Abstract
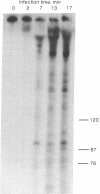
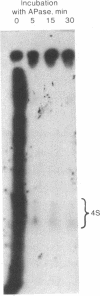
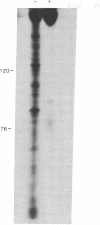
Searching for a physiological role of T4 RNA ligase [polyribonucleotide synthetase (ATP); poly(ribonucleotide):poly(ribonucleotide) ligase (AMP-forming), EC 6.5.1.3] activity, we developed an acellular system of plasmolyzed Escherichia coli cells infected by T4 bacteriophage. Upon incubation of this system with [gamma-32P]ATP, 32P was transferred into a large number of polyribonucleotides, mostly up to 300-400 residues long. The bulk of 32P in the product polyribonucleotides was found in 5'-terminal phosphate groups, suggesting that they originated by a phosphorylation reaction catalyzed by the endogenous polynucleotide kinase (EC 2.7.1.78). Indeed, these products were not seen in an acellular system from uninfected cells, and their amount and complexity increased with the progress of infection. Analysis of the 32P-labeled polyribonucleotide products by gel electrophoresis, either before or after digestion with alkaline phosphatase (EC 3.1.3.1), revealed that a small fraction of the 32P resided in phosphodiester bonds of several tRNA-sized chains. This specific 32P transfer from [gamma-32P]ATP into phosphodiester bonds was apparently catalyzed by successive polynucleotide kinase and RNA ligase reactions. The possible relationship of the 32P transfer to RNA ligase was investigated next by using a system from cells infected with T4 am M69 (an amber mutant deficient in RNA ligase). Transfer of 32P from [gamma-32P]ATP into phosphodiester bonds was not detected in the am M69 system. However, addition of purified RNA ligase to the am M69 system restored the specific 32P transfer. A system from cells infected with T4 psu-b delta 33 (a deletion mutant lacking the entire tRNA region) sustained the specific 32P transfer into tRNA-sized products, indicating that they were not derived from transcripts of T4 tRNA genes. These data may reflect a role of RNA ligase in posttranscriptional conversion of presumably host polyribonucleotides into novel tRNA species during T4 infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron V., Uhlenbeck O. C. 3'-Phosphatase activity in T4 polynucleotide kinase. Biochemistry. 1977 Nov 15;16(23):5120–5126. doi: 10.1021/bi00642a027. [DOI] [PubMed] [Google Scholar]
- Collinsworth W. L., Mathews C. K. Biochemistry of DNA-defective amber mutants of bacteriophage T4. IV. DNA synthesis in plasmolyzed cells. J Virol. 1974 Apr;13(4):908–915. doi: 10.1128/jvi.13.4.908-915.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew R. E., Cozzarelli N. R. Genetics and physiology of bacteriophage T4 3'-phosphatase: evidence for involvement of the enzyme in T4 DNA metabolism. J Virol. 1974 Apr;13(4):888–897. doi: 10.1128/jvi.13.4.888-897.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Kallenbach N. R. Determination of recognition sites of T4 RNA ligase on the 3'-OH and 5' -P termini of polyribonucleotide chains. Nature. 1975 Apr 3;254(5499):452–454. doi: 10.1038/254452a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Klein T., Littauer U. Z. T4 RNA ligase: substrate chain length requirements. FEBS Lett. 1974 Sep 15;46(1):271–275. doi: 10.1016/0014-5793(74)80385-6. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Littauer U. Z. Covalent joining of phenylalanine transfer ribonucleic acid half-molecules by T4 RNA ligase. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3741–3745. doi: 10.1073/pnas.71.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain W. H., Guthrie C., Barrell B. G. Eight transfer RNAs induced by infection of Escherichia coli with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3703–3707. doi: 10.1073/pnas.69.12.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Nishikawa S., Sugiura M., Ikehara M. Joining of ribooligonucleotides with T4 RNA ligase and identification of the oligonucleotide-adenylate intermediate. Nucleic Acids Res. 1976 Jun;3(6):1613–1623. doi: 10.1093/nar/3.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber R., Malathi V. G., Hurwitz J. Purification and properties of bacteriophage T4-induced RNA ligase. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3009–3013. doi: 10.1073/pnas.69.10.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin K., Cooley W., Runnels J., Snyder L. R. A role in true-late gene expression for the T4 bacteriophage 5' polynucleotide kinase 3' phosphatase. J Mol Biol. 1978 Aug 5;123(2):221–233. doi: 10.1016/0022-2836(78)90322-4. [DOI] [PubMed] [Google Scholar]
- Sninsky J. J., Last J. A., Gilham P. T. The use of terminal blocking groups for the specific joining of oligonucleotides in RNA ligase reactions containing equimolar concentrations of acceptor and donor molecules. Nucleic Acids Res. 1976 Nov;3(11):3157–3166. doi: 10.1093/nar/3.11.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snopek T. J., Sugino A., Agarwal K. L., Cozzarelli N. R. Catalysis of DNA joining by bacteriophage T4 RNA ligase. Biochem Biophys Res Commun. 1976 Jan 26;68(2):417–424. doi: 10.1016/0006-291x(76)91161-x. [DOI] [PubMed] [Google Scholar]
- Snopek T. J., Wood W. B., Conley M. P., Chen P., Cozzarelli N. R. Bacteriophage T4 RNA ligase is gene 63 product, the protein that promotes tail fiber attachment to the baseplate. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3355–3359. doi: 10.1073/pnas.74.8.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Snoper T. J., Cozzarelli N. R. Bacteriophage T4 RNA ligase. Reaction intermediates and interaction of substrates. J Biol Chem. 1977 Mar 10;252(5):1732–1738. [PubMed] [Google Scholar]
- Uhlenbeck O. C., Cameron V. Equimolar addition of oligoribonucleotides with T4 RNA ligase. Nucleic Acids Res. 1977 Jan;4(1):85–98. doi: 10.1093/nar/4.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C., Uhlenbeck O. C., Bedows E., Gumport R. I. T4-induced RNA ligase joins single-stranded oligoribonucleotides. Proc Natl Acad Sci U S A. 1975 Jan;72(1):122–126. doi: 10.1073/pnas.72.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Hurwitz J. DNA replication in Escherichia coli made permeable by treatment with high sucrose. Biochem Biophys Res Commun. 1972 Apr 14;47(1):202–211. doi: 10.1016/s0006-291x(72)80029-9. [DOI] [PubMed] [Google Scholar]
- Wilson J. H., Kim J. S., Abelson J. N. Bacteriophage T4 transfer RNA. 3. Clustering of the genes for the T4 transfer RNA's. J Mol Biol. 1972 Nov 28;71(3):547–556. doi: 10.1016/s0022-2836(72)80022-6. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Henninger M. Attachment of tail fibers in bacteriophage T4 assembly: some properties of the reaction in vitro and its genetic control. J Mol Biol. 1969 Feb 14;39(3):603–618. doi: 10.1016/0022-2836(69)90148-x. [DOI] [PubMed] [Google Scholar]
- Yudelevich A. Specific cleavage of an Escherichia coli leucine transfer RNA following bacteriophage T4 infection. J Mol Biol. 1971 Aug 28;60(1):21–29. doi: 10.1016/0022-2836(71)90444-x. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Kleppe K., Khorana H. G. Reversal of bacteriophage T4 induced polynucleotide kinase action. Biochemistry. 1973 Dec 4;12(25):5050–5055. doi: 10.1021/bi00749a004. [DOI] [PubMed] [Google Scholar]