, cumulative increase in TXNIP expression before any onset of overt diabetes. Moreover, insulin resistance or any increased demand on the β-cell may, via the unfolded protein response and ER stress, also lead to elevated β-cell TXNIP levels. Furthermore, the newly identified feed-forward loop suggests that any increase in TXNIP expression would be further magnified, leading to more β-cell apoptosis and further impairment in glucose homeostasis and amplification of this vicious cycle. Most importantly, deletion or inhibition of TXNIP effectively protected against type 1 and type 2 diabetes, strongly supporting the notion that TXNIP also plays a causative role in the development of diabetes.
On the other hand, it is important to remember that normal, basal levels of TXNIP expression are not detrimental to β-cell biology and that only the pathological increase as observed in diabetes causes β-cell dysfunction and β-cell death. In addition, it is tempting to speculate that, similar to the “thrifty gene” theory, basal TXNIP expression may have been beneficial in the context of prehistoric food scarcity and exposure to microbes, whereas under the current conditions of excess energy- and glucose-dense foods associated with a Western diet, the resulting increase in TXNIP starts exerting detrimental effects. In fact, TXNIP-induced oxidative stress could be viewed as a cellular antimicrobial defense mechanism, and TXNIP has been shown to play an important role in the metabolic transition from the fed to the fasting state (8). In addition, TXNIP inhibits insulin transcription, resulting in reduced insulin production, while promoting hepatic glucose production, and thereby may help prevent hypoglycemia and promote survival in the context of acute starvation. Indeed, TXNIP-null mice were found to be more sensitive to starvation and exhibited a markedly increased death rate in response to prolonged fasting (113), further supporting this notion.
In summary, a lot has been learned over the last 12 years about TXNIP in the β-cell and as a therapeutic target, but it seems that the future may still hold some exciting and potentially highly relevant TXNIP-related discoveries.
Acknowledgments
This work was supported by grants from the National Institutes of Health (Grant R01DK-078752), the American Diabetes Association (Grant 7-12-BS-167), and the Juvenile Diabetes Research Foundation and JNJSI (Grant 40-2011–1).
Disclosure Summary: The author has nothing to disclose.
Footnotes
- AMPK
- AMP-activated protein kinase
- ASK1
- apoptosis signal-regulating kinase 1
- ATF
- activating transcription factor
- ChREBP
- carbohydrate response element-binding protein
- Fox
- forkhead box
- GLP-1
- glucagon-like peptide 1
- GLUT1
- glucose transporter 1
- IAPP
- islet amyloid polypeptide
- IRE1α
- serine/threonine-protein kinase/endoribonuclease
- PERK
- protein kinase R-like ER kinase
- STZ
- streptozotocin
- TXNIP
- thioredoxin-interacting protein
- UTR
- untranslated region.
References
- 1. Chen KS, DeLuca HF. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1994;1219:26–32 [DOI] [PubMed] [Google Scholar]
- 2. Ludwig DL, Kotanides H, Le T, Chavkin D, Bohlen P, Witte L. Cloning, genetic characterization, and chromosomal mapping of the mouse VDUP1 gene. Gene. 2001;269:103–112 [DOI] [PubMed] [Google Scholar]
- 3. Nishiyama A, Matsui M, Iwata S, et al. Identification of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650 [DOI] [PubMed] [Google Scholar]
- 4. Castellani LW, Weinreb A, Bodnar J, et al. Mapping a gene for combined hyperlipidaemia in a mutant mouse strain. Nat Genet. 1998;18:374–377 [DOI] [PubMed] [Google Scholar]
- 5. Stassen AP, Groot PC, Eppig JT, Demant P. Genetic composition of the recombinant congenic strains. Mamm Genome. 1996;7:55–58 [DOI] [PubMed] [Google Scholar]
- 6. Groot PC, Moen CJ, Dietrich W, Stoye JP, Lander ES, Demant P. The recombinant congenic strains for analysis of multigenic traits: genetic composition. FASEB J. 1992;6:2826–2835 [DOI] [PubMed] [Google Scholar]
- 7. Bodnar JS, Chatterjee A, Castellani LW, et al. Positional cloning of the combined hyperlipidemia gene Hyplip1. Nat Genet. 2002;30:110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheth SS, Castellani LW, Chari S, et al. Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J Lipid Res. 2005;46:123–134 [DOI] [PubMed] [Google Scholar]
- 9. Hui TY, Sheth SS, Diffley JM, et al. Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J Biol Chem. 2004;279:24387–24393 [DOI] [PubMed] [Google Scholar]
- 10. Pajukanta P, Lilja HE, Sinsheimer JS, et al. Familial combined hyperlipidemia is associated with upstream transcription factor 1 (USF1). Nat Genet. 2004;36:371–376 [DOI] [PubMed] [Google Scholar]
- 11. Nishiyama A, Masutani H, Nakamura H, Nishinaka Y, Yodoi J. Redox regulation by thioredoxin and thioredoxin-binding proteins. IUBMB Life. 2001;52:29–33 [DOI] [PubMed] [Google Scholar]
- 12. Junn E, Han SH, Im JY, et al. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164:6287–6295 [DOI] [PubMed] [Google Scholar]
- 13. Yamanaka H, Maehira F, Oshiro M, et al. A possible interaction of thioredoxin with VDUP1 in HeLa cells detected in a yeast two-hybrid system. Biochem Biophys Res Commun. 2000;271:796–800 [DOI] [PubMed] [Google Scholar]
- 14. Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT. The interaction of thioredoxin with Txnip: evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem. 2006;281:21884–21891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forrester MT, Seth D, Hausladen A, et al. Thioredoxin-interacting protein (Txnip) is a feedback regulator of S-nitrosylation. J Biol Chem. 2009;284:36160–36166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966 [PubMed] [Google Scholar]
- 17. Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979;254:9627–9632 [PubMed] [Google Scholar]
- 18. Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noguchi T, Takeda K, Matsuzawa A, et al. Recruitment of TRAF family proteins to the ASK1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem. 2005;280:37033–37040 [DOI] [PubMed] [Google Scholar]
- 20. Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266 [DOI] [PubMed] [Google Scholar]
- 21. Schulze PC, De Keulenaer GW, Yoshioka J, Kassik KA, Lee RT. Vitamin D3-upregulated protein-1 (VDUP-1) regulates redox-dependent vascular smooth muscle cell proliferation through interaction with thioredoxin. Circ Res. 2002;91:689–695 [DOI] [PubMed] [Google Scholar]
- 22. Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem. 2004;279:30369–30374 [DOI] [PubMed] [Google Scholar]
- 23. Saxena G, Chen J, Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem. 2010;285:3997–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu G, Chen J, Jing G, Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat Med. 2013;19:1141–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patwari P, Chutkow WA, Cummings K, et al. Thioredoxin-independent regulation of metabolism by the α-arrestin proteins. J Biol Chem. 2009;284:24996–25003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polekhina G, Ascher DB, Kok SF, Waltham M. Crystallization and preliminary X-ray analysis of the N-terminal domain of human thioredoxin-interacting protein. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011;67(Pt 5):613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hwang J, Suh HW, Jeon YH, et al. The structural basis for the negative regulation of thioredoxin by thioredoxin-interacting protein. Nat. Commun. 2014;5:2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shalev A, Pise-Masison CA, Radonovich M, et al. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFβ signaling pathway. Endocrinology. 2002;143:3695–3698 [DOI] [PubMed] [Google Scholar]
- 29. Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–587 [DOI] [PubMed] [Google Scholar]
- 30. Mandrup-Poulsen T. β-cell apoptosis: stimuli and signaling. Diabetes. 2001;50 Suppl 1:S58–63 [DOI] [PubMed] [Google Scholar]
- 31. Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433–1440 [DOI] [PubMed] [Google Scholar]
- 32. Bonner-Weir S. Life and death of the pancreatic β cells. Trends Endocrinol Metab. 2000;11:375–378 [DOI] [PubMed] [Google Scholar]
- 33. Pick A, Clark J, Kubstrup C, et al. Role of apoptosis in failure of β-cell mass compensation for insulin resistance and β-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47:358–364 [DOI] [PubMed] [Google Scholar]
- 34. Mathis D, Vence L, Benoist C. β-Cell death during progression to diabetes. Nature. 2001;414:792–798 [DOI] [PubMed] [Google Scholar]
- 35. Poitout V, Robertson RP. Minireview: Secondary β-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342 [DOI] [PubMed] [Google Scholar]
- 36. Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces β-cell apoptosis. Endocrinology. 2005;146:2397–2405 [DOI] [PubMed] [Google Scholar]
- 37. Chen J, Hui ST, Couto FM, et al. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic β-cell mass and protects against diabetes. FASEB J. 2008;22:3581–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen J, Fontes G, Saxena G, Poitout V, Shalev A. Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid-induced ER stress-mediated β-cell death. Diabetes. 2010;59:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and β-cell apoptosis. Diabetes. 2008;57:938–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spindel ON, World C, Berk BC. Thioredoxin interacting protein: redox dependent and independent regulatory mechanisms. Antioxid Redox Signal. 2012;16:587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoshihara E, Masaki S, Matsuo Y, Chen Z, Tian H, Yodoi J. Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol. 2014;4:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saitoh T, Tanaka S, Koike T. Rapid induction and Ca2+ influx-mediated suppression of vitamin D3 up-regulated protein 1 (VDUP1) mRNA in cerebellar granule neurons undergoing apoptosis. J Neurochem. 2001;78:1267–1276 [DOI] [PubMed] [Google Scholar]
- 43. Kibbe C, Chen J, Xu G, Jing G, Shalev A. FOXO1 competes with carbohydrate response element-binding protein (ChREBP) and inhibits thioredoxin-interacting protein (TXNIP) transcription in pancreatic β cells. J Biol Chem. 2013;288:23194–23202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu G, Chen J, Jing G, Shalev A. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61:848–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lerner AG, Upton JP, Praveen PV, et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu N, Zheng B, Shaywitz A, et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell. 2013;49:1167–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shao W, Yu Z, Fantus IG, Jin T. Cyclic AMP signaling stimulates proteasome degradation of thioredoxin interacting protein (TxNIP) in pancreatic β-cells. Cell Signal. 2010;22:1240–1246 [DOI] [PubMed] [Google Scholar]
- 48. Robinson KA, Brock JW, Buse MG. Posttranslational regulation of thioredoxin-interacting protein. J Mol Endocrinol. 2013;50:59–71 [DOI] [PubMed] [Google Scholar]
- 49. Cha-Molstad H, Saxena G, Chen J, Shalev A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic β cells. J Biol Chem. 2009;284:16898–16905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamashita H, Takenoshita M, Sakurai M, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U S A. 2001;98:9116–9121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Poungvarin N, Lee JK, Yechoor VK, et al. Carbohydrate response element-binding protein (ChREBP) plays a pivotal role in β cell glucotoxicity. Diabetologia. 2012;55:1783–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stoltzman CA, Peterson CW, Breen KT, Muoio DM, Billin AN, Ayer DE. Glucose sensing by MondoA:Mlx complexes: a role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci U S A. 2008;105:6912–6917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Minn AH, Couto FM, Shalev A. Metabolism-independent sugar effects on gene transcription: the role of 3-O-methylglucose. Biochemistry. 2006;45:11047–11051 [DOI] [PubMed] [Google Scholar]
- 54. Svoboda M, Tastenoy M, Zhang Y, et al. d-Glucose and 3-O-methyl-d-glucose-induced upregulation of selected genes in rat hepatocytes and INS1E cells: reevaluation of the possible role of hexose phosphorylation. Mol Med Rep. 2013;8:829–836 [DOI] [PubMed] [Google Scholar]
- 55. Stoltzman CA, Kaadige MR, Peterson CW, Ayer DE. MondoA senses non-glucose sugars: regulation of thioredoxin-interacting protein (TXNIP) and the hexose transport curb. J Biol Chem. 2011;286:38027–38034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shaked M, Ketzinel-Gilad M, Cerasi E, Kaiser N, Leibowitz G. AMP-activated protein kinase (AMPK) mediates nutrient regulation of thioredoxin-interacting protein (TXNIP) in pancreatic β-cells. PLoS One. 2011;6:e28804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reich E, Tamary A, Sionov RV, Melloul D. Involvement of thioredoxin-interacting protein (TXNIP) in glucocorticoid-mediated β cell death. Diabetologia. 2012;55:1048–1057 [DOI] [PubMed] [Google Scholar]
- 58. Lekva T, Bollerslev J, Sahraoui A, et al. Thioredoxin interacting protein is a potential regulator of glucose and energy homeostasis in endogenous Cushing's syndrome. PLoS One. 2013;8:e64247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shaked M, Ketzinel-Gilad M, Ariav Y, Cerasi E, Kaiser N, Leibowitz G. Insulin counteracts glucotoxic effects by suppressing thioredoxin-interacting protein production in INS-1E β cells and in Psammomys obesus pancreatic islets. Diabetologia. 2009;52:636–644 [DOI] [PubMed] [Google Scholar]
- 60. Parikh H, Carlsson E, Chutkow WA, et al. TXNIP Regulates Peripheral Glucose Metabolism in Humans. PLoS Med. 2007;4:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen J, Couto FM, Minn AH, Shalev A. Exenatide inhibits β-cell apoptosis by decreasing thioredoxin-interacting protein. Biochem Biophys Res Commun. 2006;346:1067–1074 [DOI] [PubMed] [Google Scholar]
- 62. Chen J, Jing G, Xu G, Shalev A. Thioredoxin-Interacting Protein Stimulates its Own Expression via a Positive Feedback Loop. Mol Endocrinol. 2014;28:674–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hasegawa J, Osatomi K, Wu RF, Uyeda K. A novel factor binding to the glucose response elements of liver pyruvate kinase and fatty acid synthase genes. J Biol Chem. 1999;274:1100–1107 [DOI] [PubMed] [Google Scholar]
- 64. Wang H, Wollheim CB. ChREBP rather than USF2 regulates glucose stimulation of endogenous L-pyruvate kinase expression in insulin-secreting cells. J Biol Chem. 2002;277:32746–32752 [DOI] [PubMed] [Google Scholar]
- 65. Davies MN, O'Callaghan BL, Towle HC. Glucose activates ChREBP by increasing its rate of nuclear entry and relieving repression of its transcriptional activity. J Biol Chem. 2008;283:24029–24038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci U S A. 2001;98:13710–13715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Denechaud PD, Bossard P, Lobaccaro JM, et al. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J Clin Invest. 2008;118:956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kabashima T, Kawaguchi T, Wadzinski BE, Uyeda K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proc Natl Acad Sci U S A. 2003;100:5107–5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. 2002;277:3829–3835 [DOI] [PubMed] [Google Scholar]
- 70. Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163 [DOI] [PubMed] [Google Scholar]
- 71. Buteau J, Accili D. Regulation of pancreatic β-cell function by the forkhead protein FoxO1. Diabetes Obes. Metab. 2007;9(suppl 2):140–146 [DOI] [PubMed] [Google Scholar]
- 72. Buteau J, Spatz ML, Accili D. Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic β-cell mass. Diabetes. 2006;55:1190–1196 [DOI] [PubMed] [Google Scholar]
- 73. Kawamori D, Kaneto H, Nakatani Y, et al. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem. 2006;281:1091–1098 [DOI] [PubMed] [Google Scholar]
- 74. Okamoto H, Hribal ML, Lin HV, et al. Role of the forkhead protein FoxO1 in β cell compensation to insulin resistance. J Clin Invest. 2006;116:775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oslowski CM, Hara T, O'Sullivan-Murphy B, et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373 [DOI] [PubMed] [Google Scholar]
- 77. Sun Y, Koo S, White N, Peralta E, Esau C, Dean NM, Perera RJ. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 2004;32:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Landgraf P, Rusu M, Sheridan R, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234 [DOI] [PubMed] [Google Scholar]
- 80. Zhuo de X, Niu XH, Chen YC, Xin DQ, Guo YL, Mao ZB. Vitamin D3 up-regulated protein 1(VDUP1) is regulated by FOXO3A and miR-17–5p at the transcriptional and post-transcriptional levels, respectively, in senescent fibroblasts. J Biol Chem. 2010;285:31491–31501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Minn AH, Pise-Masison CA, Radonovich M, et al. Gene expression profiling in INS-1 cells overexpressing thioredoxin-interacting protein. Biochem Biophys Res Commun. 2005;336:770–778 [DOI] [PubMed] [Google Scholar]
- 82. Wang Y, De Keulenaer GW, Lee RT. Vitamin D3-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem. 2002;277:26496–26500 [DOI] [PubMed] [Google Scholar]
- 83. Shalev A. Lack of TXNIP protects β-cells against glucotoxicity. Biochem Soc Trans. 2008;36(Pt 5):963–965 [DOI] [PubMed] [Google Scholar]
- 84. Corbett JA. Thioredoxin-interacting protein is killing my β-cells! Diabetes. 2008;57:797–798 [DOI] [PubMed] [Google Scholar]
- 85. Courboulin A, Paulin R, Giguère NJ, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2011;301:H1798–H1809 [DOI] [PubMed] [Google Scholar]
- 87. Artner I, Hang Y, Mazur M, et al. MafA and MafB regulate genes critical to β-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826 [DOI] [PubMed] [Google Scholar]
- 89. Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jing G, Westwell-Roper C, Chen J, Xu G, Verchere CB, Shalev A. Thioredoxin-interacting protein promotes islet amyloid polypeptide expression through MIR-124A and FOXA2. J Biol Chem. 2014;289:11807–11815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140 [DOI] [PubMed] [Google Scholar]
- 92. Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300 [DOI] [PubMed] [Google Scholar]
- 93. Maedler K, Sergeev P, Ris F, et al. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. March CJ, Mosley B, Larsen A, et al. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985;315:641–647 [DOI] [PubMed] [Google Scholar]
- 96. Westwell-Roper CY, Ehses JA, Verchere CB. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1β production and β-cell dysfunction. Diabetes. 2014;63:1698–1711 [DOI] [PubMed] [Google Scholar]
- 97. Chutkow WA, Patwari P, Yoshioka J, Lee RT. Thioredoxin-interacting protein is a critical regulator of hepatic glucose production. J Biol Chem. 2008;283:2397–2406 [DOI] [PubMed] [Google Scholar]
- 98. Hotta M, Tashiro F, Ikegami H, et al. Pancreatic β cell-specific expression of thioredoxin, an antioxidative and antiapoptotic protein, prevents autoimmune and streptozotocin-induced diabetes. J Exp Med. 1998;188:1445–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chou FC, Sytwu HK. Overexpression of thioredoxin in islets transduced by a lentiviral vector prolongs graft survival in autoimmune diabetic NOD mice. J Biomed Sci. 2009;16:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Clee SM, Nadler ST, Attie AD. Genetic and genomic studies of the BTBR ob/ob mouse model of type 2 diabetes. Am J Ther. 2005;12:491–498 [DOI] [PubMed] [Google Scholar]
- 101. Chutkow WA, Birkenfeld AL, Brown JD, et al. Deletion of the alpha-arrestin protein Txnip in mice promotes adiposity and adipogenesis while preserving insulin sensitivity. Diabetes. 2010;59:1424–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yamamoto M, Yamato E, Toyoda S, et al. Transgenic expression of antioxidant protein thioredoxin in pancreatic β cells prevents progression of type 2 diabetes mellitus. Antioxid Redox Signal. 2008;10:43–49 [DOI] [PubMed] [Google Scholar]
- 103. Afzal N, Ganguly PK, Dhalla KS, Pierce GN, Singal PK, Dhalla NS. Beneficial effects of verapamil in diabetic cardiomyopathy. Diabetes. 1988;37:936–942 [DOI] [PubMed] [Google Scholar]
- 104. Cooper-Dehoff R, Cohen JD, Bakris GL, et al. Predictors of development of diabetes mellitus in patients with coronary artery disease taking antihypertensive medications (findings from the INternational VErapamil SR-Trandolapril STudy [INVEST]). Am J Cardiol. 2006;98:890–894 [DOI] [PubMed] [Google Scholar]
- 105. Cooper-DeHoff RM, Aranda JM, Jr, Gaxiola E, et al. Blood pressure control and cardiovascular outcomes in high-risk Hispanic patients—findings from the International Verapamil SR/Trandolapril Study (INVEST). Am Heart J. 2006;151:1072–1079 [DOI] [PubMed] [Google Scholar]
- 106. Xiang G, Seki T, Schuster MD, et al. Catalytic degradation of vitamin D up-regulated protein 1 mRNA enhances cardiomyocyte survival and prevents left ventricular remodeling after myocardial ischemia. J Biol Chem. 2005;280:39394–39402 [DOI] [PubMed] [Google Scholar]
- 107. Yoshioka J, Chutkow WA, Lee S, et al. Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J Clin Invest. 2012;122:267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cha-Molstad H, Xu G, Chen J, et al. Calcium channel blockers act through nuclear factor Y to control transcription of key cardiac genes. Mol Pharmacol. 2012;82:541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen J, Cha-Molstad H, Szabo A, Shalev A. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab. 2009;296:E1133–E1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shi Y, Ren Y, Zhao L, et al. Knockdown of thioredoxin interacting protein attenuates high glucose-induced apoptosis and activation of ASK1 in mouse mesangial cells. FEBS Lett. 2011;585:1789–1795 [DOI] [PubMed] [Google Scholar]
- 111. Perrone L, Devi TS, Hosoya KI, Terasaki T, Singh LP. Inhibition of TXNIP expression in vivo blocks early pathologies of diabetic retinopathy. Cell Death Dis. 2010;1:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sheth SS, Bodnar JS, Ghazalpour A, et al. Hepatocellular carcinoma in Txnip-deficient mice. Oncogene. 2006;25:3528–3536 [DOI] [PubMed] [Google Scholar]
- 113. Oka S, Liu W, Masutani H, et al. Impaired fatty acid utilization in thioredoxin binding protein-2 (TBP-2)-deficient mice: a unique animal model of Reye syndrome. FASEB J. 2006;20:121–123 [DOI] [PubMed] [Google Scholar]