Abstract
Toxoplasma gondii is an intracellular parasite that has to cope with the microbicidal actions of IFNγ. Previously we reported that parasite-mediated induction of suppressor of cytokine signaling protein 1 (SOCS1) contributes to inhibition of IFNγ signaling. However, the signaling requirements remained elusive. We now show that induction of SOCS1 and inhibition of nitric oxide production by IFNγ was independent of stimulation of Toll-like receptors. Instead, infection by T. gondii resulted in induction of egr transcription factors which have been reported to regulate SOCS expression. Indeed, induction of egr2 as well as SOCS1 was dependent on p38 MAP kinase and blockade of egr inhibited SOCS1 expression. Moreover, we found that Mic8, a previously identified invasion factor of T. gondii, was necessary for SOCS1 regulation and escape of IFNγ mediated nitric oxide secretion within macrophages. Surprisingly, when further analyzing Mic8 deficient parasites we noted that inhibition of IFNγ mediated up-regulation of MHC-class II and ICAM1 molecules was independent of cell invasion. Furthermore, these inhibitory effects were equally observed in type I and II strains of T. gondii and were dependent on excreted and secreted antigens. In contrast, only the virulent RH type I strain additionally induced SOCS1 and efficiently inhibited nitric oxide secretion by IFNγ. The results show that T. gondii makes use of two different mechanisms to escape from IFNγ activity with one mode being strain dependent and relying on active cell invasion and SOCS1 induction.
Keywords: Immune evasion, Innate Immunity, Interferon-γ, Macrophages, Suppressor of Cytokine Signaling
Introduction
Toxoplasma gondii is a member of the apicomplexan phylum that comprises medically important pathogens, e.g. plasmodia. The protozoan parasite is able to establish persistent infections in a variety of mammals and has the outstanding capability of intracellular replication within a vacuole in virtually all nucleated host cells.
Infection and cell invasion by T. gondii depend upon the stepwise secretion of specific organelles (Carruthers and Boothroyd, 2007). During gliding motility and upon host cell attachment, micronemes become secreted. During cell invasion and formation of the parasitophorous vacuole rhoptry proteins are delivered into the host cells followed by secretion of the dense granules into the parasitophorous vacuole. Micronemal protein 8 (Mic8) recently has been identified to be essential for host cell invasion (Kessler et al., 2008).
T. gondii usually induces a mild, self-limiting acute infection that only shows severe courses in immunocompromised patients and pregnant women. It ends up in a silent but chronic infection that can exacerbate with decreasing immunity. Three types of strains of differing virulence can be identified (Saeij et al., 2005). All type I strains (e.g. RH) cause lethal infections in mice whereas type II (e.g. Me49) and type III strains are less virulent. For a successful immune response to T. gondii, the presence of IFNγ is crucial (Suzuki et al., 1988). This cytokine boosts the antimicrobial activities of macrophages enabling them to limit the parasite’s proliferation and survival. During host-parasite co-evolution, T. gondii has evolved several means to evade the detrimental actions of innate and adaptive immunity finally resulting in chronic infections with tissue cysts in muscles and brain (Sacks and Sher, 2002; Laliberte and Carruthers, 2008). Immune evasion by T. gondii has been shown to encompass inhibition of innate immunity with decreased IL-12 and TNF secretion and reduced activity of the master transcription factor NFκB (Butcher et al., 2001; McKee et al., 2004; Dobbin et al., 2002). Activation of STAT3 contributes to these negative regulatory effects (Butcher et al., 2005). Moreover, full activation of infected macrophages is prevented by blocking IFNγ mediated induction of inducible nitric oxide synthase (iNOS) and antigen presentation (Luder et al., 2001; Luder et al., 2003).
Suppressor of Cytokine Signaling (SOCS) proteins have been identified as inducible feedback inhibitors of activated JAK/STAT signaling with SOCS1 serving a crucial role to limit IFNγ signaling (Alexander et al., 1999). Previously, we have shown that T. gondii induces SOCS1 and cytokine-inducible SH2-containing protein (CIS), another member of the SOCS family within infected macrophages (Zimmermann et al., 2006). However, the molecular details of SOCS induction as well as the role of SOCS for the overall interference of Toxoplasma with macrophage activation remained unclear.
We therefore set out to study the details of SOCS biology in the context of Toxoplasma infection. Our results show that SOCS1 induction as well as inhibition of nitric oxide secretion is dependent on active cell-invasion and takes place in a strain dependent manner. In contrast, cell invasion and strain dependency were not decisive for inhibition of antigen presentation.
Material and Methods
Reagents
SB203580, JNKII, UO126 and Ly294002 were obtained from Calbiochem (Darmstadt, Germany). Cytochalasin D (Alexis, Lörrach, Germany), wortmannin (Sigma, Taufkirchen, Germany), anhydrotetracycline (Clontech/Takar, Saint-Germain-en-Laye, France) and phosphothioate-modified CpG-oligonucleotide 1668 (TCC ATG ACG TTC CTG ATG CT, TIB Molbiol, Berlin) were commercially purchased, LPS from Salmonella minnesota was provided by U. Seydel (Borstel, Germany). Recombinant murine IFNγ was from Peprotech (Hamburg, Germany). All antibodies used for flow cytometry were obtained from BD Pharmingen (Heidelberg, Germany).
Plasmids constructs
CMV-Nab2 expressing WT NAB2 and pcDNA3-dnEgr(3) encoding a dominant-negative Egr were obtained from Warren G. Tourtellotte (Northwestern University, Department of Pathology, Chicago, IL).
Cell culture
RAW 264.7, a murine macrophage cell line, was cultured in DMEM supplemented with 10% FCS (Biowest, Nuaillé, France) and antibiotics (penicillin and streptomycin). Bone-marrow derived dendritic cells and bone-marrow derived macrophages were prepared from wt Balb/c, MyD88−/−, TRIF−/−/MyD88−/− or lps2 mice as described (Bode et al., 2009) and according to approved animal guidelines.
T. gondii from the strain RH (genotype I) and strain Me49 (genotype II) were genetically engineered to express GFP. Mic8 tetracycline-inducible knockout parasites in RH background are described (Kessler et al., 2008). All strains were cocultured with Vero cells (African Green monkey kidney cells).
Infection and stimulation
For infection experiments, freshly hatched parasites were used. Remaining host cells were removed by passage though a 27G needle. For some indicated stimulations, parasites were killed by three rapid freeze thaw cycles or heat-killed by boiling at 95 °C for 10 min. The Mic8 knockout was induced by addition of anhydrotetracycline which was present throughout the whole assay. Where indicated, the macrophages or the tachyzoites were pretreated with inhibitors for 1 h.
Analysis of microneme secretion
T. gondii tachyzoites were stimulated to secrete their micronemes with ethanol as described (Carruthers et al., 1999). In brief, 1×108 tachyzoites were put in 200 μl DMEM containing 1% ethanol and incubated for 2 min at 37 °C. The suspension was then cooled on ice and tachyzoites were removed by five centrifugation steps (4 times 1100×g 5 min, followed by 4000×g 5 min).
Determination of NO secretion
1.5×105 cells were infected and stimulated with IFNγ in 96 well plates. Supernatants were harvested after 40 h of stimulation and analyzed by the method of Griess. NO accumulation was measured photometrically (550 nm) by mixing equal parts of supernatant and Griess reagent (1:1 mixture of 1% sulfanilamide/5% H3PO4 and 0.1% naphthyl-ethylenediamine dihydrochloride).
Quantitative RT-PCR
Cells (5×105) were stimulated in 24-well plates. Total RNA was isolated using a HighPure RNA kit (Roche, Mannheim, Germany), which included DNase I digestion. Total RNA (1 μg) was reverse transcribed with a cDNA synthesis kit (MBI Fermentas, St. Leon-Rot, Germany). Quantification was performed with the 7900HT cycler (Applied Biosystems) using PCR-mixes either based on SYBR Green or FAM-TAMRA Taqman probes (ABgene House, Epsom, UK). The following primer pairs were used: egr1 (5′-GCC TCG TGA GCA TGA CCA AT-3′ and 5′-GCA GAG GAA GAC GAT GAA GCA-3′), egr 2 (5′-GTG TCG GAT CTG CAT GCG AAA CTT-3′ and 5′-GCA AAC TTG CGG CCA CAA TAG TCA-3′). The other primer pairs and probes are published (Dalpke et al., 2001). Threshold values were normalized to the expression of β-actin. Quantitative PCR results are expressed as relative expression (2−(Ct[target gene] − Ct[actin])).
Western Blotting
4×106 RAW264.7 cells were stimulated for 8h in 6-well plates. Subsequently, cells were were lysed with 200 μl of lysis buffer containing 50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM each of EDTA, NaF, Na3VO4, PMSF, 1 μg/ml each of aprotinin, pepstatin and leupeptin. Cleared lysates were analyzed by SDS-PAGE and immunoblotting using anti-SOCS1, clone 4H1 (Millipore, Schwalbach, Germany, 1:500 in PBS/0.05% Tween/3% milk powder) and a HRP-labeled secondary antibody (Cell Signaling Technologies, Frankfurt, Germany). As loading control anti-β-actin (1:1000, Abgene, Epson, UK) was used. Signals were detected using the ECL system Immobilon Western Chemiluminescence HRP Substrat (Millipore). Gels were imaged digitally, contrast adjustments were applied to all parts of a figure.
Flow cytometry
5×105 cells were seeded in 24 well plates or 0.4 μm transwell chambers and infected with T. gondii. At the end of the assay cells were fixed with formaldehyde and washed with 2% FCS in PBS. Then cells were stained with PE-conjugated anti I-Ad/Ed Ab, PE-conjugated anti-ICAM1 Ab or PE-conjugated anti-CD86 Ab for 45 min on ice. After staining, cells were washed and analyzed on a BD FACSCanto flow cytometer (BD, Heidelberg, Germany).
Inhibition of endogenous Egr
Transfection of RAW 264.7 cells was performed using NanoJuice™ according to the manufactureŕs protocol (Novagen, MERCK). Cells were cultured at 80% confluence in 24-well plates and were transfected with 0.25 μg of plasmid constructs. 48h post-transfection, cells were infected with T.gondii RH-GFP (MOI 6:1) for 5h or left untreated. Cells were harvested and cell lysates were used for quantitative RT-PCR analysis.
Statistics
All experiments were repeated at least two times if not indicated otherwise. Mean + standard error of the mean (SEM) is shown. Significant differences were evaluated by the unpaired Student’s t-test with two-tailed distributions within GraphPad prism 4.03 (GraphPad Software, San Diego California USA).
Results
Inhibition of nitric oxide secretion and induction of SOCS1 and CIS depend on viable T. gondii parasites
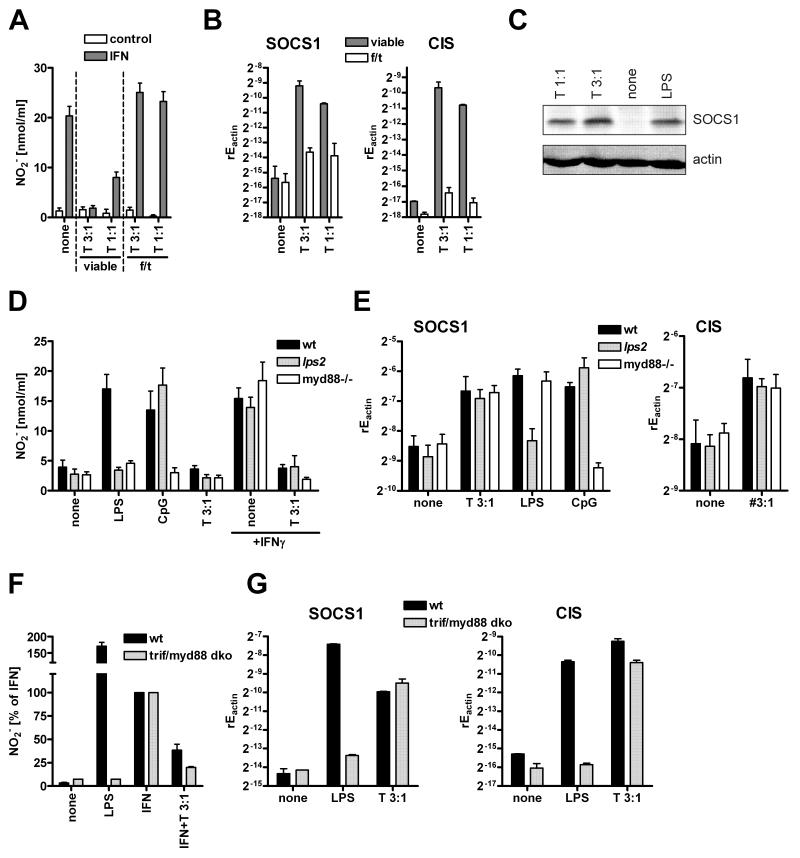
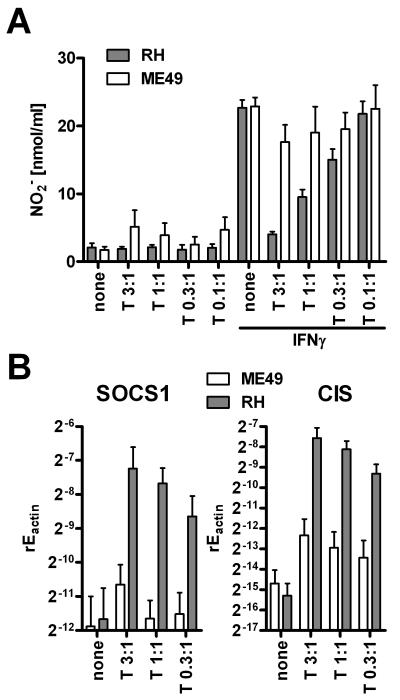
We and others have previously reported that the T. gondii strain RH can reduce NO secretion from IFNγ activated macrophages (Zimmermann et al., 2006; Luder et al., 2003) and members of the SOCS family contribute to this inhibition. We therefore addressed the parasite’s prerequisites for SOCS induction and inhibition of NO production. When freeze-thaw killed RH strain parasites were added to RAW264.7 macrophages, the IFNγ-induced NO production was not inhibited, in contrast to the results obtained with live parasites (Fig. 1A). Instead, killed parasites even showed the trend to increase NO secretion. In parallel, live T. gondii tachyzoites induced SOCS1 and CIS (Fig. 1B), whereas killed parasites did not. Similar results were obtained for primary bone marrow derived macrophages (data not shown). Using Western Blotting we confirmed that increased SOCS1 transcription by stimulation with viable Toxoplasma gondii parasites was paralleled by SOCS1 protein induction (Fig. 1C).
Figure 1. SOCS induction and nitric oxide inhibition are independent of TLR activation.
(A, B) Viable RH parasites or parasites that were killed by repeated freeze/thaw cycles (3x) were incubated at the indicated MOI with RAW264.7 macrophages. (A) Cells were analyzed for nitrite secretion after 40 h of stimulation with 50 ng/ml IFNγ(mean+SEM, N=4) and (B) were examined for SOCS1 or CIS expression by quantitative RT-PCR after 5 h of stimulation (shown is the relative expression of the target gene normalized to β-actin, mean+SEM, N=3). (C) RAW264.7 cells, stimulated as indicated with viable RH parasites or 10 ng/ml LPS for 8h, were analyzed for SOCS1 protein expression by Western Blotting. (D, E) Bone-marrow derived dendritic cells from wt, MyD88 −/− or lps2 mice were infected with RH parasites (T) at the indicated MOI. (D) Cells were stimulated with 50 ng/ml IFNγ, 100 ng/ml LPS or 100 nM CpG-ODN for 40h followed by determination of nitrite in the supernatant (mean+SEM, N=4-6). (E) Cells were infected for 5 h prior to detection of relative expression of SOCS1 or CIS by quantitative real-time PCR (mean+SEM, N=5-7). (F, G) Bone-marrow derived macrophages from wt or trif/myd88 double knockout mice were analyzed as in D and E (mean+SD, N=2).
T. gondii mediated inhibition of NO and induction of SOCS1 are independent of TLR and PI3 kinase signaling
We further wanted to delineate which cellular pathways in the host cells were responsible for SOCS induction. Members of the SOCS family can be induced by TLR signaling. Since T. gondii has been reported to trigger various TLRs (TLR11, TLR2/4, TLR9) (Minns et al., 2006; Yarovinsky et al., 2005; Debierre et al., 2007), we tested whether SOCS1 induction and inhibition of NO production were achieved by the stimulation of TLRs by T. gondii. To this, we used dendritic cells lacking the TLR adaptor proteins MyD88 or TRIF (from lps2 mice) in comparison to wildtype dendritic cells. Infection with T. gondii RH parasites led to an inhibition of IFNγ-mediated NO production, and no significant differences were observed between the knockout dendritic cells and the wildtype (Fig. 1D). Corroborating these observations, infection with T. gondii led to a similar induction of the SOCS family members SOCS1 and CIS in wildtype dendritic cells as well as in dendritic cells lacking the TLR adaptor proteins (Fig. 1E) with the control compounds for TLR4 and TLR9 showing a pattern as published (Baetz et al., 2004). To further strengthen these observations we additionally analyzed bone-marrow derived macrophages from trif/myd88 double knockout mice. Similar to the results with DCs, infection with T. gondii inhibited IFN mediated NO secretion in wildtype as well as knockout mice (Fig. 1F) and no differences could be observed for induction of SOCS1 and CIS (Fig. 1G).
It was also shown that T. gondii activates protein kinase B via phosphatidylinositol-trisphosphate (PI3) kinase (Kim and Denkers, 2006). Usage of the inhibitors wortmannin and Ly294002, which in the applied concentrations inhibited PI3 kinase effectively, did not affect the inhibitory potential of T. gondii parasites with respect to NO production (data not shown). The induction of SOCS1 by T. gondii was also not altered by pretreatment with PI3 kinase inhibitors (data not shown).
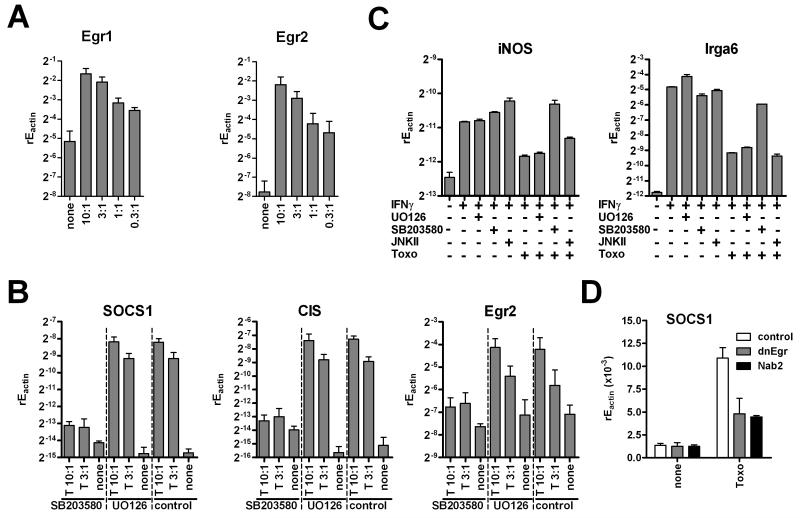
Host p38 MAPK and egr transcription factors are important for SOCS1 induction
It has been published recently that rhoptry proteins induce egr2 transcription (Phelps et al., 2008) and egr transcription factors had been linked to regulation of SOCS1 before (Mostecki et al., 2005). When we analyzed macrophages infected with RH strain parasites we observed an induction of egr1 and egr2 in a manner dependent on the number of parasites used, which also correlated well with an increase in SOCS1 (Fig. 2A). EMSA analysis confirmed increased binding activity of egr transcription factors in RH strain infected cells (data not shown). Furthermore, egr2, SOCS1 and CIS induction by RH strain parasites could be inhibited by SB203580 which blocks p38 MAPK signaling (Fig. 2B). In contrast, ERK and JNK activity were dispensable because JNKII and UO126 inhibitors did not affect induction of SOCS1, CIS or egr2 (data not shown, Fig. 2B). Again, this correlated strictly with the ability of RH strain parasites to limit IFNγ induced iNOS which was sensitive to p38, but not ERK or JNK inhibition (Fig. 2C). Moreover, egr mRNA induction always preceded SOCS1 increase in time kinetics with rapid expression 1h after infection whereas SOCS1 mRNA began to increase at 3-5h post infection (data not shown). To strengthen the link between p38 MAPK activation by RH strain parasites andegr and SOCS1 induction we made use of plasmids encoding a truncated form of egr3 or the endogenous egr corepressor Nab2 (Carter and Tourtellotte, 2007). Both plasmids previously had been shown to inhibit transcriptional activity of multiple egr factors. Expression of either of the plasmids markedly inhibited SOCS1 induction by infection with RH strain parasites (Fig. 2D).
Figure 2. T. gondii induces egr and SOCS in a p38 MAPK dependent manner.
(A) RAW264.7 macrophages were infected with RH parasites as indicated. 5h later expression of egr1 and egr2 was determined by quantitative RT-PCR (mean+SEM, N=3). (B) RAW264.7 macrophages were pre-equilibrated with 10 μM SB203580, UO126 or solvent and then were infected with RH strain parasites for 5 h. Expression of SOCS1, CIS, and egr2 was determined by quantitative real-time PCR relative to the levels of actin (mean+SEM, N=3). (C) Cells were infected as in (B) and additionally stimulated with IFNγ for 1 h. Expression of iNOS and Irga6 was analyzed by quantitative RT-PCR (mean+SD from replicates; one of two experiments is shown). (D) RAW264.7 macrophages were transfected to express dominant negative dnEgr construct, the endogenous Egr repressor Nab2 or a vector control. Cells were then infected with RH strain parasites and analyzed for expression of SOCS1 by RT-PCR (mean+SD).
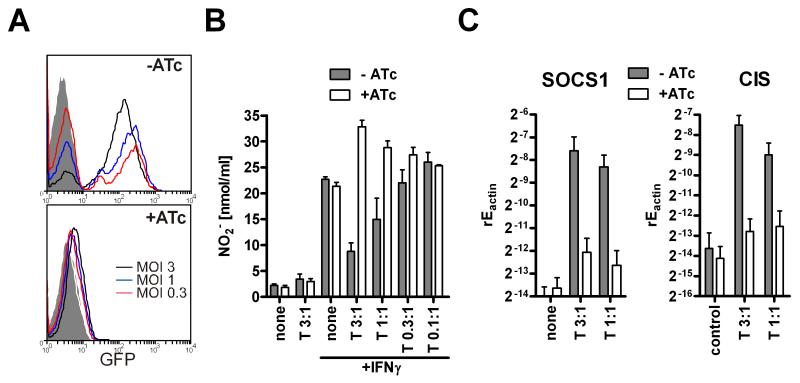
Invasion of host cells is necessary for SOCS1 and CIS induction
Next, we wanted to assess whether infection of cells was necessary or if contact between parasites and macrophages was sufficient for the inhibition of NO production. We used GFP expressing RH tachyzoites with a tetracycline-inducible knockout of Mic8. Parasites deficient of Mic8 are capable of migration and the secretion of their micronemes, but lack the ability to invade target cells (Kessler et al., 2008). We first verified that macrophages incubated with Mic8 deficient parasites are not infected as this had not been tested in detail before. Determination of infected and thus GFP positive RAW macrophages by flow cytometry proved that the anhydrotetracycline (ATc) treated parasites entirely lacked the ability to infect macrophages (Fig. 3A) at differing MOIs. Thus, we could examine the role of invasion for the inhibition of nitric oxide production and SOCS induction. ATc-induced Mic8 knockout parasites as compared to non-induced (as well as wildtype) parasites failed to inhibit IFNγ-mediated NO generation (Fig. 3B). Similarly to killed parasites, the invasion-defective parasites even enhanced NO secretion slightly. In parallel, Mic8 knockouts showed a severely diminished induction of SOCS1 and lacked significant increase in CIS mRNA (Fig. 3C). Cytochalasin D treatment which allows attachment and rhoptry secretion but blocks invasion partly impaired suppressive activity (data not shown). We conclude that viable, invasion-competent parasites are necessary for the inhibition of NO generation and the induction of SOCS molecules.
Figure 3. Cell invasion is necessary for SOCS induction and nitric oxide inhibition.
Tetracycline-induced Mic8 knockout (+ATc) and non-induced control parasites (−ATc) which expressed GFP were used for infection of RAW264.7 macrophages. (A) Infected macrophages (MOI3, 1, 0.3:1) were detected by expression of GFP by flowcytometry (gated on macrophages’ FSC/SSC characteristics). (B) Nitrite was determined in the supernatant after IFNγ stimulation and (C) SOCS1 and CIS expression was examined by quantitative RT-PCR (mean+SEM, N=4).
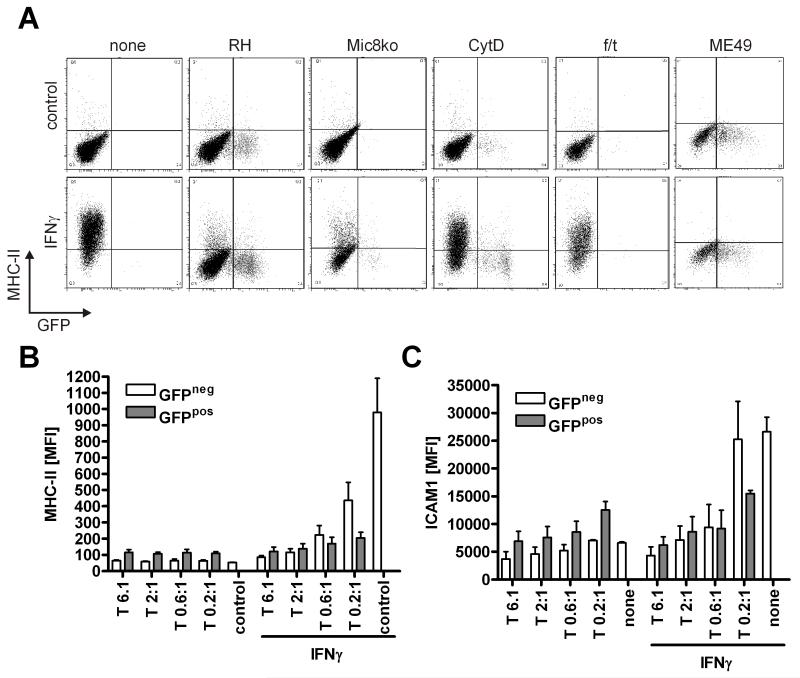
MHC-class II inhibition occurs independently of cell invasion and genotype
During our studies on T. gondii mediated inhibition of IFNγ we observed that, as analyzed by flowcytometry, GFP-RH type parasites did not only inhibit the up-regulation of MHC-class II in infected cells (as identified as being GFP positive), but also reduced the expression of MHC-class II molecules in uninfected cells (GFP negative) (Fig. 4A, B). In a similar manner, we observed that the increase in ICAM1 expression upon IFNγ stimulation was diminished in all cells irrespective whether they harbored parasites or not (Fig. 4C). The inhibitory effect on the GFP-negative cells was not due to a possible destruction of GFP which would falsely mark the cells as being uninfected, because up-regulation of CD86, which serves as a marker of cell activation and occurs as a result of infection, was only observed in GFP-positive cells (data not shown). A quantitative analysis of MHC-class II or ICAM1 expression in either infected (GFP+) or uninfected (GFP−) cells confirmed that both populations got inhibited in an MOI dependent manner (Fig. 4B, C). Of note, this kind of inhibition occurred at surprisingly low MOIs (down to 0.2:1). Infected cells were slightly more efficiently inhibited than uninfected cells. The same results were also observed in bone marrow derived macrophages (data not shown). This behavior of inhibition in both infected as well as uninfected cells contrasted the above conditions for NO inhibition and hinted towards an additional mechanism of interference with IFNγ. Further contrasting our findings on SOCS1 and NO, this kind of inhibition was not altered in invasion-deficient Mic8 knockouts (Fig. 4A). However, parasites had to be viable and motile or able to secrete micronemes as inhibition was lost upon killing by freeze/thaw cycles or cytochalasin D (CytD) treatment (which inhibits actin polymerization), respectively. In the latter case we observed that the inhibition of MHC-class II up-regulation only took place in the very few cells that, despite CytD treatment, were still infected by parasites. Further analysis suggested that secretion of CXCL9 (MIG) behaved in a similar manner (data not shown).
Figure 4. T. gondii mediated inhibition of MHC-class II and ICAM1 occurs in infected and non-infected cells independent of cell invasion.
(A) RAW264.7 cells were infected with viable RH, Mic8 knockout (grown in presence of ATc) or ME49 parasites or RH parasites that were inhibited with1 μM Cytochalasin D or killed (three freeze/thaw cycles, f/t) at a MOI 1. Cells were stimulated with 50 ng/ml IFNγ and analyzed after overnight incubation for expression of MHC-class II molecules by flowcytometry (one out of at least three experiments is shown). Similar experiments as in (A) were performed for different parasite:host cell ratios and mean fluorescence intensities of MHC-class II (B) or ICAM1 (C) expression were determined for either uninfected (GFP−) or infected (GFP+) cells (mean+SEM, N=4).
Inhibition of NO production and induction of SOCS1 and CIS, but not interference with antigen presentation, are strain specific effects
Since different genotypes of T. gondii vary in their virulence we next compared RH parasites (a genotype I strain) with the less virulent genotype II strain Me49. Infection of macrophages revealed that both, genotype I and genotype II parasites inhibited up-regulation of MHC-class II molecules by IFNγ (Fig. 4A). However, determination of IFNγ-activated production of NO radicals revealed that only the genotype I RH inhibited NO production at low MOIs (Fig. 5A). The genotype II Me49 strain only showed a trend to inhibit NO at high MOIs, but was never as efficient as RH. We then investigated whether both parasite strains induced SOCS1 and CIS. Interestingly, the findings for SOCS1 and CIS induction correlated strictly with the NO inhibition: Whereas RH tachyzoites induced high amounts of both SOCS1 and CIS mRNA, Me49 parasites only showed a trend to induce SOCS1 when used at high MOIs (Fig. 5B). CIS induction was strongly decreased as compared to RH strain infections. Thus, viability and cell invasion were necessary but not sufficient for SOCS induction and NO inhibition. Both effects occurred in a strain-specific manner.
Figure 5. Type I but not type II T. gondii induce SOCS and inhibit IFNγ mediated NO secretion.
RAW264.7 macrophages were infected with type I RH or type II Me49 Toxoplasma (T) at the indicated MOI. (A) Cells were stimulated with 50 ng/ml IFNγ either for 40 h followed by determination of nitrite in the supernatant or (B) were infected for 5 h prior to detection of SOCS1 and CIS by quantitative real-time PCR (mean+SEM, N=4).
Inhibition of MHC-class II expression by T. gondii is dependent on cell contact and secreted parasitic proteins but independent of cell invasion
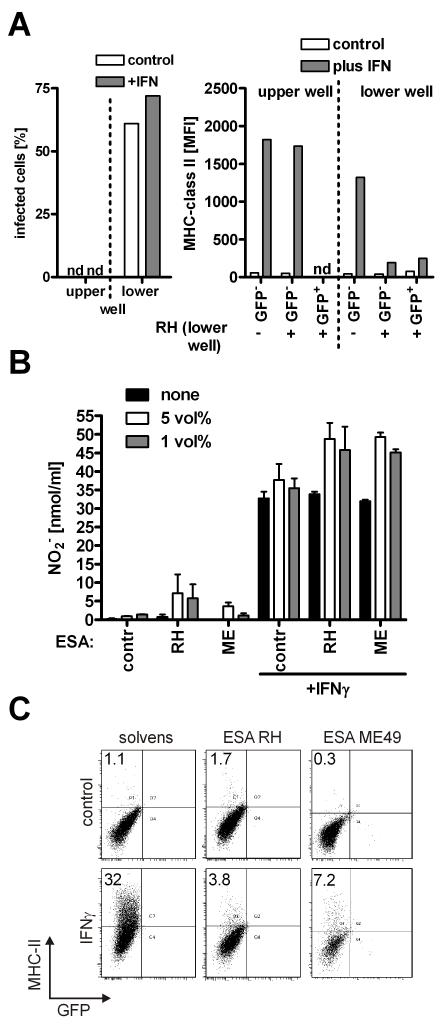
Next we analyzed the conditions that enable the parasite to interfere with IFNγ-induced MHC-class II expression. Macrophages were placed on both sides of a transwell chamber and infected only in the lower compartment. The inhibition of MHC-class II expression could only be observed in the infected compartment (again on both, GFP+ as well as GFP− cells) but not on the opposite side of the transwell (Fig. 6A). Thus, freely diffusible factors that pass the transwell chamber are not operative. We already had shown that cell invasion was not a prerequisite for MHC-class II inhibition as Mic8 deficient parasites showed no alterations (Fig. 4A). Therefore we tested whether parasitic compounds that are secreted during gliding and cell attachment (in close contact of parasites to cells) are operative for MHC-class II and ICAM1 inhibition. We generated secretory extracts by adding ethanol to parasites (Carruthers et al., 1999). We observed that those concentrated extracts as compared to solvent control did not inhibit IFN-γ mediated NO secretion (Fig. 6B) but effectively diminished MHC-class II induction (Fig. 6C). The inhibitory effects for MHC-class II induction could be observed with the extracts from both type I and II strains, in line with the previous findings (Figs. 4, 5).
Figure 6. Micronemal extracts from T. gondii mediate inhibition of MHC-class II expression and MIG but not nitric oxide secretion.
(A) RAW264.7 cells were incubated in both compartments of a transwell chamber. The lower well was infected with GFP-expressing RH parasites at MOI 1:1. After overnight incubation infected cells were determined as GFP+ cells (applying a gate on host macrophages based on FSC/SSC characteristics) (left panel). Cells were analyzed for expression of MHC-class II expression by flowcytometry. Mean fluorescence intensity was determined individually on GFP− (non-infected) as well as GFP+ (infected) cells. In the upper compartment of the transwell no infected cells could be determined (n.d.) (right panel). (B, C) Micronemal extracts (excreted/secreted antigens, ESA) were prepared from either RH or Me49 parasites (both expressing GFP) by adding ethanol. The extracts were incubated with RAW264.7 macrophages in the indicated concentrations (5 and 1%) and cells were stimulated with 50 ng/ml IFNγ. (B) Nitrite was determined after 40 h of incubation and (C) MHC-class II expression was analyzed after 24 h (mean+SEM, N=3).
Discussion
In this study we decipher the mechanisms by which T. gondii induces SOCS molecules and escapes from IFNγ activation in macrophages. Analyzing cell-invasive defective Mic8 knockout parasites we surprisingly find that at least two different mechanisms exist: Both genotype I and II strains were able to inhibit up-regulation of MHC-class II and ICAM1 following IFNγ stimulation, yet only the more virulent genotype I additionally limited NO radical production by induction of SOCS1 and CIS expression.
Inhibition of IFNγ induced up-regulation of MHC-class II by type II tachyzoites was also found previously (Luder et al., 2001). Our results add the observation that (i) both genotype I and genotype II T. gondii equally inhibit IFNγ induced up-regulation of antigen presentation and (ii) that this inhibition occurs in infected as well as uninfected cells. The transwell experiment indicates that this is not due to soluble inhibitory molecules that are secreted by infected cells. In line with these findings it has also been shown that the inhibitory cytokine IL-10 is dispensable for T. gondii mediated inhibition of IL-12 and TNF production (Butcher et al., 2005).
Instead we conclude that the parasite may employ an active mechanism to down-modulate antigen presentation and migration, thereby inhibiting the presentation of T. gondii antigens. This conclusion is additionally strengthened by the observation of Lang et al., who found that T. gondii type II lysates also inhibited MHC-class II up-regulation when added to macrophages (Lang et al., 2006). Possible candidates for such kind of inhibitory actions are secreted microneme proteins which mimicked live T. gondii effects. In contrast, rhoptries are usually secreted during and after invasion of the host cell in a process that requires functional MIC8 (Kessler et al., 2008) and dense granules are released inside the host cell only during/after invasion. Analysis of MIC8 deficient parasites showed that for interference with antigen presentation cell invasion and rhoptry discharge was dispensable. Since T. gondii leaves a trail of antigens behind when it glides through tissues (Keeley and Soldati, 2004; Jewett and Sibley, 2004), the antigens together with an adequate danger signal could activate antigen presenting cells and adaptive immunity. T. gondii has thus evolved a protein either secreted from the micronemes or present in the plasma membrane which can counteract the immunostimulatory activity of the tachyzoites and their trail of foreign antigen.
The more virulent type I strains additionally uses a second way to inhibit IFNγ-mediated NO secretion and the induction of p47 GTPases. The p47 GTPase family has been shown to possess toxoplasmacidic activity and NO is toxoplasmastatic, i.e. it inhibits the replication of the parasites and it is therefore reasonable that more virulent strains have evolved means to eliminate these factors (Seabra et al., 2002; Zhao et al., 2009). In this study, only genotype I, but not genotype II T. gondii were able to reduce NO radical production from IFNγ activated macrophages. This inhibition depended on viable, invasive parasites indicating that only infected cells get inhibited in this respect. MIC8 deficient parasites lacked infectivity for macrophages and showed abolished NO inhibition and SOCS induction. In line with the notion of two different inhibitory activities, MIC8 knockout parasites had a similar inhibitory effect as wild-type parasites on MHC-class II or ICAM1 regulation.
In contrast, the genotype II Me49 strain did not significantly inhibit NO secretion. This finding slightly contrasts another study (Luder et al., 2003) which however investigated a type II NTE patient strain and did not include a comparison with type I strains. Me49, the strain used in this study, is a widely used model for type II T. gondii. Differences in the study and the results presented here might be based on the differences in strains. Indeed, another study with Me49 parasites confirms our findings that NO production is activated and not inhibited by this strain (Dobbin et al., 2002). Interestingly, Me49 parasites induced rather than inhibited NO secretion if added to RAW264.7 macrophages at a low MOI. As T. gondii can activate signaling via TLR11, TLR2 and TLR4 as well as TLR9 (Yarovinsky et al., 2005; Minns et al., 2006; Debierre et al., 2007) it can be speculated that Me49 might stimulate these pattern recognition receptors. Indeed genotype differences in MyD88 usage have been reported (Kim et al., 2006). Genotype I T. gondii tachyzoites might not represent a very strong TLR stimulus, and activation of macrophages with IFNγ can be overcome. SOCS1 and CIS are intracellular inhibitors of IFNγ signaling known to limit JAK activation (Alexander et al., 1999; Marine et al., 1999). Hijacking the host’s suppressors of cytokine signaling is not an uncommon mechanism to evade activation of host cells (Vazquez et al., 2006; Imai et al., 2003). Thus, T. gondii might profit from inducing the expression of SOCS1 and CIS in order to inhibit IFNγ signaling. In this study, genotype I T. gondii induced SOCS1 and CIS mRNA expression, whereas type II T. gondii did not. This induction might play a role in the different abilities of the strains to inhibit NO production by macrophages.
In this work, the pathway of SOCS1 induction was investigated. Using cells from knockout mice or chemical inhibitors TLRs and PI3K could be ruled out to be involved in SOCS1 and CIS induction as well as NO inhibition. However, SOCS1 induction was dependent on p38 MAPK activity and the induction of egr2 and egr1 transcription factors. Like SOCS1, egr1 and egr2 were only induced by type I T. gondii. Egr1 has been shown to play a role in LPS-mediated induction of SOCS1 (Mostecki et al., 2005). As egr1 and egr2 share DNA binding sequences (Swirnoff and Milbrandt, 1995) and because p38 MAPK inhibition abrogated egr2 expression as well as SOCS1 induction it is probable that egr factors are downstream of p38 and upstream of SOCS1. We here report that inhibition of egr transcriptional activity by expression of a truncated, dominant negative egr3 or the corepressor Nab2 (Carter and Tourtellotte, 2007) in fact interferes with SOCS1 induction by T. gondii.
We also observed that active cell penetration was necessary. This might hint towards rhoptry proteins as responsible for the second pathway of strain-specific, cell invasion-dependent inhibition of some aspects of IFNγ signaling. Rhoptries are secreted upon invasion and would thus explain the invasion dependency which was observed. Some rhoptry proteins are delivered into the host cell’s cytoplasm or even its nucleus (Hakansson et al., 2001). Also, they have been discovered to be major virulence factors of T. gondii which would in part explain the differences in virulence between genotypes I and II. ROP16 is targeted to the host nucleus and helps to extend the signaling of STAT3 (Yamamoto et al., 2009). As SOCS1 was first described as an STAT induced inhibitor of IL-6/STAT3 signaling (Naka et al., 1997) it might be that STAT3, p38 and egr2 contribute to type I strain induced SOCS1 mRNA expression. Our data confirm on the molecular level the previously noted association of virulence with the parasite’s ability to induce SOCS1 (Saeij et al., 2007).
The findings from this study demonstrate that there are different ways by which T. gondii inhibits IFNγ activation of macrophages. Inhibition of ICAM1 and MHC-class II up-regulation upon IFNγstimulation was achieved by genotype I and II strains and was not affected in cell invasion defective MIC8 knockout strain. In contrast, cell invasion was necessary to induce egr transcription factors in a p38 MAP kinase dependent manner, to increase SOCS1 transcription and to inhibit nitric oxide secretion.
Acknowledgments
This work has been supported by grants of the German Research Foundation (DFG Da 592/3 and /4 to AD).
We acknowledge the excellent technical support from René Karayilan and Adelina Dillmann.
Abbreviations
- CIS
cytokine inducible SH2 containing protein
- Mic8
micronemal protein 8
- ROP
rhoptry protein
- SOCS
suppressor of cytokine signaling
References
- Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TW, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate Toll-like receptor signaling in innate immune cells. J. Biol. Chem. 2004;279:54708–54715. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- Bode KA, Schmitz F, Vargas L, Heeg K, Dalpke AH. Kinetic of RelA activation controls magnitude of TLR-mediated IL-12p40 induction. J. Immunol. 2009;182:2176–2184. doi: 10.4049/jimmunol.0802560. [DOI] [PubMed] [Google Scholar]
- Butcher BA, Kim L, Johnson PF, Denkers EY. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-kappa B. J Immunol. 2001;167:2193–2201. doi: 10.4049/jimmunol.167.4.2193. [DOI] [PubMed] [Google Scholar]
- Butcher BA, Kim L, Panopoulos AD, Watowich SS, Murray PJ, Denkers EY. Cutting Edge: IL-10-Independent STAT3 Activation by Toxoplasma gondii Mediates Suppression of IL-12 and TNF-{alpha} in Host Macrophages. J Immunol. 2005;174:3148–3152. doi: 10.4049/jimmunol.174.6.3148. [DOI] [PubMed] [Google Scholar]
- Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma cell invasion. Curr. Opin. Microbiol. 2007;10:83–89. doi: 10.1016/j.mib.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Carruthers VB, Moreno SN, Sibley LD. Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem. J. 1999;342(Pt 2):379–386. [PMC free article] [PubMed] [Google Scholar]
- Carter JH, Tourtellotte WG. Early growth response transcriptional regulators are dispensable for macrophage differentiation. J. Immunol. 2007;178:3038–3047. doi: 10.4049/jimmunol.178.5.3038. [DOI] [PubMed] [Google Scholar]
- Dalpke AH, Opper S, Zimmermann S, Heeg K. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. J. Immunol. 2001;166:7082–7089. doi: 10.4049/jimmunol.166.12.7082. [DOI] [PubMed] [Google Scholar]
- Debierre G, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, Mansur DS, Weingart R, Schmidt RR, Golenbock DT, Gazzinelli RT, Schwarz RT. Activation of TLR2 and TLR4 by Glycosylphosphatidylinositols Derived from Toxoplasma gondii. J Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- Dobbin CA, Smith NC, Johnson AM. Heat shock protein 70 is a potential virulence factor in murine toxoplasma infection via immunomodulation of host NF-kappa B and nitric oxide. J Immunol. 2002;169:958–965. doi: 10.4049/jimmunol.169.2.958. [DOI] [PubMed] [Google Scholar]
- Hakansson S, Charron AJ, Sibley LD. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 2001;20:3132–3144. doi: 10.1093/emboj/20.12.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Kurita-Ochiai T, Ochiai K. Mycobacterium bovis bacillus Calmette-Guerin infection promotes SOCS induction and inhibits IFN-gamma-stimulated JAK/STAT signaling in J774 macrophages. FEMS. Immunol Med. Microbiol. 2003;39:173–180. doi: 10.1016/S0928-8244(03)00231-1. [DOI] [PubMed] [Google Scholar]
- Jewett TJ, Sibley LD. The toxoplasma proteins MIC2 and M2AP form a hexameric complex necessary for intracellular survival. J. Biol Chem. 2004;279:9362–9369. doi: 10.1074/jbc.M312590200. [DOI] [PubMed] [Google Scholar]
- Keeley A, Soldati D. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 2004;14:528–532. doi: 10.1016/j.tcb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Kessler H, Herm-Gotz A, Hegge S, Rauch M, Soldati-Favre D, Frischknecht F, Meissner M. Microneme protein 8 - a new essential invasion factor in Toxoplasma gondii. J Cell Sci. 2008;121:947–956. doi: 10.1242/jcs.022350. [DOI] [PubMed] [Google Scholar]
- Kim L, Butcher BA, Lee CW, Uematsu S, Akira S, Denkers EY. Toxoplasma gondii Genotype Determines MyD88-Dependent Signaling in Infected Macrophages. J Immunol. 2006;177:2584–2591. doi: 10.4049/jimmunol.177.4.2584. [DOI] [PubMed] [Google Scholar]
- Kim L, Denkers EY. Toxoplasma gondii triggers Gi-dependent PI 3-kinase signaling required for inhibition of host cell apoptosis. J Cell Sci. 2006;119:2119–2126. doi: 10.1242/jcs.02934. [DOI] [PubMed] [Google Scholar]
- Laliberte J, Carruthers VB. Host cell manipulation by the human pathogen Toxoplasma gondii. Cell Mol. Life Sci. 2008;65:1900–1915. doi: 10.1007/s00018-008-7556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Algner M, Beinert N, Gross U, Luder CG. Diverse mechanisms employed by Toxoplasma gondii to inhibit IFN-gamma-induced major histocompatibility complex class II gene expression. Microbes. Infect. 2006;8:1994–2005. doi: 10.1016/j.micinf.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Luder CG, Algner M, Lang C, Bleicher N, Gross U. Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Int. J Parasitol. 2003;33:833–844. doi: 10.1016/s0020-7519(03)00092-4. [DOI] [PubMed] [Google Scholar]
- Luder CG, Walter W, Beuerle B, Maeurer MJ, Gross U. Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1alpha. Eur. J. Immunol. 2001;31:1475–1484. doi: 10.1002/1521-4141(200105)31:5<1475::AID-IMMU1475>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D, Yoshimura A, Ihle JN. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ. Functional Inactivation of Immature Dendritic Cells by the Intracellular Parasite Toxoplasma gondii. J Immunol. 2004;173:2632–2640. doi: 10.4049/jimmunol.173.4.2632. [DOI] [PubMed] [Google Scholar]
- Minns LA, Menard LC, Foureau DM, Darche S, Ronet C, Mielcarz DW, Buzoni-Gatel D, Kasper LH. TLR9 Is Required for the Gut-Associated Lymphoid Tissue Response following Oral Infection of Toxoplasma gondii. J Immunol. 2006;176:7589–7597. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- Mostecki J, Showalter BM, Rothman PB. Early Growth Response-1 Regulates Lipopolysaccharide-induced Suppressor of Cytokine Signaling-1 Transcription. J. Biol. Chem. 2005;280:2596–2605. doi: 10.1074/jbc.M408938200. [DOI] [PubMed] [Google Scholar]
- Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- Phelps ED, Sweeney KR, Blader IJ. Toxoplasma gondii rhoptry discharge correlates with activation of the early growth response 2 host cell transcription factor. Infect. Immun. 2008;76:4703–4712. doi: 10.1128/IAI.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 2002;3:1041–1047. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- Saeij JP, Boyle JP, Boothroyd JC. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 2005;21:476–481. doi: 10.1016/j.pt.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra SH, de Souza W, DaMatta RA. Toxoplasma gondii partially inhibits nitric oxide production of activated murine macrophages. Exp. Parasitol. 2002;100:62–70. doi: 10.1006/expr.2001.4675. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Swirnoff AH, Milbrandt J. DNA-binding specificity of NGFI-A and related zinc finger transcription factors. Mol. Cell Biol. 1995;15:2275–2287. doi: 10.1128/mcb.15.4.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez N, Greenwell-Wild T, Rekka S, Orenstein JM, Wahl SM. Mycobacterium avium-induced SOCS contributes to resistance to IFN-{gamma}-mediated mycobactericidal activity in human macrophages. J Leukoc Biol. 2006;80:1136–1144. doi: 10.1189/jlb.0306206. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Standley DM, Takashima S, Saiga H, Okuyama M, Kayama H, Kubo E, Ito H, Takaura M, Matsuda T, Soldati-Favre D, Takeda K. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J. Exp. Med. 2009;206:2747–2760. doi: 10.1084/jem.20091703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 Activation of Dendritic Cells by a Protozoan Profilin-Like Protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ferguson DJ, Wilson DC, Howard JC, Sibley LD, Yap GS. Virulent Toxoplasma gondii evade immunity-related GTPase-mediated parasite vacuole disruption within primed macrophages. J. Immunol. 2009;182:3775–3781. doi: 10.4049/jimmunol.0804190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Murray PJ, Heeg K, Dalpke AH. Induction of Suppressor of Cytokine Signaling-1 by Toxoplasma gondii Contributes to Immune Evasion in Macrophages by Blocking IFN-{gamma} Signaling. J Immunol. 2006;176:1840–1847. doi: 10.4049/jimmunol.176.3.1840. [DOI] [PubMed] [Google Scholar]