Abstract
Toxoplasma gondii establishes latent infection in the central nervous system of immunocompentent hosts. Toxoplasmic encephalitis is a life threatening reactivation of latent infection in the brain of immunocompromised patients. To further understand the mechanisms of entry into the brain of T. gondii we investigated host molecules and cells involved in the passage of the parasite through the blood–brain barrier. First, using microarrays brain endothelial cells were found to upregulate, among others, chemokines and adhesion molecules following infection with tachyzoites. Using flow cytometry we observed upregulated ICAM-1 expression on the surface of brain endothelial cells following infection; ICAM-1 expression was further increased after pre-incubation with IFN-γ. Compared to RH tachyzoites, ME49 tachyzoites induced a stronger upregulation of ICAM-1 and an earlier and stronger IL-6 and MCP-1 secretion by brain endothelial cells. Using an in vitro coculture model of the BBB (primary glia cells and brain endothelial cells) we found a stronger migration of infected antigen-presenting cells compared to lymphocytes (4.63% vs. 0.6% of all cells) across the BBB. Among all antigen-presenting cells CD11b+/CD11c+ cells showed the highest infection rate, whereas the majority of infected cells that migrated through the blood–brain barrier were CD11b+/CD11c− cells. Infection of PBMCs with type I or type II Toxoplasma strains resulted in similar patterns of cell migration across the in vitro BBB model.
In conclusion, these results suggest that T. gondii modulates gene expression of brain endothelial cells to promote its own migration through the blood–brain barrier in a ‘Trojan horse’ manner. Cells expressing CD11b either with or without CD11c are likely candidate cells for the intracellular transport of T. gondii across the BBB. T. gondii type I and type II strains induced similar migration patterns of antigen-presenting cells across the in vitro BBB.
Keywords: Toxoplasma gondii, Blood–brain barrier, Neuroinvasion, Transendothelial migration
1. Introduction
The blood–brain barrier is a selective cellular border at the level of specialized cerebral microvascular endothelial cells that protects the central nervous system (CNS) from blood-borne endangerments. The brain endothelial cells interacting with perivascular structures like pericytes, microglia and astroglia have characteristic properties defined by high transendothelial electrical resistance, the expression of tight junctions sealing the paracellular spaces, and a low pinocytotic activity (Abbott et al., 2006; Ge et al., 2005; Rubin and Staddon, 1999; Deli et al., 2005). Although the CNS is routinely surveyed by cells of the immune system, inflammatory processes can lead to an excessive leukocyte infiltration into the brain and cause pathology (Hickey, 2001; Luster et al., 2005) as in patients with multiple sclerosis or AIDS dementia (McFarland and Martin, 2007; Nottet, 1999). Under healthy conditions the endothelial cells of the blood–brain barrier express very low levels of adhesion molecules that could be used by leukocytes for transendothelial migration. Upon inflammatory stimulation, microvascular endothelial cells induce the expression of cell adhesion molecules including ICAM-1, VCAM-1, and selectins (Kadl and Leitinger, 2005; Coisne et al., 2006). In concert with the secretion of various chemokines the vascular response implicates a decrease of blood–brain barrier function. Successful leukocyte trafficking thereby demands the interaction of selectins and their ligands as well as the cooperation of cell adhesion molecules with integrins or chemokines with their receptors (Ransohoff et al., 2003; Pober and Sessa, 2007). Infections of the CNS are caused by a variety of extracellular (mostly causing meningitis) and intracellular pathogens (Kim, 2008; Marra and Brigham, 2001; Katti, 2004; Kim, 2002). Whereas extracellular pathogens are believed to use the blood-cerebrospinal fluid barrier in the choroid plexus to gain access to the brain, intracellular pathogens including Listeria monocytogenes, Cryptococcus neoformans or the human immunodeficiency virus exploit host cells to transmigrate across host barriers (Drevets et al., 2004; Charlier et al., 2009; Kanmogne et al., 2007). Infection with the protozoan parasite Toxoplasma gondii results in invasion of the brain and the formation of tissue cysts that persists throughout the life of the host without causing symptoms (Montoya and Liesenfeld, 2004). However, in immunocompromised patients, reactivation of latent infection may result in the release of rapidly multiplying tachyzoites from tissue cysts (Dellacasa-Lindberg et al., 2007) and lethal encephalitis if left untreated. Reactivated toxoplasmosis is among the most frequent CNS manifestations in seropositive AIDS and transplant patients (Montoya and Liesenfeld, 2004; Dellacasa-Lindberg et al., 2007). The mechanism(s) how Toxoplasma reaches the brain (extra- or intracellularly) during acute infection have not been elucidated in detail. Recently, parasite dissemination into the CNS inside host leukocytes has been suggested in in vivo experiments (Courret et al., 2006; Unno et al., 2008). Type I, II and III T. gondii strains differ with respect to their ability to transmigrate across cellular barriers. Whereas type I strains exhibit a higher migratory capacity than type II strains, type II strains induced superior migratory frequency and intensity of dendritic cells (Lambert et al., 2009).
In the present study, we analyzed the expression of cell adhesion molecules and cytokines by brain endothelial cells upon infection with different strains of T. gondii as a number of investigators have reported a possible role of ICAM-1, IL-6, and MCP-1 in infection with T. gondii (Barragan et al., 2005; Clahsen and Schaper, 2008; Linker et al., 2008; Aviles et al., 2008; Robben et al., 2005). Using a coculture transwell model of the BBB we then analyzed the migratory capacity of different subsets of naïve and infected peripheral blood mononuclear cell subsets through the blood–brain barrier.
2. Materials and methods
2.1. Parasites
GFP+ tachyzoites of the T. gondii RH strain were a kind gift from Prof. D. Soldati-Favre, University of Geneva, Faculty of Medicine, Switzerland, while the ME49 GFP+ tachyzoites were kindly provided by Dr. Markus Meissner, Hygiene Institute, Department of Parasitology, Heidelberg University School of Medicine, Germany.
2.2. Gene expression analysis of endothelial cells
To determine changes in transcriptional regulation profiles in brain endothelial cells 3×106 bEnd.3 cells (Montesano et al., 1990) were infected with freshly egressed GFP+ tachyzoites of the T. gondii RH strain at an MOI (multiplicity of infection) of 3 and harvested 4 and 8 h post infection. Transcription profiles were compared to uninfected cells using the Agilent Whole Mouse Genome Oligo Microarray. RNA isolation, RNA quality control, linear T7-based amplification of RNA as well as hybridization, scanning, and analysis of microarrays were performed by the gene expression profiling service of Miltenyi Biotec (Bergisch Gladbach, Germany). Gene regulations with a p<0.05 were regarded significant.
2.3. Preparation of primary glia cell cultures
The brains of 10 2–3 day old Wistar rats were separated from the meninges and choroid plexus, minced, and digested in 0.1% trypsin (Biochrom AG, Berlin, Germany) in PBS (PAA Laboratories GmbH, Cölbe, Germany) for 15 min at 37 °C. The cell suspension was pelleted at 500 g, digested with 100 μg/mL DNaseI (Roche Diagnostics, Mannheim, Germany), and subsequently washed at 300 g. The resulting pellet was suspended in D-MEM (Invitrogen GmbH, Karlsruhe, Germany) with 10% FBS (Biochrom AG), 100 μg/mL penicillin/streptomycin (Biochrom AG) and 1× non-essential amino acids (Invitrogen GmbH), and seeded in 12 well plates (Corning B.V. Life Sciences, Schiphol-Rijk, Netherlands). Medium was changed every 3 days. After 4 weeks of culture cells were used in coculture experiments with primary rat brain endothelial cells.
2.4. Preparation of rat brain endothelial cell cultures
Primary rat brain endothelial cells (pRBECs) were isolated as described previously (Deli et al., 1993; Veszelka et al., 2007). Briefly, forebrains were separated from meninges and grey matter was digested with 1 mg/mL collagenase type II (Sigma-Aldrich, Steinheim, Germany) and 15 μg/mL DNaseI for 1.5 h at 37 °C. To separate microvessels the pellet was mixed with a 20% (w/v) BSA (Sigma-Aldrich) solution and centrifuged for 20 min at 1000 g. The myelin layer on top was removed, and the pellet digested with 1 mg/mL Collagenase/Dispase (Roche Diagnostics) and 6.6 μg/mL DNaseI for 1 h at 37 °C. To remove pericytes the cell suspension was fractionated on a 33% Percoll (GE Healthcare, Munich, Germany) gradient. The endothelial cells from the layer above the erythrocyte/pericyte containing layer were collected, washed twice and plated onto 3 μm pore transwell filters (Corning B.V. Life Sciences) or cell culture flasks previously coated with 0.5 mg/mL collagen IV and 0.1 mg/mL fibronectin (both from Sigma-Aldrich). The addition of 3 μg/mL puromycin (Sigma-Aldrich) to endothelial cell medium [D-MEM containing 20% plasma-derived bovine serum (First Link Ltd., Birmingham, UK), 100 μg/mL penicillin/streptomycin, 100 μg/mL heparin (Sigma-Aldrich), and 1 ng/mL basic fibroblast growth factor (Roche Diagnostics)] for 3 days assured effective removal of contaminating brain cells (Perrière et al., 2005). Three days after isolation cells were used in mono- or coculture experiments.
2.5. Establishment of endothelial and glia cocultures
Three days after endothelial cell preparation the transwell filters containing pRBECs were placed into 12 well plates with glia cell cultures. The medium in the upper and lower compartments was replaced by endothelial cell medium with 200 ng/mL hydrocortisone (Sigma-Aldrich). The content of the upper chamber was exchanged by an endothelial cell medium containing 200 ng/mL hydrocortisone, 250 μM cAMP (Sigma-Aldrich), and 17.5 μM Ro 20–1724 (Sigma-Aldrich) 24 h before transmigration experiment to enhance BBB properties (Deli et al., 2005). The quality of the BBB model was determined by measuring the transendothelial electrical resistance (TEER, in Ω×cm2) of the monolayer as a reference for barrier tightness (Millicell-Electrical Resistance System, Millipore, Bedford, USA). The TEER of coated cell free filters was subtracted from TEER of filters with cells as a blind value. To investigate the permeability of the endothelial monolayer, the flux of selected tracers from the luminal to the abluminal compartment was measured and calculated as endothelial permeability coefficient (Deli et al., 2005). Sodium fluorescein (10 μg/mL, Sigma-Aldrich) served as paracellular, Evan’s blue-labeled albumin (EBA) (165 μg/mL, Sigma-Aldrich) as transcellular marker (Veszelka et al., 2007). Both were dissolved in D-MEM without phenol-red (Biochrom AG).
2.6. Isolation of mouse and rat PBMCs
PBMCs from Wistar rats or NMRI mice were isolated from heparinized blood with OptiPrep™ solution (Axis-Shield, Dundee, Scotland) according to the manufacturer’s recommendations. For mouse PBMCs, 5–10 mL of blood was diluted with 1/2 blood volume of TBS [0.85% NaCl (Merck, Darmstadt, Germany) and 10 mM tricine (Sigma-Aldrich), pH 7.4] and 1/8 blood volume of OptiPrep™. The solution was carefully overlaid with 0.5 mL TBS and centrifuged at 1000 g for 30 min at 20 °C. PBMCs were collected, diluted with two volumes of PBS, and pelleted at 250 g for 10 min to remove platelets. For rat PBMCs, 5–10 mL of blood was diluted with 1/8 blood volume of OptiPrep™. The solution was carefully overlaid with 0.5 mL TBS and centrifuged at 1300 g for 30 min at 20 °C. Further preparation was performed according to the preparation of mouse PBMCs described above.
2.7. Infection of brain endothelial cells and PBMCs with T. gondii tachyzoites
Infection of leukocytes or endothelial cells was performed using freshly egressed GFP+ tachyzoites of the T. gondii RH or ME49 strain. Tachyzoites were maintained by serial passage in human foreskin fibroblasts (HFF) (Amit et al., 2003). HFF monolayers and T. gondii tachyzoites were cultured in D-MEM with 10% FBS and 100 μg/mL penicillin/streptomycin. Mouse or rat PBMCs were mixed with T. gondii tachyzoites (MOI from 1 to 5) in 15 mL of medium and transferred to a 50 mL centrifugation tube (Sarstedt AG, Nümbrecht, Germany). The lid was slightly opened and the tube was incubated for 2 h at 37 °C in a slanting position. To remove extracellular parasites the cell suspension was washed twice at 200 g for 10 min in D-MEM with 10% FBS and 100 μg/mL penicillin/streptomycin. For infection of endothelial cells, pRBECs were prepared as described above. The mouse brain endothelial cell line bEnd.3 was cultured in D-MEM with 10% FBS and 100 μg/mL penicillin/streptomycin. PRBECs and bEnd.3 cells (Böggemeyer et al., 1994) were seeded onto 24 well plates (Sarstedt AG) and cultured at 37 °C and 5% CO2 atmosphere. Cells were pretreated with 100 U/mL recombinant mouse (Calbiochem, Darmstadt, Germany), or rat IFN-γ (PeproTech GmbH, Hamburg, Germany) for 48 h as indicated.
As infected endothelial cells appear GFP+ it was possible to distinguish between infected and uninfected cells of the same well. According to this the cells are designated as “T. gondii exposed and infected” or “T. gondii exposed but uninfected” cells.
2.8. Migration assay
For migration assays, filters with pRBECs were transferred to new 12 well plates containing either medium alone or medium with 100 ng/mL recombinant mouse or rat MCP-1 (both from ImmunoTools, Friesoythe, Germany) or 100 ng/mL rat recombinant TNF-α (Calbiochem). In each condition 3 × 105 PBMCs were added to the upper compartment. After 3 h at 37 °C, the content of the lower compartment was collected. The outside of the filter inserts and the inside of the plate wells were washed 3 times with ice-cold PBS and pooled with the previously collected cells. Cells obtained from 10 filters were pooled and centrifuged for 10 min at 300 g and 4 °C.
2.9. Flow cytometry
Prior to antibody staining, unspecific binding was blocked with the addition of 2.5% mouse or rat serum. The antibodies were used for flow cytometry of endothelial cells or leukocytes: biotin-conjugated α-rat-CD54, streptavidin-phycoerythrin (SA-PE), phycoerythrin (PE)-conjugated α-mouse-CD54 (Biolegend, San Diego, USA), phycoerythrin (PE)-conjugated α-rat-CD45, allophycocyanin (APC)-conjugated α-rat-CD11bc (Invitrogen GmbH), phycoerythrin (PE)-conjugated α-mouse-CD11b, allophycocyanin (APC)-conjugated α-mouse-CD11c, phycoerythrin (PE)-conjugated α-mouse-B220, and peridinin-chlorophyll-protein (PerCP)-conjugated α-mouse-CD3 (Becton Dickinson, San Jose, USA). Anti-rat-CD45 antibodies were used as pan-leukocyte markers while anti-rat-CD11bc antibodies helped to distinguish monocytes and dendritic cells from the rest of the lymphocytes.
Cells were incubated with the antibodies for 15 min at 4 °C in the dark, washed twice and subsequently fixed in 4% paraformaldehyde/PBS. Cells were suspended in storage buffer (2% FBS, 2 mM EDTA, and 0.1% sodium azide in PBS), and analyzed within 24 h by flow cytometry. For each antibody staining the corresponding isotype control staining was performed to allow accurate setting of gates and quadrants.
2.10. Immunofluorescence microscopy
Cells were fixed in 4% paraformaldehyde and labeled with the primary antibodies [rabbit-α-GFAP, rabbit-α-ZO-1, mouse-α-occludin (Invitrogen GmbH), and rabbit-α-vWF (DAKO Deutschland GmbH, Hamburg, Germany)], followed by labeling with the corresponding secondary antibodies [goat-α-rabbit-Alexa fluor 488, chicken-α-rabbit-Alexa fluor 594, and goat-α-mouse-Alexa fluor 633 (Invitrogen GmbH)] in a blocking solution of 5% FBS in PBS. DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich), and cells were mounted in DAKO mounting medium (DAKO Deutschland GmbH). Immunofluorescence pictures were acquired with an Axiovert 200 M microscope (Carl Zeiss, Jena, Germany).
2.11. Isolation of brain mononuclear cells
Anesthesized animals underwent brain perfusion with 40 mL ice-cold PBS and two brains for each condition were homogenized in a 5 mL RPMI medium with an 18 G syringe needle. The cell suspension was digested with 250 μg/mL Collagenase/Dispase (Roche) and 125 μg/mL DNase I (Roche) for 1 h at 37 °C, filtered through a cell strainer with 70 μm pore size and filled up to 50 mL with RPMI. After centrifugation for 10 min at 1000 g the pellet was suspended in a 60% (v/v) Percoll-solution and overlaid with a 30% (v/v) Percollsolution. The gradient was centrifuged for 25 min at 1000 g and brain mononuclear cells from the 60%/30% interphase were collected.
2.12. Intravenous injection of T. gondii tachyzoites
Extracellular tachyzoites were harvested after serial passage in HFF cells. A centrifugation at 200 g for 10 min pelleted remaining host cells. The supernatant was centrifuged at 350 g for 10 min and the pelleted tachyzoites were washed with PBS at the same speed. The purified tachyzoites were suspended in PBS, counted and intravenously administered to BALB/c or NMRI mice at a concentration of 107 tachyzoites/0.2 mL.
2.13. Intravenous injection of infected PBMC subsets
To separate PBMCs from NMRI mice into CD11b+ and CD11b− fractions, CD11b-coated magnetic beads (Miltenyi Biotec GmbH) were used according to the manufacturer’s recommendations. Flow cytometry revealed that the purity of the CD11b enriched fraction was approximately 85% (referred to as CD11b+ cells) while the CD11b− fraction of cells contained less than 6% CD11b+ cells (referred to as CD11b− cells). Each subset underwent 15 h incubation with GFP+ RH tachyzoites of T. gondii at an MOI of 1. Rates of infection for CD11b+ and CD11b− cells were determined by flow cytometry and NMRI mice were injected intravenously with 10,000 infected cells of either population in 0.2 mL PBS.
2.14. PCR
T. gondii DNA in brain tissue was quantified using LightCycler FastStart DNA Master Hybridization Probes-Kit (Roche Diagnostics), primers for amplification of a 162 bp fragment of T. gondii cryptic gene (TOX-9: 5′-AGGAGAGATATCAGGACTGTAG-3′, TOX-10as: 5′-GCGTCGTCTCGTCTAGATCG-3′) (TIB MolBiol, Berlin, Germany) and fluorescent hybridization oligonucleotides (TOX-HP-1: 5′-GAGTCG-GAGAGGGAGAAGATGTT-FAM-3′, TOX-HP-2: 5′-RED-640-CCGGCTTGGCTGCTTTTCCTG-PH-3′) (TIB MolBiol) on a LightCycler® (Roche Diagnostics). 50 ng of template DNA, 1 μL 10× enzyme mix, 2 mM MgCl2, 1 mM of each oligonucleotide primer and 0.2 nM of each hybridization probe were adjusted to a final volume of 10 μL in capillaries (Genaxxon BioScience GmbH, Biberach, Germany). Amplification of T. gondii DNA was performed after an initial denaturation step of 95 °C for 1 min in 50 cycles of 95 °C for 10 s, 52 °C for 30 s, and 72 °C for 30 s with data acquisition. Each PCR assay included a negative control with distilled water instead of template DNA. With each run, a standard curve of T. gondii DNA (2.5 pg to 250 pg) was generated. Fluorescence-related amount of T. gondii DNA was calculated with LightCycler Data Analysis Software 3.5 (Roche Diagnostics).
2.15. Statistics
Differences between groups were determined by Student’s t-test for normally distributed data. The Mann–Whitney-test was used for all other data using GraphPad InStat3 Software. P-values of <0.05 were considered significant.
3. Results
3.1. Changes in gene expression in murine brain endothelial cells infected with T. gondii tachyzoites
During acute infection T. gondii crosses the blood–brain barrier to gain access to the neuronal tissues where it resides in its cyst form during latent infection. We therefore investigated the effects of infection with the type I RH strain of T. gondii on the transcriptome of murine brain endothelial cells. Compared to uninfected cells transcriptional regulation was observed at 4 and/or 8 h post infection for cytokines, chemokines and their respective receptors as well as for molecules involved in cell signaling, clusters of differentiation, cell adhesion, and tight junction formation (Fig. 1). Genes related to cell adhesion, tight junctions, and chemokines showed the strongest regulation. Four hours post infection we found a 10.88-fold upregulation of JAM4 while CD80 was more than 9-fold downregulated. At 8 h post infection the chemokines CCL7 and CX3CL1 were upregulated 8.84 and 6.18-fold respectively while the expression of the signaling molecule STAT4 was reduced 4.69-fold. These results indicate that infection with T. gondii results in a major regulation of the transcriptome. Regulated genes suggest that neuroinvasion may involve exploitation of leukocyte transendothelial migration pathways.
Fig. 1.
Transcriptional response of bEnd.3 cells infected with T. gondii for 4 and 8 h. Transcriptional regulation of selected genes in murine endothelial (bEnd.3) cells infected with GFP+ tachyzoites of the T. gondii RH strain (MOI 3) for 4 and 8 h. Analysis was performed by expression profiling service of Miltenyi Biotec as described in Materials and methods. Up- or downregulation is shown as a multiple of expression levels observed in uninfected control cells.
3.2. Induction of endothelial ICAM-1 expression by IFN-γ and infection with T. gondii tachyzoites
Since ICAM-1 binds to leukocyte ligands to facilitate the entry of activated leukocytes into the CNS (Dietrich, 2002) and since ICAM-1 gene expression was upregulated following infection with T. gondii we investigated the impact of parasite infection on the expression of ICAM-1 in a murine brain endothelial cell line and in primary rat brain endothelial cells. We measured the mean fluorescence intensity of cells expressing ICAM-1 since even before infection more than 80% of bEnd.3 and primary rat endothelial cells already expressed ICAM-1.
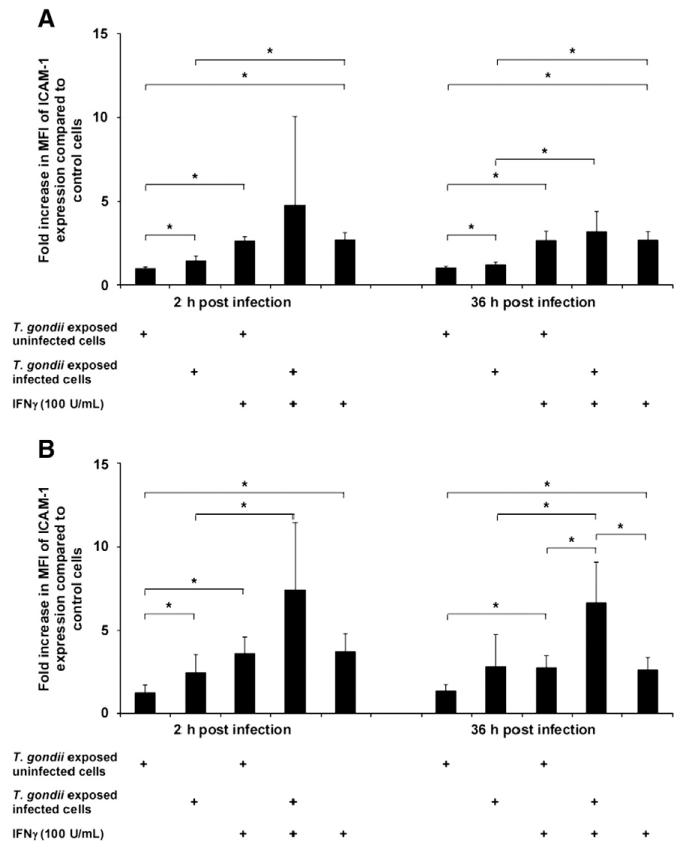
Infection of murine brain endothelial cells with T. gondii at a parasite:cell ratio of 1:1 resulted in up to 1% infected primary rat endothelial cells and up to 12% infected bEnd.3 cells. Results are presented separately for infected vs. uninfected cells in the same well following incubation with T. gondii; compared to naïve cells we observed a significant upregulation of ICAM-1 expression at 2 and 36 h post infection in infected and uninfected cells exposed to T. gondii (Fig. 2A). The ICAM-1 expression was significantly higher among infected vs. uninfected cells in the same well. Expression of ICAM-1 was further increased in infected and uninfected exposed cells after pre-incubation with IFN-γ.
Fig. 2.
ICAM-1 upregulation of endothelial cells is induced by infection with T. gondii. Endothelial cells were treated with IFN-γ or left untreated for 48 h; thereafter, cells were incubated with GFP+ tachyzoites of the T. gondii RH strain (MOI 1). As infected endothelial cells appear GFP+ we distinguished between infected and uninfected cells of the same well. According to this the cells are designated “T. gondii exposed and infected” or “T. gondii exposed but uninfected” cells. The mean fluorescence intensity (MFI) of ICAM-1 expression was analyzed at 2 and 36 h after infection and is shown as a multiple of the levels observed in untreated and uninfected control cells as determined by flow cytometry. After incubation with tachyzoites, the infected and uninfected cells of the same well were analyzed. A) MFI of ICAM-1 expression in murine brain endothelial cells (bEnd.3), B) MFI of ICAM-1 expression in rat brain endothelial cells (pRBECs). Results shown are pooled data from 3 experiments performed. *p<0.05.
Similarly, infection of primary rat brain endothelial cells with the same parasite:cell ratio led to a significant upregulation of ICAM-1 expression at 2 and 36 h post infection compared to naïve control cells (Fig. 2B); at 2 h post infection T. gondii infected cells showed a significantly higher ICAM-1 expression than uninfected cells in the same well. Pre-incubation of rat brain endothelial cells with IFN-γ led to a significant increase in ICAM-1 expression compared to naïve control cells; IFN-γ incubation resulted in a further increase in ICAM-1 expression in both infected and uninfected cells from wells exposed to T. gondii. Similar results were obtained at a higher parasite:cell ratio of 5:1 (data not shown).
These results suggest that T. gondii induces the upregulation of ICAM-1. The increased ICAM-1 expression in infected cells may facilitate leukocyte migration across endothelial barriers.
3.3. Upregulation of endothelial ICAM-1 and secretion of pro-inflammatory molecules after infection with RH and ME49 tachyzoites
Since upregulation of ICAM-1 and secretion of pro-inflammatory molecules by endothelial cells enhance the extravasation of leukocytes, and since type I and type II strains of T. gondii differ in their migratory capacities (Barragan and Sibley, 2002), we investigated ICAM-1 expression and secretion of IL-6 and MCP-1 by bEnd.3 cells following infection with the RH (type I) and ME49 (type II) strains of the parasite. IL-6 and MCP-1 production by endothelial cells may regulate early expression of adhesion molecules at the blood–brain barrier and induce the recruitment of leukocytes important for resistance to T. gondii. More than 88% of all endothelial cells showed a constitutive ICAM-1 expression. When brain endothelial cells were infected with tachyzoites at an MOI of 1, type II ME49 tachyzoites yielded significantly higher initial infection rates than infection with type I RH tachyzoites (Fig. 3A). At 36 h post infection, the percentage of cells infected with RH tachyzoites had further increased. Maximum percentages of infected cells were approx. 11% and were similar at 48 h after infection for both T. gondii strains.
Fig. 3.
ICAM-1 expression and cytokine secretion of endothelial cells after infection with T. gondii. bEnd.3 cells were incubated with GFP+ tachyzoites of either the T. gondii RH or ME49 strain at an MOI of 1. A) Percentages of infected bEnd.3 cells following infection with RH or ME49 strain tachyzoites at an MOI of 1. B) Mean fluorescence intensity (MFI) of ICAM-1 expression in bEnd.3 cells was measured by flow cytometry at indicated time points and is shown as a multiple of the MFI of untreated uninfected control cells obtained at the same time points. We distinguished between uninfected (white bars) and RH (black bars) or ME49 (grey bars) infected cells from the same well. C) IL-6 production of bEnd.3 cells after infection with RH and ME49 strain T. gondii tachyzoites determined by ELISA. D) MCP-1 production of bEnd.3 cells after infection with T. gondii RH and ME49 tachyzoites determined by ELISA. Results shown are pooled data from 3 experiments performed. *p<0.05, **p=0.053 (tendency to significance).
Compared to uninfected control cells in the same well, cells infected with the RH strain showed significantly increased ICAM-1 expression at 2 to 24 h whereas cells infected with the ME49 strain showed significantly increased ICAM-1 expression at all time points compared to uninfected cells in the same well (Fig. 3B). Infection of cells with the ME49 strain resulted in a significantly higher expression of ICAM-1 between 12 and 48 h post infection compared to RH infection.
ME49 tachyzoites also induced an earlier and stronger IL-6 and MCP-1 secretion compared to RH tachyzoites (Fig. 3C,D). The significantly increased secretion of IL-6 and MCP-1 by the ME49 compared to the RH strain was also observed when concentrations of IL-6 or MCP-1 were normalized to the infection rate of the respective T. gondii strain (data not shown). At an MOI of 5 the expression of ICAM-1 and the secretion of pro-inflammatory markers were similar to that observed at an MOI of 1 (data not shown). Taken together, these results suggest that ME49 tachyzoites have an increased infectivity early after infection, induce a stronger upregulation of ICAM-1, and secrete higher levels of pro-inflammatory molecules compared to RH tachyzoites.
3.4. Validation of the in vitro coculture model of the BBB
To allow analysis of a role for leukocytes in neuroinvasion by the parasite, we established an in vitro model of the BBB using the transwell coculture of rat glia and endothelial cells (Veszelka et al., 2007). Immunological staining with antibodies against von Willebrand factor, ZO-1, occludin and GFAP confirmed the purity of endothelial and glia cell cultures in the transwell coculture (data not shown). An incubation of primary endothelial cells with recombinant rat TNF-α induced an increased ICAM-1 expression and proofed that the cells are functional after the preparation process (data not shown). Treatment of cells with hydrocortisone, pCPT-cAMP, and Ro 20-1724 resulted in an increase in net transendothelial electrical resistance to 200-250 Ω; permeability coefficients for albumin and sodium fluorescein ranged between 2×10−6 and 5×10−6 cm/s, respectively. In addition to the physical and chemical validation, the migration of freshly isolated naïve rat PBMCs towards a MCP-1 gradient was used for biological validation. PBMCs were composed of 79.84% CD45+/CD11bc− (most likely lymphocytes) and 20.16% CD45+/CD11bc+ (most likely antigen-presenting cells) cells as determined by flow cytometry (Fig. 4A). After migration across the in vitro BBB relative percentages of CD45+/CD11bc+ cells recovered from the lower chamber increased to 34.26%; a further increase to 69.11% was found when MCP-1 was added to the lower chamber during migration. The addition of anti-ICAM-1 mAbs into the upper chamber inhibited the migration of cells by approx. 90% (data not shown).
Fig. 4.
Migration of rat PBMCs across the in vitro coculture model of the BBB. Rat PBMCs were added to the upper compartment of the in vitro model of the BBB and after 3 h of incubation the content of the lower compartment was collected and analyzed by flow cytometry. Black bars represent CD45+/CD11bc− cells, white bars CD45+/CD11bc+ cells. A) For the validation of the transwell system a chemotactic gradient was established by the addition of 100 ng/mL recombinant rat MCP-1 to the lower compartment. The percentage of CD45+/CD11bc− (most likely lymphocytes) and CD45+/CD11bc+ (antigen-presenting) cells was determined by flow cytometry. B) Rat PBMCs were incubated for 2 h with GFP+ T. gondii RH strain tachyzoites (MOI 1) and allowed to migrate through the BBB model. Before and after migration percentages of CD45+/CD11bc+ and CD45+/CD11bc− cells were evaluated by flow cytometry; percentages of infected and uninfected cells were measured from the same wells. C) Percentages of infected cells of the respective cell subsets from B) before and after migration. Results shown are pooled data from 7 experiments. In each condition, the migrated cells of 10-12 filters were pooled. *p<0.05.
Based on the combined physical, chemical, and biological validation, the coculture model shows typical characteristics of the BBB as a selective barrier.
3.5. Leukocyte migration across the BBB in an in vitro coculture model
Following validation of the in vitro model of the BBB we determined the effect of type I RH strain tachyzoites of T. gondii on the migration of rat PBMCs through the BBB. Following infection of PBMCs at an MOI of 1 we found 0.75±1.14% infected CD45+/CD11bc− (lymphocytes) and 0.36±0.17% infected CD45+/CD11bc+ (antigen-presenting) cells among all cells of the population (Fig. 4B). Three hours after addition of PBMCs onto the upper compartment of the BBB the percentage of infected CD45+/CD11bc− cells recovered from the lower compartment was 0.6±0.63% and did not differ significantly from the percentage of these cells before migration; in contrast, the percentage of infected CD45+/CD11bc+ cells increased significantly to 4.86±2.63% (a 13-fold increase) after migration. Thus, the ratio of infected CD45+/CD11bc+ vs. infected CD45+/CD11bc− cells was 8-fold higher after migration (Fig. 4B). Whereas absolute numbers of infected CD45+/CD11bc− and CD45+/CD11bc+ cells before migration were 2228.93±3404.36 and 1067.59±519.67, numbers of infected CD45+/CD11bc+ cells predominated after migration (18.5±20.91 vs. 2.38±2.83), indicating that more infected CD45+/CD11bc+ than CD45+/CD11bc− cells migrated through the BBB.
Analysis of infection rates of these subpopulations before and after migration underlined the preferential induction of migration of antigen-presenting cells by T. gondii. Before migration, 0.85% of all CD45+/CD11bc− cells were infected while the infection rate for CD45+/CD11bc+ cells was 2.88% (Fig. 4C). After migration, the infection rate of CD45+/CD11bc+ cells increased to 16.05% (a 5-fold increase) and was 18-fold higher than that of CD45+/CD11bc− cells (infection rates remained unchanged at levels below 1% before and after migration). Interestingly, following infection of PBMCs with tachyzoites, not only infected but also uninfected CD45+/CD11bc+ cells showed enhanced migratory capacities; the percentage of uninfected CD45+/CD11bc+ cells increased from 12.07±4.52% to 25.42 ± 6.68% (Fig. 4B). Among control PBMCs that were left unexposed to T. gondii, a preferential migration of CD45+/CD11bc+ cells was also observed compared to CD45+/CD11bc− cells, even though this was less pronounced than for T. gondii exposed cells (data not shown).
The migration of infected PBMCs across the BBB model was not altered by addition of MCP-1 to the lower compartment of the transwell system (data not shown). Further analysis of subpopulations of antigen-presenting cells was impossible since the commercially available rat antibodies against cell surface markers tested did not allow to distinguish between subpopulations of antigen-presenting cells (data not shown).
These results suggest that infection of antigen-presenting cells with T. gondii increases their preferential migratory capacity compared to lymphocytes; antigen-presenting cells thereby are likely candidates for the transport of T. gondii across the BBB.
3.6. Migration of mouse PBMCs across an in vitro model of the BBB
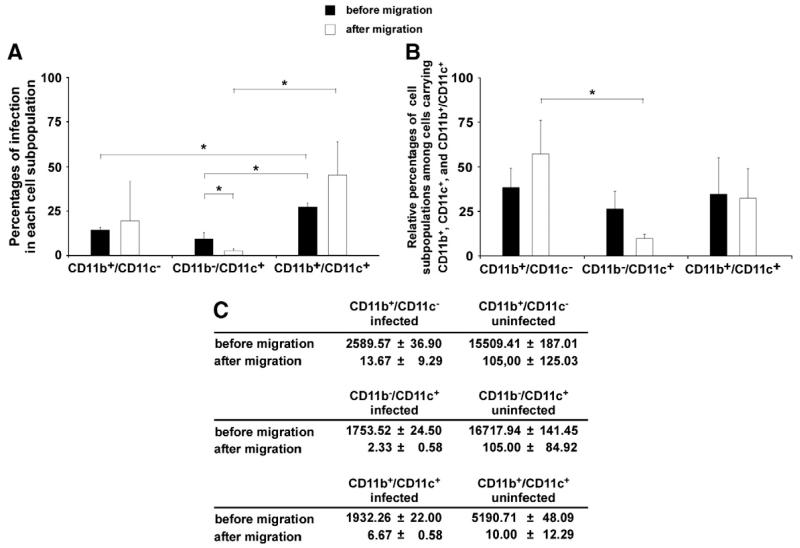
Since the lack of appropriate cell surface markers did not allow to define subpopulations of antigen-presenting cells among PBMCs we used mouse PBMCs in a separate set of experiments. First, a chemokine gradient was established by the addition of 100 ng/mL recombinant mouse MCP-1 to the lower compartment of the rat in vitro model of the BBB. As shown above for the migration of rat PBMCs the addition of MCP-1 resulted in increased migration of CD11b+ and CD11c+ cells compared to the migration of cells in the absence of a chemokine gradient (data not shown). Since addition of murine cells to the rat coculture model did not appear to alter migration characteristics we next incubated mouse PBMCs with T. gondii tachyzoites at an MOI of 5. Using flow cytometry we observed that CD11b+/CD11c+ double-positive cells showed significantly higher infection rates before and after migration compared to CD11b+/CD11c− and CD11b−/CD11c+ cells (Fig. 5A). Among all infected antigen-presenting cells (CD11b+, CD11c+, and double-positive cells), the CD11b+/CD11c− cells outnumbered CD11b−/CD11c+ and CD11b+/CD11c+ cells (before migration: 38.62±10.95% CD11b+/CD11c− cells vs. 26.53±9.92% CD11b−/CD11c+ cells and 34.85±20.52% CD11b+/CD11c+ cells; after migration: 57.59±18.7% CD11b+/CD11c− cells vs. 9.89±2.39% CD11b−/CD11c+ cells and 32.61 ±16.58% CD11b+/CD11c+ cells) (Fig. 5B).
Fig. 5.
Migration of mouse PBMCs across the in vitro coculture model of the BBB. Mouse PBMCs were incubated for 2 h with GFP+ tachyzoites of the RH strain (MOI 5) and added to the upper compartment of the in vitro coculture model of the BBB. After 3 h of incubation the content of the lower compartment was collected and analyzed by flow cytometry. Black bars represent cell populations before migration, white bars cell populations after migration. A) The percentages of infected antigen-presenting cells (CD11b+, CD11c+ and double-positive cells) were determined before and after migration. B) Composition of the infected antigen-presenting cells before and after migration. All infected antigen-presenting cells were considered 100%. C) Absolute numbers of cells before and after migration. In each condition, the migrated cells of 10-12 filters were pooled. Results are pooled data of 3 independent experiments. *p<0.05.
Before migration the absolute number of infected CD11b+/CD11c− cells (2589.57±36.90) exceeded that of infected CD11b−/CD11c+ (1753.52±24.50) or infected CD11b+/CD11c+ cells (1932.26±22) (Fig. 5C); after migration, the predominance of CD11b+/CD11c− cells was more pronounced (13.67±9.29 CD11b+/CD11c− vs. 2.33±0.58 CD11b−/CD11c+ cells vs. 6.67±0.58 CD11b+/CD11c+ cells).
The ratios of absolute numbers of infected CD11b+/CD11c−, CD11b−/CD11c+, and CD11b+/CD11c+ cells before vs. after migration were 194.79, 768.47, and 316.55, respectively. Similarly, ratios of absolute numbers of uninfected CD11b+/CD11c−, CD11b−/CD11c+, and CD11b+/CD11c+ cells before vs. after migration were 151.72, 164.60, and 564.14 (data not shown). These results indicate that upon infection with T. gondii CD11b+ cells (more so those CD11c− than those CD11c+) are the predominant cell population migrating through the BBB. Therefore, CD11b+ cells appear to play a major role in the neuroinvasion by T. gondii.
3.7. Migration across the BBB of leukocytes infected with T. gondii type I or type II tachyzoites
As type I and type II strains of T. gondii were shown to induce different migratory responses in cells upon infection (Lambert et al., 2009) we compared the migration of PBMC subsets infected with either the T. gondii RH type I or ME49 type II strain. Tachyzoites of the ME49 strain showed a tendency towards higher infection rates for cells carrying CD11b, CD11c or both markers before migration compared to tachyzoites of the RH strain (data not shown); both strains yielded the highest infection rates in CD11b+/CD11c+ double-positive cells, but no preferential migration of leukocytes infected with RH or ME49 tachyzoites could be detected.
The infection rates of individual subpopulations among all cells carrying CD11b or/and CD11c surface markers were also evaluated. Infection rates of cell subpopulations expressing CD11b, CD11c or both did not differ following infection with the RH or ME49 strain. In addition, the percentages of infected cell subpopulations did not differ before and after migration indicating that type I or type II Toxoplasma strains induce similar patterns of cell migration across the in vitro BBB.
3.8. Dissemination and neuroinvasion of T. gondii in vivo
Finally, to confirm our in vitro findings of a preferential migration of CD11b+ cells in the neuroinvasion of the parasite in vivo we determined the migration of CD11b+ cells across the BBB in mice. Preliminary experiments revealed that i. v. infection of mice with extracellular tachyzoites induced an accumulation of CD11b+ cells in the brain 96 h after injection (Fig. 6A) but Toxoplasma-infected leukocytes could not be detected in the brains by flow cytometry (data not shown); in addition, T. gondii-specific DNA was not detected consistently in brains (even when 6 individual pieces of each brain were processed separately) (data not shown).
Fig. 6.
In vivo approach to study neuroinvasion by T. gondii. A) BALB/c mice were intravenously injected with 107 extracellular GFP+ T. gondii tachyzoites of the RH strain. Mononuclear cells of the brains were harvested at 1.5, 24 and 96 h post infection. B) NMRI mice were infected with 10,000 intracellular GFP+ T. gondii tachyzoites of a CD11b+ (black bars) or CD11b− (white bars) mouse PBMC population. One, three and seven days post infection the amount of T. gondii-specific DNA in total brain DNA was evaluated by quantitative PCR. Results shown are pooled data from 2 independent experiments.
Therefore, we chose a different approach and separated mouse PBMCs into a CD11b+ fraction (purity of at least 85% CD11b+ cells) and a CD11b− fraction (containing not more than 6% CD11b+ cells) before infection with RH tachyzoites at an MOI of 1. The infection rate of the CD11b+ fraction was markedly higher than that of the CD11b− fraction (44.71 vs. 10.46%, respectively). Then, 10,000 infected cells of each of the CD11b+ or the CD11c− population were injected intravenously into the tail vein of mice. Low concentrations of T. gondii DNA were detected in brains of mice injected with infected CD11b+ or CD11b− cells at 24 h after injection. At 3 and 7 days after injection increasing T. gondii DNA concentrations were detected in brains but concentrations of DNA in mice infected with CD11b+ or CD11b− cells did not differ significantly at any time point (Fig. 6B).
In conclusion, whereas in vitro experiments pointed towards CD11b+ cells as the main cell population carrying T. gondii across the BBB, we were unable to detect a preferential migration of CD11b+ compared to CD11b− cells in vivo.
4. Discussion
Infection with the parasite T. gondii is characterized by the entry of parasites into the brain and the persistence of parasites in cysts. Acute infection in immunocompetent subjects rarely causes symptoms but tissue cysts develop in the brain. These cysts are the likely origin of reactivated disease in immunocompromised patients that progresses to lethal encephalitis. The early events that enable the parasite to cross the blood–brain barrier are poorly understood and it remains to be shown whether neuroinvasion by the parasite occurs mainly intracellularly or extracellularly. Improvements in our knowledge on the interplay of host cellular mechanisms and the parasite during neuroinvasion by T. gondii may help in the development of strategies to prevent this fatal brain disease.
In the present study we therefore examined molecules and cells involved in the neuroinvasion by T. gondii. We observed a marked change in the transcriptome of brain endothelial cells upon infection with T. gondii and identified CD11b+ cells as the main cell population exploited by the parasite during neuroinvasion. In line with previous reports (Kim et al., 2006; Robben et al., 2004) we confirm the superior inflammatory potential of mouse-avirulent type II compared to type I strains but also provide new evidence that type II and type I strains do not differ in their capacity to infect CD11b+ and/or CD11c+ cell populations and to migrate across the BBB.
Using mouse whole genome arrays we observed a marked change of transcriptional regulation in brain endothelial cells following infection with the parasite. Genes differentially regulated corresponded to molecules that are involved in the interaction between immune cells and host barrier functions thus pointing towards a role for immune cells in neuroinvasion by the parasite. Adhesion molecules including E- and P-selectin and ICAM-1 as well as cytokines, chemokines, signaling molecules, surface molecules or toll-like receptor (TLR) 4 showed upregulated expression after infection with T. gondii. As E-selectin, P-selectin and ICAM-1 are known to support the migration of immune cells to sites of inflammation (Ley et al., 1995; Venturi et al., 2003; Rahman and Fazal, 2008) their upregulation is important for an effective immune response. The upregulation of TLR4 in our experiment is in line with a previous report by Debierre-Grockiego et al. (2007) who postulate that TLR4 is involved in the recognition of T. gondii glycosylphosphatidylinositols (GPI) and may contribute to resistance against T. gondii. In addition, we observed the upregulation of cytokines and chemokines including IL-6 that was previously shown to act as an acute phase protein in the immune response and hematopoiesis (Akira et al., 1990; Suzuki et al., 1997); chemokines including CCL2 (MCP-1), CCL7 (MCP-3), CXCL1 (GRO-1), CXCL2 (GRO-2) and CX3CL1 (fractalkine) were upregulated upon infection with the parasite and are known to favor chemotactic attraction of immune cells (Stein and Nombela-Arrieta, 2005). Tight junction molecules and junctional adhesion molecules efficiently seal the spaces between adjacent endothelial cells in healthy conditions and thereby influence the blood–brain barrier permeability (Zlokovic, 2008). The reduced expression of claudin 8 may represent a parasite-induced mechanism to support the paracellular entry of immune cells as claudins are effectively sealing the tight junctions (Ohtsuki et al., 2008; Krause et al., 2008). Both the downregulation of the transcription factor STAT4 and the upregulation of SOCS molecules interfere with the induction of IFN-γ production (Murray, 2007; Cai et al., 2000) and activation of type I and type II cytokine receptors (Dalpke et al., 2008) thereby contributing to immune evasion by the parasite. Of interest, designed to act as part of the host’s immune responses against parasite replication many of the above mentioned molecules at the same time facilitate the entry of infected leukocytes into the brain thereby acting as double-edged swords.
ICAM-1 was reported to be essential for the transendothelial migration of naïve and infected leukocytes due to its interaction with the parasite adhesin MIC2 (Rahman and Fazal, 2008; Barragan et al., 2005; Turowski et al., 2005). Since the expression of ICAM-1 was increased after infection with T. gondii we investigated the effects of mouse-virulent type I (RH) and mouse-avirulent type II (ME49) T. gondii tachyzoites on the expression of ICAM-1. Upon exposure to the parasite both infected and – with delay – uninfected brain endothelial cells upregulated ICAM-1 on their surface. It remains to be shown whether this is a direct or a bystander effect due to cytokine production of the infected cells. In this regard, IL-6 and MCP-1 production were also increased following infection of brain endothelial cells with T. gondii. Proinflammatory cytokines including TNF-α, IFN-γ and IL-1β have been shown to enhance ICAM-1 expression (Dietrich, 2002) and T. gondii influences the transcription of endothelial cell genes with immunomodulatory functions including IL-8, IP-10, MCP-1, GM-CSF, COX-2 and iNOS (Taubert et al., 2006).
The increased ICAM-1 expression and cytokine secretion of infected cells are most likely mediated by T. gondii-associated molecular patterns (Gazzinelli and Denkers, 2006). Toxoplasma GPIs were shown to induce TNF-α production in macrophages through TLR2 and TLR4 while Toxoplasma cyclophilin 18 activates DCs through TLR11 to induce IL-12 production (Debierre-Grockiego et al., 2007, 2003; Yarovinsky et al., 2005).
Differences in ICAM-1 expression and the secretion of IL-6 and MCP-1 between type I and type II strains of T. gondii in our experiments are in agreement with previous reports on the differential IL-12 production by host cells following infection with type I or type II strains (Robben et al., 2004; Kim et al., 2006). The differences in adhesion molecule or cytokine production presumably account for diverging virulence features (Barragan and Sibley, 2002). This might be due to diverse induction of signaling cascades by type I and type II strains, respectively (Saeij et al., 2007; Taylor et al., 2006).
In addition to challenging endothelial cells with viable Toxoplasma tachyzoites further experiments should also examine the effect of inactivated parasites on cytokine production and adhesion molecule expression in endothelial cells.
Pathogens with neurotropic and neurovirulent potential can gain entry to the CNS in a ‘Trojan horse’-like manner. L. monocytogenes preferentially infects Ly6C+ blood mononuclear cells to reach the CNS (Drevets et al., 2004; Join-Lambert et al., 2005). The human immunodeficiency virus and C. neoformans likewise take advantage of an intracellular transportation inside monocytic cells (Nottet et al., 1996; Persidsky et al., 1997; Eugenin et al., 2006; Charlier et al., 2009) while Aspergillus spp. as well as Listeria can use DCs to disseminate through the body of the host (Pron et al., 2001; Bozza et al., 2002). Using rat PBMCs in a well characterized rat coculture model of the BBB (Perrière et al., 2005; Veszelka et al., 2007) we identified CD11bc+ cells as the main cell population carrying RH tachyzoites through the BBB. This finding confirms results reported by Courret et al. (2006). In an elegant series of experiments, these authors showed that CD11b+ and CD11c+ cells infected in the periphery of the body may transport T. gondii into the brain. Furthermore, Channon et al. (2000) found that monocytic and dendritic cells are predominant targets.
Since specific antibodies to characterize subpopulations of rat CD11bc+ cells were not available we then used mouse PBMCs in the rat coculture model. Leukocytes were differentiated into CD11b+ and CD11c+ or CD11b+/CD11c+ cells. Although the CD11b+/CD11c+ cells are a minor cell population in the blood, T. gondii showed the highest infection rate in these cells compared to CD11b- and CD11c-single-positive cells. Interestingly, Bierly et al. (2008) recently described a plasmacytoid dendritic cell (pDC)-like population co-expressing CD11c, CD11b, Gr-1, and PDCA-1 that is the predominant population among T. gondii infected cells in the spleen. It remains to be shown whether these cells are also found in the peripheral blood and may be involved in the entry of T. gondii into the brain.
Infected CD11b+/CD11c− cells - most likely monocytic cells - dominated the infected cell population after migration through the in vitro BBB. Even though these cells showed lower overall infection rates compared to the CD11b+/CD11c+ cells this was compensated by their larger absolute numbers. Interestingly, CD11b+/CD11c− cells showed increased migration potential compared to other PBMC subpopulations. In the blood of infected C57BL/6 mice CD11b+ cells were the main T. gondii reservoir (Courret et al., 2006); 7 days after infection mice showed an accumulation of CD11b+ cells in the brains and these cells represented 50% of all cells that had migrated to the brain (Courret et al., 2006).
Monocytic cells of the peripheral blood can be distinguished not only by the expression of certain antigens but also by their respective physiologic activity. Monocytes are separated according to their chemokine receptors or CD antigens into inflammatory or resident cells. While resident monocytes are CCR2−, CX3CR1hi, CD11b+, CD62L− and Ly6C−, the expression of CCR2, low CX3CR1, CD11b, CD62L, and Ly6C is characteristic for inflammatory monocytes (Gordon and Taylor, 2005). The CD11b+/CD11c− subpopulation described in the current study may represent inflammatory monocytes whereas CD11b+/CD11c+ cells could be inflammatory DCs that express CD11c+, CD11b+ and Gr-1+ as described by Nakano et al. (2009) in cells infected with influenza virus.
Other findings also support the idea of intracellular dissemination and entry into the brain of T. gondii. Upon infection with T. gondii dendritic cells acquire a state of hypermotility and migrate without the addition of chemoattractants (Lambert et al., 2006). Unno et al. (2008) compared the neuroinvasive capacity of intracellular vs. extracellular tachyzoites and found considerably better conditions for survival and dissemination of intracellular compared to extracellular tachyzoites since intracellular parasites greatly outnumbered extracellular tachyzoites in various organs. This can be due to the fact that dissemination in the bloodstream inevitably requires contact with hostile serum components like immunoglobulins (Kaneko et al., 2004; Gama et al., 2004). It seems more likely that the oral infection with cysts is followed by an infection of leukocytes in the lamina propria than by a dissemination of extracellular parasites in the bloodstream (Courret et al., 2006).
We cannot exclude that extracellular parasites migrate through the BBB but in preliminary experiments we calculated that almost none (<0.001%) of the parasites used for infection of cells in the upper compartment migrated through the BBB when incubated with the BBB but in the absence of leukocytes. Results presented in this study therefore focused on the passage of intracellular parasites.
The use of hydrocortisone to strengthen the endothelial barrier and increase the TEER represents a limitation of our model for transendothelial cell migration as corticosteroids may have an impact on gene expression in endothelial cells. Nevertheless the mentioned concentration of hydrocortisone is a physiological dose and it is used by many groups as an indispensable “goldstandard” to improve barrier functions in BBB models (Hoheisel et al., 1998; Deli et al., 2005).
We observed low numbers of migrating cells in the present study. We postulate that these low numbers represent a physiological finding that mirrors the low numbers of infected cells migrating into the brain in vivo. When the time of incubation of PBMCs was expanded from 3 to 24 h we observed a 15-fold increase in the numbers of migrating cells; however, the composition of migrated cells was very similar to the composition of the original PBMC population added to the transwells. Thus, the intact barrier appears to have been destroyed between 3 and 24 h after incubation.
We detected a higher infection rate of PBMC subsets by type II than by type I strain tachyzoites. Yet, the overall composition of the infected antigen-presenting cell population (CD11b+, CD11c+ and double-positive cells) did not differ after infection with type I or type II strains. The higher infection rates observed in antigen-presenting cells after infection with type II strain tachyzoites may result in a stronger neuroinvasive potential of the type II strain. It has been reported that cells infected with type II strain parasites show higher migration frequencies than type I infected cells; however, genotype-associated differences were not reported to be due to differences in infection rates (Lambert et al., 2009).
Finally, we were unable to confirm the dominant role of CD11b+ cells in the in vitro neuroinvasion by T. gondii in vivo. As reported by Courret et al. (2006) numbers of CD11b+ cells increased in brains of infected mice 7 days after infection. However, injection of infected CD11b+ vs. infected CD11b− cells into the bloodstream of mice did not result in preferential neuroinvasion of CD11b+ compared to CD11b− cells in the present study. This might be due to a lack of sensitivity of the detection method as the total number of tachyzoites in the brain is low. Future studies will be focused on the identification of infected CD11b+ and CD11b− cells ex-vivo using alternative techniques such as (immuno−) histology.
However, only the injection of intracellular but not that of extracellular parasites into the bloodstream resulted in the detection of reproducible levels of parasite DNA in the brains of infected animals thereby further supporting the likelihood of an intracellular rather than extracellular migration of the parasite into the brain. Nevertheless an adequate animal model is needed to study the in vivo migration of Toxoplasma-infected leukocytes and the neuroinvasive potential of T. gondii.
In summary, results of the present study suggest that T. gondii exploits monocytic or dendritic cells as ‘Trojan horses’ to enter the CNS. The increased expression of adhesion molecules and the T. gondii-induced secretion of chemokines by endothelial cells support a role for transendothelial migration of intracellular parasites. Type I and type II strains of T. gondii appear to be equally capable of crossing the BBB.
Acknowledgements
This study was supported by the Deutsche Forschungsgemeinschaft, FG463 (to O. L.). S. L. was supported by a grant of the Sonnenfeld-Stiftung, Berlin. We appreciate the expert help of Berit Söhl-Kielczynski and the staff of the animal facilities of the Charité.
References
- Abbott N. Joan, Rönnbäck Lars, Hansson Elisabeth. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4(11):2860–2867. [PubMed] [Google Scholar]
- Amit M, Margulets V, Segev H, Shariki K, Laevsky I, Coleman R, Itskovitz-Eldor J. Human feeder layers for human embryonic stem cells. Biol Reprod. 2003;68(6):2150–2156. doi: 10.1095/biolreprod.102.012583. [DOI] [PubMed] [Google Scholar]
- Aviles Hernan, Stiles Jonathan, O’Donnell Phyllis, Orshal Julia, Leid Jeffrey, Sonnenfeld Gerald, Monroy Fernando. Kinetics of systemic cytokine and brain chemokine gene expression in murine Toxoplasma infection. J Parasitol. 2008;94(6):1282–1288. doi: 10.1645/GE-1309.1. [DOI] [PubMed] [Google Scholar]
- Barragan Antonio, Sibley L.David. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J Exp Med. 2002;195(12):1625–1633. doi: 10.1084/jem.20020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan Antonio, Brossier Fabien, Sibley L.David. Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell Microbiol. 2005;7(4):561–568. doi: 10.1111/j.1462-5822.2005.00486.x. [DOI] [PubMed] [Google Scholar]
- Bierly Allison L., Shufesky William J., Sukhumavasi Woraporn, Morelli Adrian E., Denkers Eric Y. Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. J Immunol. 2008;181(12):8485–8491. doi: 10.4049/jimmunol.181.12.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böggemeyer E, Stehle T, Schaible UE, Hahne M, Vestweber D, Simon MM. Borrelia burgdorferi upregulates the adhesion molecules E-selectin, P-selectin, ICAM-1 and VCAM-1 on mouse endothelioma cells in vitro. Cell Adhes Commun. 1994;2(2):145–157. doi: 10.3109/15419069409004433. [DOI] [PubMed] [Google Scholar]
- Bozza Silvia, Gaziano Roberta, Spreca Antonio, Bacci Angela, Montagnoli Claudia, di Francesco Paolo, Romani Luigina. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol. 2002;168(3):1362–1371. doi: 10.4049/jimmunol.168.3.1362. [DOI] [PubMed] [Google Scholar]
- Cai G, Radzanowski T, Villegas EN, Kastelein R, Hunter CA. Identification of STAT4-dependent and independent mechanisms of resistance to Toxoplasma gondii. J Immunol. 2000;165(5):2619–2627. doi: 10.4049/jimmunol.165.5.2619. [DOI] [PubMed] [Google Scholar]
- Channon JY, Seguin RM, Kasper LH. Differential infectivity and division of Toxoplasma gondii in human peripheral blood leukocytes. Infect Immun. 2000;68(8):4822–4826. doi: 10.1128/iai.68.8.4822-4826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier Caroline, Nielsen Kirsten, Daou Samira, Brigitte Madly, Chretien Fabrice, Dromer Françoise. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77(1):120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clahsen Thomas, Schaper Fred. Interleukin-6 acts in the fashion of a classical chemokine on monocytic cells by inducing integrin activation, cell adhesion, actin polymerization, chemotaxis, and transmigration. J Leukoc Biol. 2008;84(6):1521–1529. doi: 10.1189/jlb.0308178. [DOI] [PubMed] [Google Scholar]
- Coisne Caroline, Faveeuw Christelle, Delplace Yannick, Dehouck Lucie, Miller Florence, Cecchelli Roméo, Dehouck Bénédicte. Differential expression of selectins by mouse brain capillary endothelial cells in vitro in response to distinct inflammatory stimuli. Neurosci Lett. 2006;392(3):216–220. doi: 10.1016/j.neulet.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Courret Nathalie, Darche Sylvie, Sonigo Pierre, Milon Geneviève, Buzoni-Gâtel Dominique, Tardieux Isabelle. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107(1):309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpke Alexander, Heeg Klaus, Bartz Holger, Baetz Andrea. Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology. 2008;213(3-4):225–235. doi: 10.1016/j.imbio.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Debierre-Grockiego Françoise, Azzouz Nahid, Schmidt Jörg, Dubremetz Jean-François, Geyer Hildegard, Geyer Rudolf, Weingart Ralf, Schmidt Richard R., Schwarz Ralph T. Roles of glycosylphosphatidylinositols of Toxoplasma gondii. Induction of tumor necrosis factor-alpha production in macrophages. J Biol Chem. 2003;278(35):32987–32993. doi: 10.1074/jbc.M304791200. [DOI] [PubMed] [Google Scholar]
- Debierre-Grockiego Françoise, Campos Marco A., Azzouz Nahid, Schmidt Jörg, Bieker Ulrike, Resende Marianne Garcia, Mansur Daniel Santos, Weingart Ralf, Schmidt Richard R., Golenbock Douglas T., Gazzinelli Ricardo T., Schwarz Ralph T. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179(2):1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- Deli MA, Joó F, Krizbai I, Lengyel I, Nunzi MG, Wolff JR. Calcium/calmodulin-stimulated protein kinase II is present in primary cultures of cerebral endothelial cells. J Neurochem. 1993;60(5):1960–1963. doi: 10.1111/j.1471-4159.1993.tb13429.x. [DOI] [PubMed] [Google Scholar]
- Deli Máiria A., Abrahám Csongor S., Kataoka Yasufumi, Niwa Masami. Permeability studies on in vitro blood–brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25(1):59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellacasa-Lindberg Isabel, Hitziger Niclas, Barragan Antonio. Localized recrudescence of Toxoplasma infections in the central nervous system of immunocompromised mice assessed by in vivo bioluminescence imaging. Microbes Infect. 2007;9(11):1291–1298. doi: 10.1016/j.micinf.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Dietrich Jean-Bernard. The adhesion molecule ICAM-1 and its regulation in relation with the blood–brain barrier. J Neuroimmunol. 2002;128(1-2):58–68. doi: 10.1016/s0165-5728(02)00114-5. [DOI] [PubMed] [Google Scholar]
- Drevets Douglas A., Dillon Marilyn J., Schawang Jennifer S., Nico Van Rooijen, Ehrchen Jan, Sunderkötter Cord, Leenen Pieter J.M. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J Immunol. 2004;172(7):4418–4424. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- Eugenin Eliseo A., Osiecki Kristin, Lopez Lillie, Goldstein Harris, Calderon Tina M., Berman Joan W. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood–brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4):1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama Leonardo M., Da Ribeiro-Gomes, Lima Flávia, Guimarães Ubirajara, Arnholdt Andrea C. Vetö. Reduction in adhesiveness to extracellular matrix components, modulation of adhesion molecules and in vivo migration of murine macrophages infected with Toxoplasma gondii. Microbes Infect. 2004;6(14):1287–1296. doi: 10.1016/j.micinf.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Gazzinelli Ricardo T., Denkers Eric Y. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol. 2006;6(12):895–906. doi: 10.1038/nri1978. [DOI] [PubMed] [Google Scholar]
- Ge Shujun, Song Li, Pachter Joel S. Where is the blood–brain barrier ... really? J Neurosci Res. 2005;79(4):421–427. doi: 10.1002/jnr.20313. [DOI] [PubMed] [Google Scholar]
- Gordon Siamon, Taylor Philip R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36(2):118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, Galla HJ. Hydrocortisone reinforces the blood–brain barrier properties in a serum free cell culture system. Biochem Biophys Res Commun. 1998;244(1):312–316. doi: 10.1006/bbrc.1997.8051. [DOI] [PubMed] [Google Scholar]
- Join-Lambert Olivier F., Ezine Sophie, Monnier Alban Le., Jaubert Francis, Okabe Masaru, Berche Patrick, Kayal Samer. Listeria monocytogenes-infected bone marrow myeloid cells promote bacterial invasion of the central nervous system. Cell Microbiol. 2005;7(2):167–180. doi: 10.1111/j.1462-5822.2004.00444.x. [DOI] [PubMed] [Google Scholar]
- Kadl Alexandra, Leitinger Norbert. The role of endothelial cells in the resolution of acute inflammation. Antioxid Redox Signal. 2005;7(11-12):1744–1754. doi: 10.1089/ars.2005.7.1744. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Takashima Y, Xuaun X, Igarashi I, Nagasawa H, Mikami T, Otsuka H. Natural IgM antibodies in sera from various animals but not the cat kill Toxoplasma gondii by activating the classical complement pathway. Parasitology. 2004;128(Pt 2):123–129. doi: 10.1017/s0031182003004414. [DOI] [PubMed] [Google Scholar]
- Kanmogne Georgette D., Schall Kathy, Leibhart Jessica, Knipe Bryan, Gendelman Howard E., Persidsky Yuri. HIV-1 gp120 compromises blood–brain barrier integrity and enhances monocyte migration across blood–brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27(1):123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katti Muralidhar K. Pathogenesis, diagnosis, treatment, and outcome aspects of cerebral tuberculosis. Med Sci Monit. 2004;10(9):RA215–RA229. [PubMed] [Google Scholar]
- Kim Kwang Sik. Strategy of Escherichia coli for crossing the blood–brain barrier. J Infect Dis. 2002 Dec;186(Suppl 2):220–224. doi: 10.1086/344284. [DOI] [PubMed] [Google Scholar]
- Kim Kwang Sik. Mechanisms of microbial traversal of the blood–brain barrier. Nat Rev Microbiol. 2008;6(8):625–634. doi: 10.1038/nrmicro1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Leesun, Butcher Barbara A., Lee Chiang W., Uematsu Satoshi, Akira Shizuo, Denkers Eric Y. Toxoplasma gondii genotype determines MyD88-dependent signaling in infected macrophages. J Immunol. 2006;177(4):2584–2591. doi: 10.4049/jimmunol.177.4.2584. [DOI] [PubMed] [Google Scholar]
- Krause Gerd, Winkler Lars, Mueller Sebastian L., Haseloff Reiner F., Piontek Jörg, Blasig Ingolf E. Structure and function of claudins. Biochim Biophys Acta. 2008;1778(3):631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Lambert Henrik, Hitziger Niclas, Dellacasa Isabel, Svensson Mattias, Barragan Antonio. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol. 2006;8(10):1611–1623. doi: 10.1111/j.1462-5822.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- Lambert Henrik, Vutova Polya P., Adams William C., Loré Karin, Barragan Antonio. The Toxoplasma gondii-shuttling function of dendritic cells is linked to the parasite genotype. Infect Immun. 2009;77(4):1679–1688. doi: 10.1128/IAI.01289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Bullard DC, Arbonés ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995;181(2):669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker Ralf A., Lühder Fred, Kallen Karl-Josef, Lee De-Hyung, Engelhardt Britta, Rose-John Stefan, Gold Ralf. IL-6 transsignalling modulates the early effector phase of EAE and targets the blood–brain barrier. J Neuroimmunol. 2008;205(1-2):64–72. doi: 10.1016/j.jneuroim.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Luster Andrew D., Alon Ronen, von Andrian Ulrich H. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6(12):1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- Marra A, Brigham D. Streptococcus pneumoniae causes experimental meningitis following intranasal and otitis media infections via a nonhematogenous route. Infect Immun. 2001;69(12):7318–7325. doi: 10.1128/IAI.69.12.7318-7325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland Henry F., Martin Roland. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8(9):913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- Montesano R, Pepper MS, Möhle-Steinlein U, Risau W, Wagner EF, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62(3):435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- Murray Peter J. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178(5):2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- Nakano Hideki, Lin Kaifeng Lisa, Yanagita Manabu, Charbonneau Chantal, Cook Donald N., Kakiuchi Terutaka, Gunn Michael D. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10(4):394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottet HS. Interactions between macrophages and brain microvascular endothelial cells: role in pathogenesis of HIV-1 infection and blood–brain barrier function. J Neurovirol. 1999;5(6):659–669. doi: 10.3109/13550289909021294. [DOI] [PubMed] [Google Scholar]
- Nottet HS, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, Sharer LR, McComb RD, Swindells S, Soderland C, Gendelman HE. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156(3):1284–1295. [PubMed] [Google Scholar]
- Ohtsuki Sumio, Yamaguchi Hirofumi, Katsukura Yuki, Asashima Tomoko, Terasaki Tetsuya. mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J Neurochem. 2008;104(1):147–154. doi: 10.1111/j.1471-4159.2007.05008.x. [DOI] [PubMed] [Google Scholar]
- Perrière N, Demeuse Ph., Garcia E, Regina A, Debray M, Andreux J-P, Couvreur P, Scherrmann J-M, Temsamani J, Couraud P-O, Deli MA, Roux F. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood–brain barrier-specific properties. J Neurochem. 2005;93(2):279–289. doi: 10.1111/j.1471-4159.2004.03020.x. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M. A model for monocyte migration through the blood–brain barrier during HIV-1 encephalitis. J Immunol. 1997;158(7):3499–3510. [PubMed] [Google Scholar]
- Pober Jordan S., Sessa William C. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- Pron B, Boumaila C, Jaubert F, Berche P, Milon G, Geissmann F, Gaillard JL. Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell Microbiol. 2001;3(5):331–340. doi: 10.1046/j.1462-5822.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Rahman Arshad, Fazal Fabeha. Hug Tightly and Say Goodbye: Role of Endothelial ICAM-1 in Leukocyte Transmigration. Antioxid Redox Signal. 2008 Sep; doi: 10.1089/ars.2008.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff Richard M., Kivisäkk Pia, Kidd Grahame. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3(7):569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Robben Paul M., Mordue Dana G., Truscott Steven M., Takeda Kiyoshi, Akira Shizuo, Sibley L.David. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol. 2004;172(6):3686–3694. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- Robben Paul M., LaRegina Marie, Kuziel William A., Sibley L.David. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201(11):1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL, Staddon JM. The cell biology of the blood–brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- Saeij JJP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445(7125):324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein Jens V., Nombela-Arrieta César. Chemokine control of lymphocyte trafficking: a general overview. Immunology. 2005;116(1):1–12. doi: 10.1111/j.1365-2567.2005.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Rani S, Liesenfeld O, Kojima T, Lim S, Nguyen TA, Dalrymple SA, Murray R, Remington JS. Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infect Immun. 1997;65(6):2339–2345. doi: 10.1128/iai.65.6.2339-2345.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert Anja, Zahner Horst, Hermosilla Carlos. Dynamics of transcription of immunomodulatory genes in endothelial cells infected with different coccidian parasites. Vet Parasitol. 2006;142(3-4):214–222. doi: 10.1016/j.vetpar.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj H.El., Jerome M, Behnke MS, White M, Wootton JC, Sibley LD. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314(5806):1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- Turowski Patric, Adamson Peter, Greenwood John. Pharmacological targeting of ICAM-1 signaling in brain endothelial cells: potential for treating neuroinflammation. Cell Mol Neurobiol. 2005;25(1):153–170. doi: 10.1007/s10571-004-1380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno Akihiro, Suzuki Kazuhiko, Xuan Xuenan, Nishikawa Yoshifumi, Kitoh Katsuya, Takashima Yasuhiro. Dissemination of extracellular and intracellular Toxoplasma gondii tachyzoites in the blood flow. Parasitol Int. 2008;57(4):515–518. doi: 10.1016/j.parint.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Venturi Guglielmo M., Tu LiLi, Kadono Takafumi, Khan Adil I., Fujimoto Yoko, Oshel Philip, Bock Cheryl B., Miller Ann S., Albrecht Ralph M., Kubes Paul, Steeber Douglas A., Tedder Thomas F. Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity. 2003;19(5):713–724. doi: 10.1016/s1074-7613(03)00295-4. [DOI] [PubMed] [Google Scholar]
- Veszelka Szilvia, Pásztói Mária, Farkas Attila E., Krizbai István, Ngo Thi Khue, Dung Niwa, Masami Abrahám, Csongor S, Deli Mária A. Pentosan polysulfate protects brain endothelial cells against bacterial lipopolysaccharide-induced damages. Neurochem Int. 2007;50(1):219–228. doi: 10.1016/j.neuint.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Yarovinsky Felix, Zhang Dekai, Andersen John F., Bannenberg Gerard L., Serhan Charles N., Hayden Matthew S., Hieny Sara, Sutterwala Fayyaz S., Flavell Richard A., Ghosh Sankar, Sher Alan. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308(5728):1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- Zlokovic Berislav V. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]