Abstract
Many infectious diseases that affect global health are most accurately diagnosed through nucleic acid amplification and detection. However, existing nucleic acid amplification tests are too expensive and complex for most low-resource settings. The small numbers of centralized laboratories that exist in developing countries tend to be in urban areas and primarily cater to the affluent. In contrast, rural area health care facilities commonly have only basic equipment and health workers have limited training and little ability to maintain equipment and handle reagents.1 Reliable electric power is a common infrastructure shortfall. In this paper, we discuss a practical approach to the design and development of non-instrumented molecular diagnostic tests that exploit the benefits of isothermal amplification strategies. We identify modular instrument-free technologies for sample collection, sample preparation, amplification, heating, and detection. By appropriately selecting and integrating these instrument-free modules, we envision development of an easy to use, infrastructure independent diagnostic test that will enable increased use of highly accurate molecular diagnostics at the point of care in low-resource settings.
Keywords: Low-resource settings, point of care diagnostics, non-instrumented diagnostics, infection detection, nucleic acid amplification, molecular diagnostics, sample preparation, laminar flow
1. INTRODUCTION
1.1 The ASSURED criteria
One of the challenges to infectious disease diagnosis in low-resource settings (LRS) is that many infections occur far away from centralized laboratories where diagnostic tests are routine. Unfortunately, few diagnostic tests can be performed in LRS. In response to the need for improved diagnostic tests for the developing world, the World Health Organization (WHO) Sexually Transmitted Diseases Diagnostics Initiative (SDI) has identified appropriate diagnostic test attributes to address disease control needs for LRS. These attributes, refered to as the “ASSURED” criteria can be summarized as: sensitive, specific, user-friendly, rapid and robust, equipment-free and deliverable to end-users.2 (see Figure 1)
Figure 1.

WHO SDI “ASSURED” criteria.
1.2 Strip tests: a prototypical ASSURED solution
For the past decade, immunochromatographic strip (ICS) tests have been one of the few diagnostic technologies to approach the ASSURED criteria and achieve successful uptake in the developing world. ICS tests provide point-of-care (POC) diagnosis in areas without access to well-equipped and well-staffed clinical laboratories. Because they rely on inexpensive, off-the-shelf components and reagents, they are affordable, in many cases costing less than US$2 to the end user. They can be formatted for detection of antigens or antibodies and are usable with a wide range of specimens, making them adaptable to a wide range of applications.3,4 ICS strips require relatively little and sometimes no sample processing, and they do not require an external instrument. Figure 2 depicts the key features of an ICS.5
Figure 2.
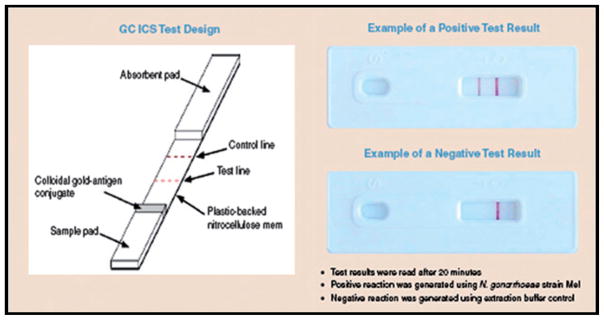
Schematic of an ICS test for gonorrhea specifically developed for low-resource settings (reproduced from Weigl et al.; 200810).
ICS are stable for many months at ambient temperatures—a critical feature for settings in which the electrical supply is inconsistent and temperature control is difficult. Because of their low cost, small size and stability, strip tests may also be used by epidemiological surveillance teams in the field to gather baseline data or to assess the effect of public health interventions. Despite these positive attributes, ICS also frequently suffer from limited sensitivity and specificity. While some commercialized lateral flow assays can achieve sensitivities and specificities in the range of 90% or higher, others have sensitivity lower than 70%. Additionally, antigen polymorphisms can result in varying performance in different settings.6,7,8 The persistence of some antigens beyond clearance of infection can decrease test specificity, while other antigens with shorter life times in the blood have lower sensitivities.6,7,9 While ICS performance values represent an improvement over syndromic diagnosis, tests with high performance (characterized by higher sensitivity, higher specificity and lower limits of detection) are needed.
1.3 Design opportunity
Nucleic acid amplification tests (NAATs) are more sensitive and specific than their immunoassay counterparts and have much lower limits of detection. Nonetheless, in mapping currently available NAATs and ICS tests against the high accuracy and low infrastructure demands of LRS, Figure 3 demonstrates there is still an unmet need where existing NAAT technologies fail to meet the demands of decentralized POC settings. This gap represents a design opportunity. A NAAT platform that obviates the need for an instrument entirely would greatly enhance LRS POC testing capabilities in decentralized areas while providing the sensitivity, specificity, and limit of detection required for accurate diagnosis. Developing such a test that has minimal complexity comparable to ICS, requires careful consideration of each process involved.
Figure 3.
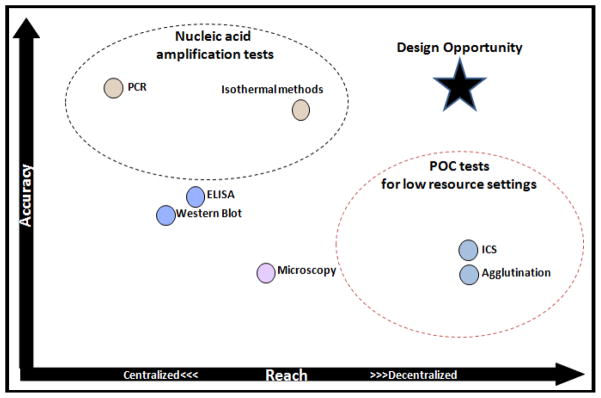
The design opportunity where currently available NAATs and ICS fail to meet the demands of POC testing in LRS.
Currently available polymerase chain reaction (PCR)-based NAATs are not amenable to most LRS because they require complex, electrically-powered equipment for thermal-cycling of the sample/reagent mixture. Additionally, operation of PCR equipment requires highly trained staff to prepare reactions and interpret results. Recently, there have been significant developments in a class of NAATs that do not involve temperature cycling.11,12,13,14 Several isothermal methods have demonstrated a high yield of DNA and robust function over a range of temperatures. Some have high sensitivity on par with PCR but are much less susceptible to inhibitors than PCR.15 In some cases, the sample preparation steps required for PCR can be eliminated.16 In general these isothermal methods are less comlex than PCR. Amplification occurs at a constant temperature, reagents and workflow are relatively simple, and direct visual detection is possible. While many isothermal assays for LRS applications are under development,16,17,18,19 these tests have not yet made an impact on diagnostics designed for LRS. There are several reasons for this. First, isothermal NAATs still rely on electrical heat input. Second, the specificity of common isothermal amplification and detection schemes has been variable.16,20,21,22,23 Finally, most isothermal amplification schemes require high heat treatment of the raw specimen and are reliant upon solid phase purification with either a centrifuge or vacuum manifold.24 To date, these factors have all limited the penetration of isothermal amplification technologies into LRS. The design opportunity for a LRS-appropriate NAAT can be described simply: as accurate as PCR—and as fast, easy to use, and inexpensive as ICS diagnostics.
1.4 Modular approach
The modular approach to developing an instrument-free NAAT is built upon the simplicity of isothermal NAAT methods. Individual component design improvements can be integrated to achieve a non-instrumented solution. PATH and collaborators are advancing modular non-instrument nucleic acid amplification (NINA) technologies that leverage the benefits of isothermal amplification chemistries for POC testing. These benefits include reduced sensitivity to sample contaminants and simple thermal profile requirements. Thus samples can be minimally processed without the need for a centrifuge or vacuum manifold as the sample preparation module is simplified using buffer chemistries, detergents, FTA cards, or other low-infrastructure compatible methods. Furthermore, the complex, closed loop, thermo-cycling electric module common to PCR heating can be replaced by an isothermal chemical heating module using compact exothermic cartridges instead of electricity. Finally, we are advancing non-instrumented detection module technologies by exploiting nucleic acid laminar flow (NALF), that benefits from the easy-to-read, non-ambiguous ICS detection and by investigating dyes such as hydroxy naphthol blue (HNB) that provide naked-eye colorimetric detection.
In general, the steps for performing any NAAT include: specimen collection and metering, sample processing (removal of inhibitory compounds and analyte enrichment), incubation; signal amplification, and signal detection. Careful selection of instrument-free modules that perform each of these steps is a requirement toward the development and commercialization of an instrument- free NAAT platform. For all these steps, there are a relatively small number of instrument-free components to choose from. To date, a product that successfully combines all necessary processes in an instrument free manner has not been demonstrated. In this paper, we highlight several promising technologies that may be combined as modules of the first completely non-instrumented NAAT platform that performs sample collection and processing, amplification, and detection.
2. INTRUMENT-FREE NUCLEIC ACID AMPLIFICATION MODULES
2.1 Sample collection and transfer module
Sample collection and transfer often relies upon the use of standard pipette techniques. There are several inexpensive, disposable alternatives for metering sample and reagent volumes. First, is the Microsafe collection and dispensing tube by Safe-Tec Clinical Products Inc, Ivyland, PA, which is commercially available in 5 μL to 100 μL volumes. These are designed to collect and dispense blood samples at a preset volume using capillary action and a volume controlling air vent.25 Second is the Exact Volume Pipette manufactured by Poly-Pipets, Inc.26 These single-dose disposable pipets are available in a range of sizes from 20 μL to 250 μL. They are designed to dispense the exact volume held in the stem. Any excess fluid from the draw remains in the bottom bulb. In addition, PATH has developed a single-use, calibrated blood collection and transfer device. This device depicted in Figure 4 is designed to collect 100 μL of blood from a fingerstick and transfer diluted blood to a microfluidic diagnostic disposable.
Figure 4.
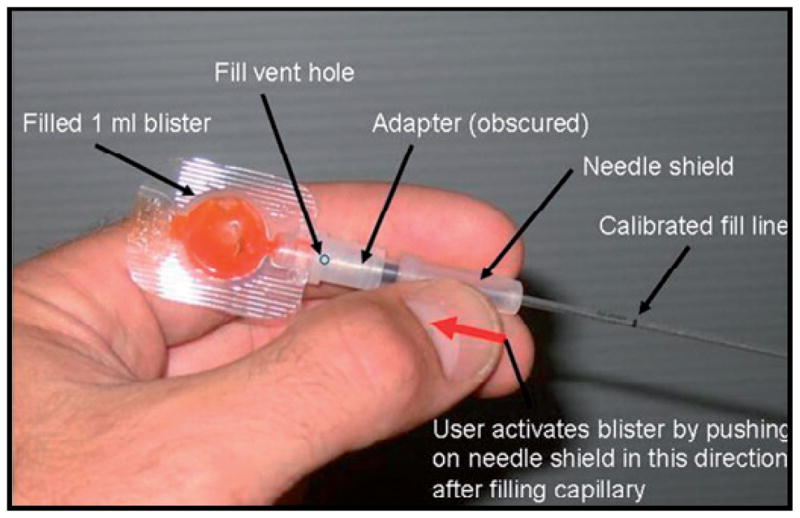
A prototype device designed to collect 100 mL of blood from a fingerstick and transfer diluted blood to a microfluidic diagnostic disposable (reproduced with permission from Weigl et al; 200810).
2.2 Sample preparation module
For POC testing, ideally results should be obtained and reported to the individual at the initial visit. However, time-consuming sample preparation techniques are considered the rate-limiting step for NAATs.27 Electricity-dependent sample preparation is also a barrier to effective use of most NAATs in LRS where electricity may not be reliable. Several techniques have emerged that can enable instrument-free sample preparation. These including the use of proviral DNA capture cards (PDCCs), FTA cards, and simplified purification techniques. Additionally, several isothermal methods, such as loop mediated isothermal amplification (LAMP) are unique in their ability to amplify directly from clinical specimens,18,19,28,29,30 exhibiting less sensitivity to inhibitory compounds than PCR.31
2.3 PDCC for infant human immunodeficiency virus (HIV) testing
HIV disease progresses rapidly in undiagnosed infants with over 50 percent mortality rate in the first two years of life. Therefore, determining HIV status and initiating treatment in the first months of life could lower the mortality rate. However, infant HIV diagnosis by standard immunoassay methods is complicated by the persistence of maternal antibodies in infants for 12 to 18 months after birth. Commercially available infant HIV tests nucleic acid amplification tests are complex, expensive, and typically operated from central laboratories. Many countries are now utilizing dried blood spots (DBS) to facilitate collection and transport of specimens from remote areas to central laboratories.32,33,34 Yet, the handling of DBS in the laboratory is cumbersome and labor-intensive or expensive if large automated systems are purchased to perform testing. The overall time taken to ship, test and report back the specimens test results plays a very important role in preventing loss to follow up of HIV-1 infected children; longer turn-around times increase the chance that infected infant swill not receive treatment.35,36,37 Thus, there is an urgent need to develop better tools for HIV testing in infants at or closer to the POC.
To address this need for improved diagnosis of HIV in infants, PATH is developing the PDCC, a product which may simplify sample collection and reduce the labor required by laboratory personnel to perform the HIV test algorithm. The principle of the PDCC is to capture the peripheral blood mononuclear cells (PBMC) population (including HIV-1 infected T lymphocytes containing HIV-1 proviral DNA) from a drop of whole blood on a porous membrane while the red blood cells are washed away via a simple wash step as shown in Figure 5. The membrane is then dried to stabilize the genetic material during shipping. At the test laboratory, technicians use forceps to peel away the membrane where the PMMC’s are captured and place it directly in the PCR reaction. No further processing of the PDCC is required prior to the PCR amplification step. The only equipment required to set up reactions are sterile forceps. The PDCC may be deployed as a stand-alone product to support existing, centralized laboratory testing systems. Additionally, the PDCC could be deployed in concert with POC DNA amplification and detection technologies to facilitate LRS infant HIV testing. Development of the PDCC is currently on-going.38 Preliminary data suggests that the PDCC technology has similar performance to DBS but at with potentially lower cost and with reduced complexity. The PDCC maintains the fundamental value provided by DBS (ease of collection, inactivation of the viral particles and stability of genetic material during transport from remote settings) while reducing the costs and turnaround time of performing the test in the laboratory. A similar technology, filtration isolation of nucleic acids, (FINA) has already been developed, and demonstrates this approach to be feasible.39
Figure 5.
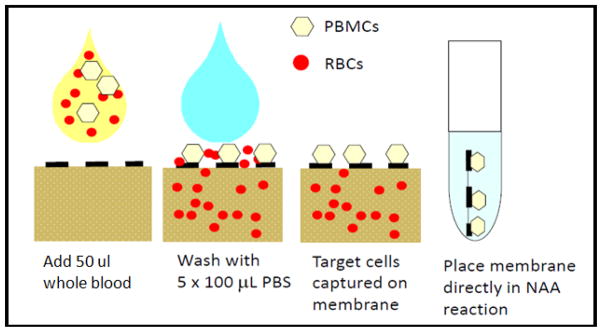
A cartoon demonstrating the principles of the PDCC.38
2.4 FTA cards
“Fast Technology for Analysis,” a technology better known by its acronym moniker, FTA card, and also sometimes referenced as “Flinders Technology Associates” was originally developed by Dr. Leigh Burgoyne as a convenient medium that would preserve nucleic acids and facilitate fast analysis of multiple samples using a robot. The FTA (available as the FTA® membrane; Whatman, United Kingdom) card is a chemically-treated filter paper that can lyse cells, denature proteins, and protect nucleic acids from nucleases, oxidative, and ultra violet (UV) damage allowing the rapid isolation and partial purification of nucleic acids. When samples are applied to the FTA card, cell lysis occurs and nucleic acids are immobilized within the matrix. The nucleic acids captured by the membrane are used directly as templates for amplification without elution, thus simplifying the assay flow. When using whole blood, the washing of FTA membranes enables the removal of heme and other potent inhibitors that can dramatically improve the sensitivity of test reactions.
The use of the FTA card requires minimal training but involves several processing steps. The specimen is blotted and then dried onto the FTA paper. The blood spots can then be stored at room temperature until DNA extraction. The blood spot is cut out with a punch and washed three times with FTA purification reagent (Whatman, United Kingdom). The washed piece of FTA card can be directly added to an amplification reaction.21,40 Therefore, FTA cards can provide a relatively fast, easy, and instrument-free method to prepare DNA from whole blood specimens for PCR and isothermal amplification.21 Furthermore, FTA cards have been assessed for their DNA extraction potential. Despite lower analytical sensitivity than the gold standard Qiagen extraction kit, FTA cards may also be considered as a potentially valuable alternative viral extraction technique when more traditional techniques are not possible due to the demands of the POC testing.41 To date, the only isothermal method that has been demonstrated with FTA preparation is loop mediated isothermal amplification (LAMP).21,42
2.5 Simplified extraction techniques
PATH and other groups such as Catherine Klapperich’s group at Boston University are developing technologies that enable nucleic acid purification at the POC and remove the requirement for cold chain preservation that is the current HIV-1 viral load testing paradigm.43,44 Such technologies would address the bottleneck of RNA purification from clinical specimens and stabilization of viral RNA in peripheral laboratories.45 The goal of this work is to compliment emerging low-cost, low-complexity NAATs and remove the stringent requirement of cold chain in centralized viral load testing systems.
2.6 Purification-free chemistries
Several researchers have been successful in minimizing sample preparation through novel assay chemistries. Recent studies from the CDC16 have demonstrated a LAMP assay formulation where all of the components required for amplification and detection of HIV-1 in whole blood can be added to the reaction tube simultaneously, with the exception of the fluorescence quenching reagent. Using a combination of lysis buffer added directly to the reverse transcriptase-LAMP (RT-LAMP) reaction tube, researchers demonstrated a simplified, purification-free and thermal-lysis-free workflow. The RT-LAMP procedure enables the direct detection of HIV-1 nucleic acids from plasma, serum and whole blood samples, eliminating the need for an additional purification step and reducing the overall procedure time to approximately 90 minutes.16,18
DNA amplification has also been performed successfully using urine and plasma without any processing steps. Even though unprocessed, freshly-voided urine is considered to contain PCR inhibitors, Bista et al. achieved successful amplification results with BK virus LAMP assays. Some loss of sensitivity was noted with the use of unprocessed samples, however, with further optimization, the purification-free assay may be relevant in clinical situations.46
2.7 Isothermal strategies anchor the amplification module
There are a number of methods for detecting clinically-relevant nucleic acids that do not involve cycling the sample temperature as is necessary for PCR. The obvious advantage of these methods over PCR is that they are isothermal and therefore may be more amenable to non-instrumented POC diagnostics. The various isothermal approaches have been recently reviewed (Table 1). Several of the methods discussed below are currently being used in US Food and Drug Administration (USFDA)-cleared or USFDA-approved diagnostic tests. Other methods are at earlier stages of development, but are promising nevertheless. Most of these methods use DNA polymerases and/or reverse transcriptases to amplify nucleic acids which are then detected directly.
Table 1.
Current isothermal nucleic acid amplification methods (reproduced from Neimz et al.; 2011)47
| Assay | Reaction temperature (°C)a | Reaction duration (min)a | Multiplexb | Rapid detection formatsc | Target | Amplification product |
|---|---|---|---|---|---|---|
| Methods based on RNA transcription | ||||||
| NASBA | 41d | 105 | Y | RTF, NALF | RNA (DNA) | RNA, DNA |
| TMA | 60d | 140 | Y | RTF | RNA(DNA) | RNA, DNA |
| SMART | 41d | 180 | N/A | RTF | RNA, DNA | RNA |
| Methods based on DNA replication with enzymatic duplex melting/primer annealing | ||||||
| HDA | 65 | 75–90 | Y | RTF, NALF | DNAe | DNA |
| RPA | 30–42 | 20 | Y | RTF, NALF | DNAe | DNA |
| Methods based on DNA-polymerase-mediated strand displacement from linear or circular targets | ||||||
| LAMP | 60–65d | 60–90 | N/A | RTF, NALF, RTT, TE | DNAe | DNA |
| CPA | 65 | 60 | N/A | RTF, NALF | DNA | DNA |
| SMART-AMP | 60 | 45 | N/A | RTF | DNe | DNA |
| RCA | 65 | 60 | N/A | RTF | DNAe | DNA |
| RAM | 63d | 120–180 | N/A | RTF | DNAe | DNA |
| Methods based on polymerase extension/strand displacement, plus a single strand cutting event | ||||||
| SDA | 37 | 120 | Y | RTF, NALF | DNAe | DNA |
| NEAR | 55 | 10 | Y | RTF, NALF | DNAe | DNA |
| NEMA | 65 | 30 | N/A | NALF | DNA | DNA |
| ICA | 60 | 60 | N/A | RTF | DNA | DNA |
| EXPAR | 55 | 10–20 | Y | RTF, NALF | DNA | DNA |
| BAD AMP | 40 | 40 | N/A | RTF | DNA | DNA |
| PG-RCA | 60 | 60–120 | N/A | RTF | DNA | DNA |
Key:
Typical incubation temperature and reaction time, variability might exist.
Capability to multiplex, defined as the ability to amplify simultaneously at least two different targets (or one target and an internal control): yes (Y) or data not available (N/A).
Most commonly reported formats only. Other formats might exist, but are not included here.
Initial incubation at a higher temperature is sometimes recommended.
RNA can be the target if a reverse transcriptase step to generate cDNA is first included.
Abbreviations: RTF, real-time fluorescence; RTT, real-time turbidity; TE, turbidity-related endpoint, including fluorescence and colorimetric enhancement.
Nucleic acid sequence-based amplification (NASBA), transcription-mediated amplification (TMA), LAMP, and helicase dependent amplification (HDA) are used in a number of commercially-available tests. These proven technologies may be good candidates for a non-instrumented POC device. NASBA and TMA generally require a short (2 to10 minute) melting step at high temperature (65°C to 95°C) followed by a longer (30 to 90 minute) incubation at their extension temperature (40°C to 65°C). HDA avoids the initial melting step by using heat stable helicase to unwind the double stranded DNA.48,49 This allows the reaction to be performed without an initial melting step but incurs a reduction in sensitivity of approximately one log as compared to the same test conducted with an initial melting step. Another particularly promising, but less-proven, technology is LAMP. While LAMP has also been demonstrated without the high temperature incubation,50 it appears to be less susceptible to inhibitors and has been demonstrated with FTA card preparation.
2.8 Instrument-free heat
Adoption of isothermal amplification strategies enable replacement of the complex, closed loop, thermo-cycling, electric module common to PCR heating with a simple, disposable chemical heating module that uses compact exothermic cartridges instead of electricity. PATH has been leading development of technologies that facilitate electricity-free, instrument-free amplification heating.
The development of the prototype NINA heating devices represents the first example of using an exothermic chemical reaction and engineered phase change material (EPCM) in a NAAT. In the NINA heater for LAMP, we used the exothermic reaction of calcium oxide (CaO, or quicklime) and water to generate the necessary heat:
To keep the isothermal device within the temperature band required for LAMP, the reaction chambers were surrounded with an engineered fat-based compound with a high specific heat capacity and specific melting range centered around 65°C. While several prototype heater designs have been explored, the optimized heater uses an off-the-shelf insulated food storage container to provide an insulated housing with two chambers. The bottom chamber contains the exothermic reaction, and the upper chamber contains the EPCM and reaction wells. To facilitate directed heat transfer to the reaction wells, an aluminum honeycomb material is added to the upper chamber prior to introduction of the EPCM. The machined reaction wells, sized to closely fit a standard 200 μL PCR tube, are embedded in the EPCM. Three reaction wells are used for most prototypes (one for a positive control, one for a negative control, and one for an unknown specimen). The existing prototype however, can easily be modified to accept up to 16 tubes without loss of performance. An inexpensive spring timer with an audible “ready” indicator was affixed to the lid of the heater unit for added electricity-free functionality. PATH has demonstrated the use of this configuration to stabilize a heat mixture within a narrow temperature range (65°C ± 0.5°C) suitable for LAMP.51,52,53,54 NINA heater prototypes were used to demonstrate proof of principle, amplifying genomic malaria DNA.
2.9 Non-instrument detection strategies
A number of sensitive detection methods have been employed with the amplification methods described above. Many of these real time detection schemes include measurement of reaction fluorescence via intercalating dyes or cleavage/hybridization of fluorescent probes. These require relatively complex optical instrumentation. There are however, a few non-instrumented endpoint detection options that provide simpler outputs for qualitative interpretation. These instrument-free options such as a combined turbidity and fluorescence shown in Figure 8a, naked eye-detectable dyes shown in Figure 8b and nucleic acid lateral flow (NALF) shown in Figure 8c may be valuable to an integrated POC NAAT for LRS.
Figure 8a, 8b, 8c.
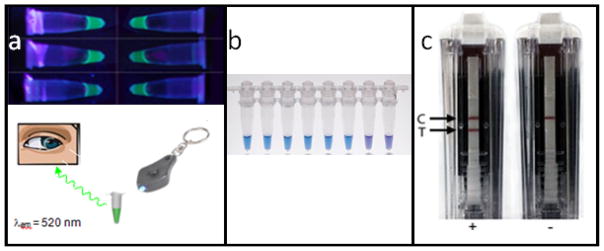
Non-instrumented detection strategies. 8a. Combined turbidity and fluorescence (top) with optional (minimally instrumented) LED keychain excitation source (bottom). 8b. Naked eye-detectable HNB dye: visualization on a light box. The color changes from violet (negative reaction) to sky blue (positive reaction).72 8c. NALF showing the control and test lines of a laminar flow strip contained in a BESt cassette.73
Turbidity via the precipitation of manganese pyrophosphate precipitation has been demonstrated for visual endpoint detection of LAMP reactions55 although our experience indicates that endpoint detection with turbidity alone is user-subjective. Through inclusion of a dye, such as calcein, turbidity can be enhanced via fluorescence allowing simple and accurate naked-eye determinations of reaction results.17 The addition of a small keychain LED excitation source further improves endpoint recognition. Precipitates of different colors can be obtained through the addition of cationic polymers in conjunction with fluorescently labeled primers and probes.56 Colorimetric detection can be achieved through the addition of hydroxyl napthol blue (HNB).57,58,59
NALF offers another endpoint detection option for instrument-free NAATs. 60,61,62,63 The advantage of NALF over most molecular diagnostic readouts is that results can be visually interpreted by untrained operators with no additional instrumentation. Unlike antigen/antibody ICS tests, the release of amplification products into the test environment can cause extensive contamination problem. To mitigate this Ustar (China) have developed an enclosed NALF detection cassette for the interrogation of amplification results with cross-priming amplification (CPA) and in addition HDA and LAMP have been demonstrated with this same technology.2 NALF tests have yet to have significant impact at the POC due to the relatively few commercial options for and high cost of containment solutions. The BESt Cassette which detects labeled amplicons via antibody-dependent NALF, is designed with detection-integrated containment to minimize the risk of carryover contamination.64 NALF is amenable to low-level multiplexing and semi-quantitative readout, and has been coupled with PCR,65,66 NASBA,67 HDA,68,69 recombinase-polymerase amplification (RPA),70 LAMP,63 and CPA.71
3. DISCUSSION
3.1 Cold chain considerations
Many NAAT diagnostics require a reliable cold chain for reagent storage—another barrier to adoption in LRS. The gold standard for NAAT reagent shipping and storage is still considered to be −20°C. Thus, many diagnostic test manufacturers are developing dried-down formulations of their assays to improve stability. There are several technologies available for stabilizing reagents and eliminating the need for reagent cold storage and transportation, potentially saving on large capital investments and enabling access in LRS. These include lyophilization17 and glassification.74 For easy adoption of NAATs in the developing world, assay components must be stable when stored for extended periods outside the cold chain at extreme ambient conditions such as those encountered in sub-Saharan Africa. A poorly functioning cold chain may result in autolysis and sample damage.75 Through development of an innovative NAAT platform that eliminates the need for cold chain, assay kits can be shipped at ambient temperatures and used at the POC with far less expense and infrastructure investment.
3.2 Potential applications
There are many infectious diseases that are endemic in LRS where the lack of simple, instrument-free, NAATs is a critical barrier to timely diagnosis and treatment. These diseases include malaria, HIV, tuberculosis, influenza, and others.1 Millions of lives and disability adjusted life years are lost through inadequate treatment and diagnosis of these diseases.1 In many cases the need for rapid diagnostics appropriate for these LRS is so severe that mediocre performance tests are preferred to less accessible better performing tests.76 The logistics of operating and maintaining complex instrumentation, sample collection, preparation, and testing prevent the timely reporting of test results back to individuals at the POC, thus preventing or delaying follow-up treatment.
Extension of a non-instrumented NAAT platform to multiple infectious diseases in LRS may represent a clinical advance for POC diagnostics similar to the advent of the first rapid immunodiagnostic strip assays a generation ago. Other potential applications of this instrument-free nucleic acid amplification platform may include rapid field identification of bioterror or biowarfare agents in emergency scenarios, outbreak management following natural disasters, forward- deployed military medical kits, rapid field identification and differentiation of Anopheles mosquitos in LRS to increase understanding and control of this malaria vector,77 and detection of other dangerous protozoans in drinking water.78
3.3 Integrating the modules
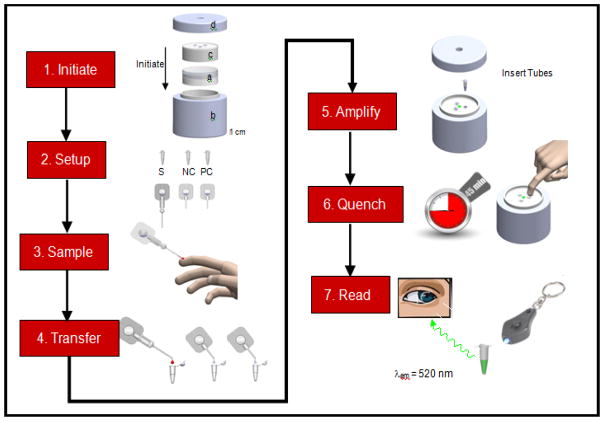
The current paradigm in NAATs, involves discrete steps for sample preparation, NA amplification, and amplicon detection -each with its own reagents, labware, and manual manipulation. As a result, the operator must be highly trained. Even when training is adequate, the laborious process creates potential for cross-contamination of reactions due to multiple re-entries into the reaction vessel. Integrating sample preparation with nucleic acid amplification and detection in a cost-effective, robust, LRS-appropriate and user-friendly format remains challenging. However, combining non-instrumented modules for sample handling, amplification, and detection in an easy to use disposable package can greatly simplify use in LRS. PATH is working to advance multiple technologies within these modules so that a simplified workflow such as that illustrated in Figure 9 may soon be possible.
Figure 9.
The workflow of a conceptual instrument-free, electricity-free, whole-blood compatible, temperature stable, contamination-containing NAAT. 1. Initiate the NINA heater by installing a heater cartridge (a) into insulated housing, (b) add EPCM module, (c) and lid (d). 2. Set up for assay by opening single assay subkit. 3. Sample blood to calibrated line on collection capillary. 4. Transfer blood and blister contents to “S” tube and prefilled diluent to “NC” and “PC” tubes and mix all. 5. Amplify. Verify temperature ready indication on the NINA device through transparent view port in the lid, remove the lid, add the three tubes to the NINA heater, and replace lid. Incubate 45 minutes. Verify temperature is still in range through transparent view port (process control). 6. Quench to all three tubes by pushing cap to burst frangible seal and transfer ~10 μL diluted quencher to the amplified mixture. Mix. 7. Read each result immediately via naked eye (non-instrumented) or by illuminating at an excitation wavelength with an LED penlight (minimally instrumented).
Note: In this conceptual NINA kit for HIV-1 detection, we envision 1 reusable NINA heater, 100 assays, 100 rechargeable CaO modules, and 1 LED keychain. Each single assay subkit contains the following: 3 reaction tubes individually marked S, NC, and PC, (each tube contains a lyophilized mastermix containing a reporter [FAM-labeled primer] and the other reagents required for LAMP and whole-blood lysis, and the tube marked PC also contains purified ACH2 genomic DNA at a concentration corresponding to a very high viral load); a prefilled precise blood collection, dilution, and transfer device; two prefilled 100 μL diluent dispensers marked NC and PC; and one finger-prick device.
A non-instrumented NAAT platform has the potential to shift clinical practice paradigms. We envision shifting accurate diagnosis of the disease from centralized laboratories and closer to the POC in LRS by creating a platform that eliminates the major barriers to achieving this. Currently, most POC assays for infectious disease diagnostics lack the accuracy and performance of NAATs. By creating an innovative NAAT platform that eliminates the need for instrumentation, electrical power, and sample extraction, diagnostic testing with gold-standard sensitivity and specificity will become practical at the POC. Future non-instrumented platforms will improve diagnosis of infectious diseases in LRS, enhance treatment of diseases, reduce the costs associated with inappropriate treatment, prevent the spread of drug-resistant disease strains due to overuse of medicines, and diagnose additional patients in need of treatment.
Figure 7a, 7b, 7c.
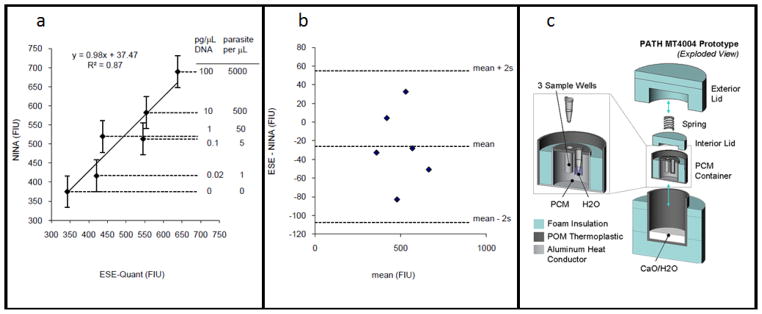
The NINA test bed. LAMP assays were performed for a dilution series of P. falciparum genomic DNA with amplification performed in both the NINA heater and a reference instrument (ESE-Quant Tube Scanner, set at 63°C) for the same amount of time. Fluorescence intensity of the Calcein dye was then read on the SpectraMax M2 plate reader with λex=485 nm and λem=515 nm. 7a. Linear regression analysis of the method comparison. The error bars represent ±2s using the best unbiased estimate for replicate noise available from the data set. 7b. Bland-Altman analysis of the same data. 7c. An exploded CAD drawing of the prototype device used to generate the results.
References
- 1.Burgess D, Wasserman J, Dahl C. Determining the global health impact of improved diagnostic technologies for the developing world. Nature (London) 2006;S1 [Google Scholar]
- 2.www.who.int/std_diagnostics
- 3.Glynou K, Ioannou PC, Christopoulos TK, et al. Analytical Chemistry. 2003;75:4155. doi: 10.1021/ac034256+. [DOI] [PubMed] [Google Scholar]
- 4.Seal J, Braven H, Wallace P. IVD. Technology Magazine. 2006;41 [Google Scholar]
- 5.Burgess D, Odugwu S, Matsyshen D, et al. Evaluation of a new rapid test for Gonorrhea in high risk populations in Johannesburg, Republic of South Africa. 2008 [Google Scholar]
- 6.Lee N, Baker J, Andrews KT, et al. Effect of sequence variation in Plasmodium falciparum histidine-rich protein 2 on binding of specific monoclonal antibodies: Implications for rapid diagnostic tests for malaria. Journal of Clinical Microbiology. 2006;44(8):2773–2778. doi: 10.1128/JCM.02557-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee N, Baker J, Bell D, et al. Assessing the genetic diversity of the aldolase genes of Plasmodium falciparum and Plasmodium vivax and its potential effect on performance of aldolase-detecting rapid diagnostic tests. Journal of Clinical Microbiology. 2006;44(12):4547–4549. doi: 10.1128/JCM.01611-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray CK, Gasser RA, Jr, Magill AJ, et al. Update on rapid diagnostic testing for malaria. Clinical Microbiology Reviews. 2008;21(1):97–110. doi: 10.1128/CMR.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moody A. Rapid diagnostic tests for malaria parasites. Clinical Microbiology Reviews. 2002;15(1):66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigl BH, Domingo GJ, LaBarre PD, et al. Towards non- and minimally instrumented, microfluidics-based diagnostic devices. Lab Chip. 2008;8(12):1999–2014. doi: 10.1039/b811314a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori Y, Notomi T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. Journal of Infection and Chemotherapy. 2009;15(2):62–69. doi: 10.1007/s10156-009-0669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andresen D, von Nickisch-Rosenegk M, Bier FF. Helicase-dependent amplification: use in OnChip amplification and potential for point-of-care diagnostics. Expert Review of Molecular Diagnostics. 2009;9(7):645–650. doi: 10.1586/erm.09.46. [DOI] [PubMed] [Google Scholar]
- 13.Wu WJ, Tang YW. Emerging molecular assays for detection and characterization of respiratory viruses. Clinics in Laboratory Medicine. 2009;29(4):673. doi: 10.1016/j.cll.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morisset D, Stebih D, Cankar K, et al. Alternative DNA amplification methods to PCR and their application in GMO detection: a review. European Food Research and Technology. 2008;227(5):1287–1297. [Google Scholar]
- 15.Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Research. 2000;28(12):E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis KA, Rudolph DL, Owen SM. Sequence-specific detection method for reverse transcription, loop-mediated isothermal amplification of HIV-1. J Med Virol. 2009;81(6):966–972. doi: 10.1002/jmv.21490. [DOI] [PubMed] [Google Scholar]
- 17.Boehme CC, Nabeta P, Henostroza G, et al. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. Journal of Clinical Microbiology. 2007;45(6):1936–1940. doi: 10.1128/JCM.02352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis KA, Rudolph DL, Owen SM. Rapid detection of HIV-1 by reverse-transcription, loop-mediated isothermal amplification (RT-LAMP) Journal of Virological Methods. 2008;151(2):264–270. doi: 10.1016/j.jviromet.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Han ET, Watanabe R, Sattabongkot J, et al. Detection of four Plasmodium species by genus- and species-specific loop-mediated isothermal amplification for clinical diagnosis. Journal of Clinical Microbiology. 2007;45(8):2521–2528. doi: 10.1128/JCM.02117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inacio J, Flores O, Spencer-Martins I. Efficient identification of clinically relevant Candida yeast species by use of an assay combining panfungal loop-mediated isothermal DNA amplification with hybridization to species-specific oligonucleotide probes. Journal of Clinical Microbiology. 2008;46(2):713–720. doi: 10.1128/JCM.00514-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuboki N, Sakurai T, Di Cello F, et al. Loop-mediated isothermal amplification for detection of African trypanosomes. Journal of Clinical Microbiology. 2003;41(12):5517–5524. doi: 10.1128/JCM.41.12.5517-5524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng PH, Chen CL, Sung PF, et al. Specific detection of reverse-transcription loop-mediated isothermal amplification amplicons for Taura syndrome virus by colorimetric dot-blot hybridization. Journal of Virological Methods. 2007;146(1–2):317–326. doi: 10.1016/j.jviromet.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Yeh HY, Shoemaker CA, Klesius PH. Evaluation of a loop-mediated isothermal amplification method for rapid detection of channel catfish Ictalurus punctatus important bacterial pathogen Edwardsiella ictaluri. Journal of Microbiological Methods. 2005;63(1):36–44. doi: 10.1016/j.mimet.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Poon LLM, Wong BWY, Ma EHT, et al. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clinical Chemistry. 2006;52(2):303–306. doi: 10.1373/clinchem.2005.057901. [DOI] [PubMed] [Google Scholar]
- 25.Safe-Tec Clinical Products website. Microsafe page. http://www.safe-tecinc.com/microsafe.htm.
- 26.Poly-Pipets Incorporated website. http://www.polypipets.com/pagetwo.html.
- 27.Dineva MA, MahiLum-Tapay L, Lee H. Sample preparation: a challenge in the development of point-of-care nucleic acid-based assays for resource-limited settings. Analyst. 2007;132(12):1193–1199. doi: 10.1039/b705672a. [DOI] [PubMed] [Google Scholar]
- 28.Ihira M, Akimoto S, Miyake F, et al. Direct detection of human herpes virus 6 DNA in serum by the loop-mediated isothermal amplification method. Journal of Clinical Virology. 2007;39(1):22–26. doi: 10.1016/j.jcv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Njiru ZK, Mikosza AS, Armstrong T, et al. Loop-mediated isothermal amplification (LAMP) method for rapid detection of Trypanosoma brucei rhodesiense, PLoS Neglected Tropical Diseases. 2008;2(1):e147. doi: 10.1371/journal.pntd.0000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paris DH, Imwong M, Faiz AM, et al. Loop-mediated isothermal PCR (LAMP) for the diagnosis of falciparum malaria. The American Journal of Tropical Medicine and Hygiene. 2007;77(5):972–976. [PubMed] [Google Scholar]
- 31.Kaneko H, Kawana T, Fukushima E, et al. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. Journal of Biochemical and Biophysical Methods. 2007;70(3):499–501. doi: 10.1016/j.jbbm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Creek T, Tanuri A, Smith M, et al. Early diagnosis of human immunodeficiency virus in infants using polymerase chain reaction on dried blood spots in Botswana’s national program for prevention of mother-to-child transmission. Pediatr Infect Dis J. 2008;27(1):22–26. doi: 10.1097/INF.0b013e3181469050. [DOI] [PubMed] [Google Scholar]
- 33.Patton JC, Akkers E, Coovadia AH, et al. Evaluation of dried whole blood spots obtained by heel or finger stick as an alternative to venous blood for diagnosis of human immunodeficiency virus type 1 infection in vertically exposed infants in the routine diagnostic laboratory. Clin Vaccine Immunol. 2007;14(2):201–203. doi: 10.1128/CVI.00223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherman GG, Stevens G, Jones SA, et al. Dried blood spots improve access to HIV diagnosis and care for infants in low-resource settings. J Acquir Immune Defic Syndr. 2005;38(5):615–617. doi: 10.1097/01.qai.0000143604.71857.5d. [DOI] [PubMed] [Google Scholar]
- 35.Elbeik T, Delassandro R, Chen Y, et al. Global cost modeling analysis of HIV-1 and HCV viral load assays. Expert Reviews in Pharmacoeconomics Outcomes Res. 2003;3(4):383–407. doi: 10.1586/14737167.3.4.383. [DOI] [PubMed] [Google Scholar]
- 36.Elbeik T, Charlebois E, Nassos P, et al. Quantitative and cost comparison of ultrasensitive human immunodeficiency virus type 1 RNA viral load assays: Bayer bDNA quantiplex versions 3.0 and 2.0 and Roche PCR Amplicor monitor version 1.5. J Clin Microbiol. 2000;38(3):1113–1120. doi: 10.1128/jcm.38.3.1113-1120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elbeik T, Dalessandro R, Loftus R, et al. HIV-1 and HCV viral load cost models for bDNA: 440 Molecular System versus real-time PCR AmpliPrep/TaqMan test. Expert Reviews in Molecular Diagnostics. 2007;7(6):723–753. doi: 10.1586/14737159.7.6.723. [DOI] [PubMed] [Google Scholar]
- 38.Stevens D, Barfied C, Emery, et al. Simple, inexpensive pro-viral DNA preparation at the point-of-care. Oakridge. 2010 [Google Scholar]
- 39.Jangam S, Yamada D, McFall S, Kelso D. Rapid, point-of-care extraction of human immunodeficiency virus type 1 proviral DNA from whole blood for detection by real-time PCR. J Clin Microbiol. 2009 Aug;:2363–8. doi: 10.1128/JCM.r00092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.http://www.whatman.com/References/FTAforBloodDNA%20DataSheet%20FINAL%203.25.10%20LR.pdf
- 41.Brassard J, Lamoureux L, Gagne M, et al. Comparison of commercial viral genomic extraction kits for the molecular detection of foodborne viruses. Canadian Journal of Microbiology. 2009 Aug;55(8):1016–1019. doi: 10.1139/w09-054. [DOI] [PubMed] [Google Scholar]
- 42.Yamamura M, Makimuri K, Ota Y. Evaluation of new rapid molecular diagnostic system for plasmodium falciparum combined with DNA filter paper, loop mediated isothermal amplification, and melting curve analysis. Japanese Journal of Infectious Disease. 2009:20–25. [PubMed] [Google Scholar]
- 43.Beddoe A, Hubbard L, Stevens D, Gerlach J, De los Santos T, LaBarre P. Affordable point-of-care specimen processing: A viral RNA extraction and stabilization platform. Poster presented at: World Health Innovation and Technology Conference; November 2009; Washington, DC. [Google Scholar]
- 44.http://www.technologyreview.com/biomedicine/22980/
- 45.Puren A, Gerlach J, Weigl B, Kelso D, Domingo G. Laboratory operations, specimen processing, and handling for viral load testing and surveillance. J Infect Dis. 2010 Apr 15;201(Suppl 1):S27–36. doi: 10.1086/650390. Review. [DOI] [PubMed] [Google Scholar]
- 46.Bista B, Ishwad C, Wadowsky R. Development of a Loop-Mediated Isothermal Amplification Assay for Rapid Detection of BK Virus. Journal of Clinical Microbiology. 2007 May;:1581–1587. doi: 10.1128/JCM.01024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niemz A, Ferguson T, Boyle D. Point of care nucleic acid tests for infectious disease. Trends in Biotechnology. 2011 doi: 10.1016/j.tibtech.2011.01.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An L, Tang W, Ranalli TA, et al. Characterization of a Thermostable UvrD Helicase and Its Participation in Helicase-dependent Amplification. J Biol Chem. 2005;280(32):28952–8. doi: 10.1074/jbc.M503096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent M, Xu Y, Kong H. Helicase Dependent Isothermal Amplification. EMBO Reports. 2004;5:795–800. doi: 10.1038/sj.embor.7400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LaBarre P, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Weigl B. Poster Abstracts 41st Annual Oak Ridge Conference; April 16–17, 2009. [Google Scholar]
- 51.LaBarre P, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Weigl B. Poster Abstracts 41st Annual Oak Ridge Conference; April 16–17, 2009. [Google Scholar]
- 52.LaBarre P, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Weigl B. Non-instrumented nucleic acid amplification (NINA): Instrument-free molecular malaria diagnostics for low-resource settings. Proc. 32nd Annu. Int. Conf. Institute of Electrical and Electronics Engineers (IEEE) Engineering in Medicine and Biology Society (EMBS),; Aug. 2010; [DOI] [PubMed] [Google Scholar]
- 53.LaBarre P, Hawkins K, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Boyle D, Weigl B. A Simple, Inexpensive Device that Performs Nucleic-Acid Amplification without Electricity – Enabling Instrument-Free Molecular Diagnostics in Low Resource Settings. PLoS One. 2011 May;6:1–8. doi: 10.1371/journal.pone.0019738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaBarre P. Engineering Global Health. Institute of Electrical and Electronic Engineers (IEEE) Pulse. 2011 Jan#x02013;feb;2(1):18–25. doi: 10.1109/MPUL.2010.939605. [DOI] [PubMed] [Google Scholar]
- 55.Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loopmediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun. 2001;289:150–4. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- 56.Mori Y, et al. Sequence specific visual detection of LAMP reactions by addition of cationic polymers. BMC Biotechnol. 2006;6:3. doi: 10.1186/1472-6750-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goto M, et al. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 2009;46:167–172. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- 58.Bearinger J, Dugan L, Baker B, Hall S, Ebert K, Mioulet V, Madi M, King D. Development and initial results of a low cost, disposable, point-of-care testing device for pathogen detection. IEEE Transactions on Biomedical Engineering. 2011 Mar;58(3):805. doi: 10.1109/TBME.2010.2089054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harper S, Ward L, Clover G. Development of LAMP and Real-Time PCR Methods for the Rapid Detection of Xylella fastidiosa for Quarantine and Field Applications. The American Phytopathological Society. 2010 Dec;100(12):1282–1288. doi: 10.1094/PHYTO-06-10-0168. [DOI] [PubMed] [Google Scholar]
- 60.Jaroenrama W, Kiatpathomchai W, Flegel T. Rapid and sensitive detection of white spot syndrome virus by loop-mediated isothermal amplification combined with a lateral flow dipstick. Molecular and Cellular Probes. 2009;23:65–70. doi: 10.1016/j.mcp.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Kiatpathomchaia W, Jaroenram W, Arunruta N, Jitrapakdeec S, Flegel T. Shrimp Taura syndrome virus detection by reverse transcription loop-mediated isothermal amplification combined with a lateral flow dipstick. Journal of Virological Methods. 2008;153:214–217. doi: 10.1016/j.jviromet.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 62.Nimitphaka T, Kiatpathomchaia W, Flegel T. Shrimp hepatopancreatic parvovirus detection by combining loop-mediated isothermal amplification with a lateral flow dipstick. Journal of Virological Methods. 2008;154:56–60. doi: 10.1016/j.jviromet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 63.Puthawiboola T, Senapina S, Kiatpathomchaia W, Flegel T. Detection of shrimp infectious myonecrosis virus by reverse transcription loop-mediated isothermal amplification combined with a lateral flow dipstick. Journal of Virological Methods. 2009;156:27–31. doi: 10.1016/j.jviromet.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Goldmeyer J, et al. Identification of Staphylococcus aureus and determination of methicillin resistance directly from positive blood cultures by isothermal amplification and a disposable detection device. J Clin Microbiol. 2008;46:1534–1536. doi: 10.1128/JCM.02234-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dineva M, et al. Simultaneous visual detection of multiple viral amplicons by dipstick assay. J Clin Microbiol. 2005;43:4015–4021. doi: 10.1128/JCM.43.8.4015-4021.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mens P, et al. Molecular diagnosis of malaria in the field: development of a novel 1-step nucleic acid lateral flow Immunoassay for the detection of all 4 human Plasmodium spp. and its evaluation in Mbita, Kenya. Diagn Microbiol Infect Dis. 2008;61:421–427. doi: 10.1016/j.diagmicrobio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 67.Mugasa C, et al. Nucleic acid sequence-based amplification with oligochromatography for detection of Trypanosoma brucei in clinical samples. J Clin Microbiol. 2009;47:630–635. doi: 10.1128/JCM.01430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chow W, McCloskey C, Tong Y, Hu L, You Q, Kelly C, Kong H, Tang Y, Tang W. Application of Isothermal Helicase-Dependent Amplification with a Disposable Detection Device in a Simple Sensitive Stool Test for Toxigenic Clostridium difficile. Journal of Molecular Diagnostics. 2008;10(5) doi: 10.2353/jmoldx.2008.080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldmeyer J, et al. Identification of Staphylococcus aureus and determination of methicillin resistance directly from positive blood cultures by isothermal amplification and a disposable detection device. J Clin Microbiol. 2008;46:1534–1536. doi: 10.1128/JCM.02234-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Piepenburg O, et al. DNA detection using recombination proteins. PLoS Biol. 2006;4:e204. doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang R, et al. Cross-priming amplification for rapid detection of Mycobacterium tuberculosis in sputum specimens. J Clin Microbiol. 2009;47:845–847. doi: 10.1128/JCM.01528-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goto M, et al. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 2009;46:167–172. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- 73.2010 May 1; http://www.biohelix.com/products/IsoAmp_Cdif_Kit.asp.
- 74.2010 May 1; https://www.who.int/medical_devices/poster_a10.pdf.
- 75.Chung P. Global Influenza Program, World Hlth Org. Expert consultation on diagnosis of H5N1 avian influenza infections in humans. Influenza and Other Respiratory Viruses. 2007;1(4):131–138. doi: 10.1111/j.1750-2659.2007.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rafael M, Taylor T, Magill A, Lim Y, Girosi F, Allan R. Reducing the burden of childhood malaria in Africa: the role of improved diagnostics. Nature. 2006;444(Suppl 1):39–48. 17159893. doi: 10.1038/nature05445. [DOI] [PubMed] [Google Scholar]
- 77.Bonizzoni M, Afrane Y, Yan G. Loop-mediated isothermal amplification (LAMP) for rapid identification of Anopheles gambiae and Anopheles arabiensis Mosquitoes. American Journal of Tropical Medicine and Hygiene. 2009;81(6):1030–1034. doi: 10.4269/ajtmh.2009.09-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Plutzer J, Torokne A, Karanis P. Combination of ARAD microfibre filtration and LAMP methodology for simple, rapid and cost-effective detection of human pathogenic Giardia duodenalis and Cryptosporidium spp. in drinking water. Letters in Applied Microbiology. 2010;50(1):82–88. doi: 10.1111/j.1472-765X.2009.02758.x. [DOI] [PubMed] [Google Scholar]