Abstract
Background and Purpose
Patients with intracerebral hemorrhage (ICH) who present with a spot sign on CT angiography (CTA) are at increased risk of hematoma expansion and poor outcome. Since primary ICH is the acute manifestation of chronic cerebral small vessel disease, we investigated whether different clinical or imaging characteristics predict spot sign presence, using ICH location as a surrogate for arteriolosclerosis- and cerebral amyloid angiopathy-related ICH.
Methods
Patients with primary ICH and available CTA at presentation were included. Predictors of spot sign were assessed using uni- and multivariable regression, stratified by ICH location.
Results
741 patients were eligible, 335 (45%) deep and 406 (55%) lobar ICH. At least one spot sign was present in 76 (23%) deep and 102 (25%) lobar ICH patients. In multivariable regression, warfarin (OR 2.42, 95% CI 1.01–5.71; p=0.04), baseline ICH volume (OR 1.20, 95% CI 1.09–1.33, per 10 mL increase; p<0.001), and time from symptom onset to CTA (OR 0.89, 95% CI 0.80–0.96, per hour; p=0.009) were associated with the spot sign in deep ICH. Predictors of spot sign in lobar ICH were warfarin (OR 3.95, 95% CI 1.87–8.51; p<0.001) and baseline ICH volume (OR 1.20, 95% CI 1.10–1.31, per 10 mL increase; p<0.001).
Conclusions
The most potent associations with spot sign are shared between deep and lobar ICH, suggesting that the acute bleeding process that arises in the setting of different chronic small vessel diseases shares commonalities.
Keywords: intracerebral hemorrhage, CT angiography spot sign, cerebral small vessel disease, hypertension, cerebral amyloid angiopathy
Introduction
Primary intracerebral hemorrhage (ICH)occurs as the acute manifestation of chronic small vessel disease. The most common pathological findings are cerebral amyloid angiopathy (CAA) for lobar ICH, and arteriolosclerosis, for non-lobar or deep ICH.1 Although deep and lobar ICH share several risk factors, these conditions are generally associated with different chronic small vessel diseases.1
The CT angiography (CTA) spot sign is a strong predictor of hematoma expansion and clinical outcome.2 Several risk factors for the spot sign have been identified, including larger initial hematoma volume, anticoagulation, early presentation, and APOE ε2 allele suggesting that underlying features of the brain or the blood vessels may impact its development.3 Of note, APOE ε2 is associated with spot sign in lobar and not deep ICH.4 However, whether there are other factors that influence the spot sign in a location-specific manner is not known. We therefore investigated whether clinical or imaging characteristics are associated with spot sign in a location-specific manner, using ICH location as a surrogate for arteriolosclerosis- and CAA-related ICH.
Methods
This is a retrospective analysis of an ongoing cohort study.5 This study was approved by the institutional review board, and written informed consent was obtained from all participants or their next of kin, or consent was waived by a protocol-specific allowance. Inclusion criteria comprised primary supratentorial ICH and CTA performed within 72 hours of symptom onset. Exclusion criteria were infratentorial ICH, multiple hemorrhages, primary intraventricular hemorrhage (IVH), and secondary causes of ICH.
ICH location was determined on admission CT according to published methods.4 Hemorrhages involving both lobar and deep regions (n=5) were excluded. CTAs were reviewed by two independent, blinded readers.6 Hematoma volumes were measured using Analyze 10.0 (Mayo Clinic, Rochester, MN) software.6 Hematoma expansion, defined as absolute growth >6 mL or a relative increase >33% as compared to the baseline CT was evaluated in subjects with an available follow-up CT.7
We performed univariable and multivariable regression, stratified by ICH location. Covariates with p<0.05 in univariable analyses were entered into the model and backward eliminated to p<0.2. Collinearity was assessed through the variance inflation factor. Statistical analyses were performed using R version 3.0.1. (R Foundation for Statistical Computing) with significance threshold p<0.05.
Results
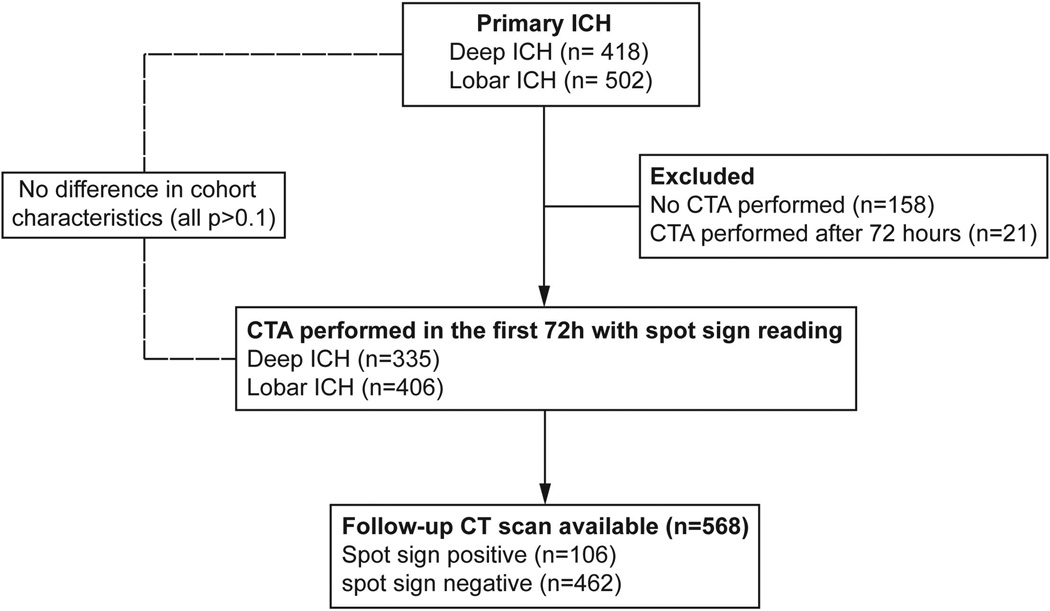
Of the initial 920 patients, 741 were included in final analysis (Figure 1). Cohort characteristics are presented in Supplemental Table I. Subjects excluded because of lack of CTA, or CTA performed after 72 hours (19%) had similar characteristics to included individuals (Supplemental Table II). At least one spot sign was observed in 178 (24%) patients; 76 (23%) in deep and 102 (25%) in lobar ICH (p=0.49).
Figure 1.
Cohort Flowchart
Spot sign in deep ICH
Predictors of spot sign in deep ICH identified through univariable analysis were male sex, atrial fibrillation, antiplatelet therapy, warfarin, international normalized ratio (INR), IVH, larger baseline ICH volume, and shorter time to CTA (Supplemental Table III). In multivariable analysis, warfarin (odds ratio [OR] 2.42; p=0.04), larger baseline ICH volume (OR 1.20, per 10 mL increase; p<0.001), and time to CTA (OR 0.89, per hour; p=0.009) remained associated with spot sign (Table 1).
Table 1.
Multivariable regression analysis of spot sign in deep ICH
| Covariate | OR [95% CI] | p |
|---|---|---|
| Male sex | 1.40 [0.71–2.81] | 0.33 |
| Antiplatelet | 1.51 [0.78–2.94] | 0.21 |
| Warfarin | 2.42 [1.01–5.71] | 0.04 |
| Time to CTA, per hour | 0.89 [0.80–0.96] | 0.009 |
| Intraventricular extension | 1.26 [0.63–2.53] | 0.51 |
| ICH volume, per 10 mL | 1.20 [1.09–1.33] | 0.0002 |
CTA, computed tomography angiography; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; mL, milliliter
Spot sign in lobar ICH
In univariable analysis, male sex, coronary artery disease, atrial fibrillation, antiplatelet therapy, warfarin, systolic blood pressure, INR, IVH, larger baseline ICH volume, and time to CTA were associated with spot sign presence (Supplemental Table III). After adjustment, an independent association with spot sign was found for warfarin (OR 3.95; p<0.001) and baseline ICH volume (OR 1.20, per 10 mL increase; p<0.001) (Table 2).
Table 2.
Multivariable regression analysis of spot sign in lobar ICH
| Covariate | OR [95% CI] | p |
|---|---|---|
| Male sex | 1.26 [0.68–2.33] | 0.45 |
| Coronary artery disease | 1.40 [0.61–3.14] | 0.42 |
| Antiplatelet | 1.47 [0.78–2.79] | 0.23 |
| Warfarin | 3.95 [1.87–8.51] | 0.0003 |
| Time to CTA, per hour | 0.97 [0.93–1.01] | 0.25 |
| Intraventricular extension | 1.30 [0.65–2.57] | 0.45 |
| ICH volume, per 10 mL | 1.20 [1.10–1.31] | 4.3×10−5 |
CTA, computed tomography angiography; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; mL, milliliter
Spot sign and hematoma expansion
Follow up CT were available in 568 (69%) subjects. As expected, spot sign was a strong predictor of hematoma expansion in both deep (OR 3.95; p<0.001) and lobar ICH (OR 6.80; p<0.001). After adjusting for age, sex, warfarin, and ICH volume, the spot sign remained associated with hematoma expansion in both deep (OR 3.20; p=0.002) and lobar ICH (OR 5.38; p<0.001).
Discussion
This study identifies risk factors for the presence of the spot sign in each of the two ICH subtypes: arteriolosclerosis- and CAA-related ICH. These results suggest that the underlying pathophysiology of the spot sign shares common features across different cerebral vasculopathies.
Anticoagulant therapy and baseline hematoma volume have been consistently associated with the spot sign,8 and this analysis confirmed these associations in both ICH subtypes. Patients with larger hematoma volumes are more likely to have prolonged bleeding, irrespective of the underlying mechanisms of vascular damage. The same holds true for anticoagulation, although the effect may be more pronounced in lobar ICH. The association of shorter time to CTA and spot sign only in deep ICH is likely reflective of more pronounced symptoms in these patients, as active bleeding and subsequent expansion lead to more rapid deterioration and consequently earlier presentation.
Our study is limited by the fact that as an observational study, not all patients underwent CTA, and by using one definition of the spot sign, and the limited ability to assess the clinical outcomes associated with hematoma expansion according to ICH location.
In conclusion, baseline ICH volume and anticoagulation treatment are shared risk factors between deep and lobar ICH, suggesting that spot sign occurring in ICH caused by different disease processes share common features.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by grants R01NS073344, R01NS059727, and 5K23NS059774 from the NIH–National Institute of Neurological Disorders and Stroke (NIH-NINDS) and, 0755984T from American Heart Association.
Disclosures
C.D.A received research grants from NIH-NINDS, American Brain Foundation, and Massachusetts General Hospital Institute for Heart, Vascular and Stroke Care. J.N.G. received a research grant from NIH. J.R. received research grants from the NIH, and is a consultant to Boehringer Ingelheim.
References
- 1.Martini SR, Flaherty ML, Brown WM, Haverbusch M, Comeau ME, Sauerbeck LR, et al. Risk factors for intracerebral hemorrhage differ according to hemorrhage location. Neurology. 2012;79:2275–2282. doi: 10.1212/WNL.0b013e318276896f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowlatshahi D, Wasserman JK, Momoli F, Petrcich W, Stotts G, Hogan M, et al. Evolution of computed tomography angiography spot sign is consistent with a site of active hemorrhage in acute intracerebral hemorrhage. Stroke. 2014;45:277–280. doi: 10.1161/STROKEAHA.113.003387. [DOI] [PubMed] [Google Scholar]
- 3.Brouwers HB, Goldstein JN, Romero JM, Rosand J. Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage: A review. Stroke. 2012;43:3427–3432. doi: 10.1161/STROKEAHA.112.664003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwers HB, Biffi A, McNamara KA, Ayres AM, Valant V, Schwab K, et al. Apolipoprotein E genotype is associated with CT angiography spot sign in lobar intracerebral hemorrhage. Stroke. 2012;43:2120–2125. doi: 10.1161/STROKEAHA.112.659094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genes for Cerebral Hemorrhage on Anticoagulation Collaborative G. Exploiting common genetic variation to make anticoagulation safer. Stroke. 2009;40:S64–S66. doi: 10.1161/STROKEAHA.108.533190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: The spot sign score. Stroke. 2009;40:2994–3000. doi: 10.1161/STROKEAHA.109.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): A prospective observational study. Lancet Neurol. 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JN, Fazen LE, Snider R, Schwab K, Greenberg SM, Smith EE, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.