Abstract
This case report demonstrates the feasibility of a single-lead peripheral nerve stimulation system for the treatment of pain secondary to chronic subacromial impingement syndrome. The participant was a 57-year-old man who experienced persistent pain from subacromial impingement syndrome for 20 months despite having undergone conservative therapy of steroid injection and physical therapy. After study enrollment, a single intramuscular lead was placed percutaneously into the deltoid muscle of the affected shoulder. He was treated 6 hours per day for 3 weeks and the lead was removed. The primary outcome measure was the Brief Pain Inventory (Short-Form) Question 3 (BPI 3), which queries the worst pain in the last week on a 0–10 numeric rating scale. At baseline, BPI 3 was an 8. At the end of treatment and at 4 and 12 weeks post treatment, BPI 3 scores were 2, 0, and 0, respectively. Substantial improvements in shoulder impairment, quality of life, and shoulder disability measures were also observed. Additional studies are needed to further demonstrate safety and efficacy, determine optimal dose, define optimal prescriptive parameters, expand clinical indications, and demonstrate long-term effect.
Keywords: subacromial impingement syndrome, shoulder pain, electrical stimulation
Introduction
Shoulder pain is a common problem in the adult population, with 1 year prevalence being up to 47%.1 The etiologies of shoulder pain vary depending on operational definitions and methodological approaches; however, up to half are due to subacromial impingement syndrome (SIS), the most common cause of shoulder pain.2 Anatomically, SIS impingement syndrome refers to the supraspinatus tendon impinging on the undersurface of the anterior acromion as the arm is raised overhead.3 Typically, pain is generated with elevation of the arm above the head though it can occur with rest. Multiple pathologies, such as subacromial bursitis, rotator cuff tendinopathy, partial rotator cuff tears, and even small tears can coexist to create the syndrome of subacromial impingement.5
Approximately half of the patients with SIS will respond to conservative management.6 When conservative treatment has failed and pain persists patients are often referred for surgical management through subacromial decompression.7 Unfortunately, randomized controlled trials have not shown surgical management of SIS to be better than conservative therapy.8, 9 This leaves a large number of people with refractory shoulder pain from SIS without options for effective treatment.
Percutaneous peripheral nerve stimulation (PNS) is a treatment that has shown success in those who have refractory hemiplegic shoulder pain (HSP).10, 11 Similarities exist between SIS and HSP. Studies have shown that up to half of those with HSP have a clinical syndrome consistent with SIS.12, 13 In a prior trial PNS in those with HSP it was found that subjects who had subluxation and experienced pain relief did not experience reduction in subluxation, nor did they experience improvements in range of motion or spasticity.14 These observations suggested that the effect of PNS therapy on shoulder pain in HSP may be through the central nervous system, such as central hypersensitivity.15 Evidence of central hypersensitivity has been found in SIS. These observations led us to believe that PNS may be appropriate for non-stroke patients with shoulder pain due to SIS. This is the first case to describe a patient treated with PNS for persistent shoulder pain due to SIS.
Case Presentation
Pre-intervention Clinical Course
The subject is a 57-year-old man who developed neck and left shoulder pain with radiation to his left arm as a result of a motor vehicle collision 20 months prior to enrollment. On initial presentation, the distribution the pain was described as being intermittent and variable in intensity, located in his left shoulder and left neck. Subjectively, pain was provoked with movement of his left arm and was relieved with rest. On physical examination he had tenderness to palpation of anterior and posterior shoulder. He had full range of motion of the shoulder with pain in abduction and flexion. He had full strength and reduced sensation to pin prick in his left thumb. On provocative testing he had a positive Hawkin’s sign16, positive empty can sign17, and negative Neer’s sign.4 He underwent x-ray imaging studies of his cervical spine and left shoulder, both of which were without acute or degenerative abnormality. No evidence of radiculopathy or plexopathy was found on electromyographic study of his left arm. With ultrasound examination, it was noted that he had tendinopathy of the midsubstance of the supraspinatus. He underwent an ultrasound guided subacromial injection of kenalog and lidocaine 16.5 months before enrollment. He experienced mild relief as a result of the injection but his left shoulder pain persisted and he was referred for physical therapy. He completed six visits of physical therapy and was discharged 12 months before enrollment with a home exercise program consisting of rotator cuff strengthening exercises and shoulder stretches. Five months before enrollment his pain worsened in spite of continued home exercise program and lack of trauma or other inciting event. On pre-procedure examination the subject exhibited no shoulder tenderness. He had full strength in internal rotation, external rotation, and abduction. On Neer’s impingement test4, he experienced a 6 out of 10 pain (0 no pain, 10 pain worst imaginable) that reduced to 0 of 10 with subacromial injection of 5 cc of 2% lidocaine. Baseline pain, pain interference, shoulder disability, and range of motion (ROM) are shown in Table 1.
Table 1.
Baseline, during treatment, and post-treatment outcome measures.
| Baseline | Start of Stimulation | week 1 | week 2 | week 3 | 1 week post | 4 weeks post | 12 weeks post | |
|---|---|---|---|---|---|---|---|---|
| BPI-3 | 8 | 7 | 6 | 3 | 2 | 1 | 0 | 0 |
| BPI-9 | 5.7 | 5.4 | 4 | 0.7 | 0 | 0.7 | 0 | 0 |
| DASH | 34.2 | 0.8 | 1.7 | 0 | 0.8 | |||
| Shoulder Flexion (degrees) | 129 | 155 | 170 | 165 | 180 | |||
| Shoulder Abduction (degrees) | 108 | 173 | 170 | 180 | 180 | |||
| Shoulder Ext Rotation (degrees) | 76 | 75 | 80 | 83 | 79 | |||
| PPT Affected Deltoid (kg/cm2) | 5.6 | 9.0 | 8.1 | 9.3 | 5.9 | 5.9 | ||
| PPT Contralateral Deltoid (kg/cm2) | 5.7 | 7.4 | 9.3 | 6.2 | 6.4 | 6.9 | ||
| PPT Contralateral Tibialis Anterior (kg/cm2) | 6.4 | 6.1 | 8.2 | 8.8 | 4.5 | 8.4 |
BPI-3: Worst pain in the last week, 0 (None) – 10 (Worst imaginable)
BPI-9: Average of the scores for the seven domains on a 0 (no interference)-10 (complete interference) with general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life during the last week
DASH: a measure of physical function and symptoms in people with musculoskeletal disorders of the upper limb ranging from 0 (no disability) to 100 (complete disability
Shoulder Flexion: Measured by handheld goniometer with patient standing
Shoulder Abduction: Measured by handheld goniometer with patient standing
Shoulder External Rotation: Measured by handheld goniometer with lying supine, starting position of hand on abdomen
PPT: Pressure-Pain Thresholds – The amount of pressure (kg/cm2) from a handheld algometer where a sensation of pressure first changes to pain. The average of 3 measurements at each location is reported.
Intervention Protocol
The intervention protocol was approved by the local institutional review board. The 4-month intervention protocol included lead implantation, 1-wk of lead stabilization, 3 weeks of PNS treatment, and 3 months of follow-up. The primary outcome was the Brief Pain Inventory Short-form Question 3 (BPI 3), which rates the “worst pain” in the prior week on a 0–10 numeric rating scale, where 0 indicates “no pain” and 10 indicates “pain as bad as you can imagine.” Secondary outcomes were: 1) Brief Pain Inventory Short-form Question 9 (BPI 9), a measure of pain interference with seven daily activities on a 0–10 numeric rating scale, where 0 indicates “does not interfere” and 10 indicates “completely interferes.” The BPI 9 score is the average of the scores for the seven domains; 2) the Disabilities of Shoulder, Arm, and Hand (DASH) questionnaire, a measure of physical function and symptoms in people with musculoskeletal disorders of the upper limb ranging from 0 (no disability) to 100 (complete disability); 3) the Patient Global Impression of Change (PGIC), a 7-point subjective measure of change in quality of life since the beginning of treatment, with responses ranging from “very much worse” to “very much improved”; 4) pain-free range of motion of the glenohumeral joint (internal rotation, external rotation, and abduction); and, 5) Pressure-pain threshold measurement (PPT) of the deltoid of the affected shoulder, contralateral shoulder, and contralateral tibialis anterior. The PPT is a measure of deep somatic tissue sensitivity, indicated by the amount of pressure (kg/cm2) from a handheld algometer where a sensation of pressure first changes to pain. The average of 3 measurements at each location is reported.
The skin overlying the deltoid muscle was cleaned with povidone-iodine topical antiseptic. Monopolar needle electrodes were inserted perpendicular to the skin surface at the motor points of the middle and posterior deltoids. Motor points were confirmed by stimulating each muscle separately and demonstrating strong contraction of the middle and posterior deltoids. A third needle electrode was placed at the midpoint between the two motor points to provide peripheral nerve stimulation to branches of the axillary nerve. The position and depth of the electrode and the pulse duration of the stimulation were iteratively adjusted until strong contraction of both heads was achieved.
A 20-gauge insulated introducer loaded with a percutaneous lead (Figure 1) was then inserted perpendicular to the skin surface to the depth and location indicated by the third needle electrode. The characteristics of the percutaneous lead have been previously described.18 The percutaneous lead, introducer and connecting cable were sourced from NDI Medical (Cleveland, OH). Stimulation was delivered to verify proper position. Pressure was maintained at the skin surface to anchor the electrode’s barb in the belly of the muscle and the introducer was withdrawn leaving the electrode in place. Stimulation was delivered again to ensure proper placement. A dry sterile dressing was placed over the lead and an occlusive dressing was applied. Prior to leaving the clinic, the subject was instructed on the proper care of the lead exit site. He returned 48 hours later for examination of the skin.
Figure 1.
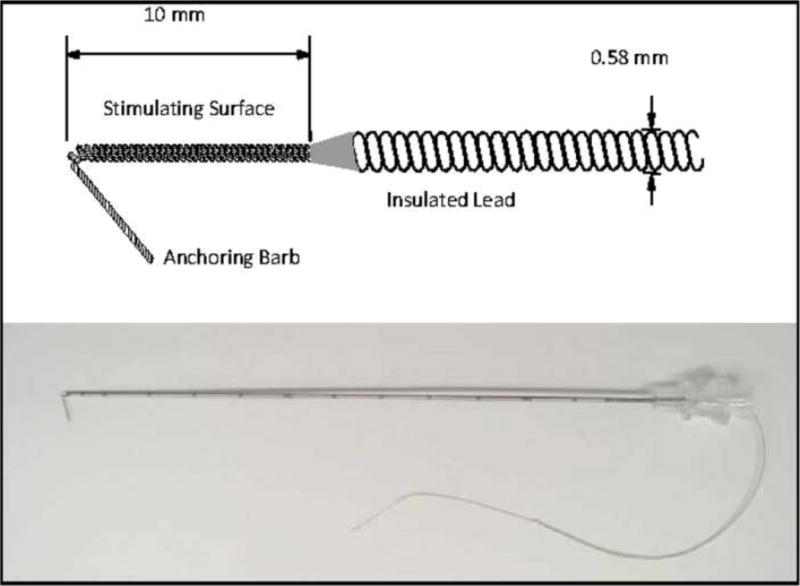
Schematic of percutaneous intramuscular lead (top) and actual lead loaded in the percutaneous insulated needle introducer (bottom).
Treatment parameters were based on those used in prior studies.10, 11 Following a one week lead stabilization period, the stimulator (Rehabilicare NT2000, Empi, Inc., St. Paul, MN) was connected to the lead and parameters were set to stimulate the middle and posterior deltoids at 12 Hz frequency and 20 mA amplitude with a pulse duration of 60 μs. The stimulation provided strong contraction of both deltoids. The subject was prescribed 6 hours of stimulation per day. The stimulator completed a cycle every 30 seconds consisting of 5 seconds ramp up, 10 seconds maximum stimulation, 5 seconds ramp down, and 10 seconds relaxation. During the 3-wk stimulation phase he was contacted by telephone weekly and queried for pain intensity, adverse events, and medication usage. The PNS system can be seen in Figure 2.
Figure 2.
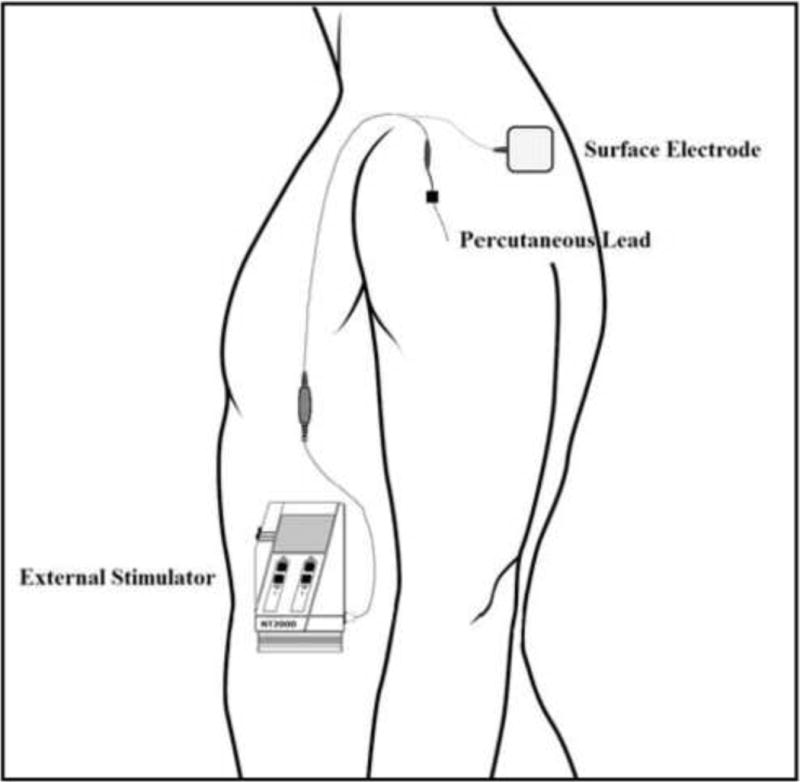
Schematic of the single-lead peripheral nerve stimulator system.
At the end of the 3-wk stimulation phase the subject returned for evaluation of primary and secondary endpoints. The electrode was then removed by gently pulling on the exposed end of the lead. As part of the protocol he underwent anterior-posterior and scapular-Y view radiographs of the shoulder for surveillance for retained electrode fragments. He returned at 1, 4, and 12-wks post-treatment for skin evaluation and outcomes assessments.
Results
The subject tolerated the implantation and stimulation test procedure well. The 3-wk stimulation protocol was completed. The following adverse events occurred: mild discomfort when flexing his shoulder simultaneously with receiving stimulation; and, a localized tissue inflammation at the site of the lead that resolved by his 1-month follow-up. The outcome measures for this case are listed in table 1. The subject experienced 75.0% and 100% reduction in pain (BPI 3) relative to baseline at end of treatment and at 3 months post-treatment, respectively. He experienced improvements in pain interference, arm function, quality of life, and an increase in PPTs.
Discussion
This is the first case to describe a patient treated with PNS for persistent shoulder pain due to SIS. After three weeks of electrical stimulation, he experienced substantial pain reduction that was maintained for at least three months after completion of treatment. The BPI 9, DASH, PGIC, pain free ROM, and PPT data suggest that the intervention might also reduce impairment, improve function, and improve quality of life.
The mechanism of pain relief for this subject is not known. An emerging hypothesis is that PNS might reduce chronic pain by altering maladaptive neuroplasticity in the central nervous system that causes central hypersensitivity. Evidence of central hypersensitivity has been demonstrated by lower local and distal PPTs in those with chronic SIS compared to controls.19 This subject had improvements in local and distal PPTs, raising the possibility that a central mechanism is being modulated through PNS. Further research of this hypothesis is warranted.
While single lead PNS was associated with improvements in pain, function, and quality of life in this subject, it is not yet reasonable to generalize this outcome to others with chronic shoulder pain from SIS. It is necessary to note that this subject experienced adverse events of temporary discomfort when flexing his shoulder during stimulation and a localized tissue inflammation at the site of the lead that resolved after 1 month. Other potential adverse events, that could occur, but were not experienced by this subject, are local infection, electrode fragmentation, or other unexpected complications. This study is limited by the 3-month follow-up period, which was shorter than the 5 months of improvement the subject experienced after prior treatment. Further, it is important to note that recurrent shoulder pain deserves a full evaluation for other sources of shoulder pain along with imaging studies. In this case, the subject’s symptoms and physical examination were straight-forward and the diagnostic injection was positive. Therefore, the authors proceeded with PNS for treatment. Further studies are needed to determine efficacy and safety, determine the mechanism of action, define optimal prescriptive parameters, expand clinical indications, and demonstrate long-term effect.
Acknowledgments
This publication was, in part, made possible by the Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health and NIH roadmap for Medical Research. Secure data storage was made possible through grants M01 RR00080 and UL1 RR024989 from NCRR/NIH. The stimulation device, percutaneous electrode, and associated supplies were donated by NDI Medical, Cleveland, OH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: SPR Therapeutics donated supplies for this study has a commercial interest in the device presented in this case report. John Chae, MD is a consultant to SPR Therapeutics, Maria Bennett is an employee of SPR Therapeutics.
Device status: An investigational percutaneous peripheral nerve stimulation system (NDI Medical) including a percutaneous intramuscular lead and a commercially available external stimulator (Rehabilicare® NT2000, Empi, Inc, St. Paul, MN) was used during this clinical study.
Contributor Information
Richard D. Wilson, Cleveland Functional Electrical Stimulation Center, Case Western Reserve University, Cleveland, OH, Department of Physical Medicine and Rehabilitation, Case Western Reserve University at MetroHealth Medical Center, Cleveland, OH.
Michael A. Harris, Department of Physical Medicine and Rehabilitation, Case Western Reserve University at MetroHealth Medical Center, Cleveland, OH.
Maria E. Bennett, SPR Therapeutics (subsidiary of NDI Medical), Cleveland, OH.
John Chae, Cleveland Functional Electrical Stimulation Center, Case Western Reserve University, Cleveland, OH, Department of Physical Medicine and Rehabilitation, Case Western Reserve University at MetroHealth Medical Center, Cleveland, OH, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH.
Bibliography
- 1.Luime JJ, Koes BW, Hendriksen IJ, Burdorf A, Verhagen AP, Miedema HS, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scandinavian journal of rheumatology. 2004;33(2):73–81. doi: 10.1080/03009740310004667. [DOI] [PubMed] [Google Scholar]
- 2.van der Windt DA, Koes BW, de Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Annals of the rheumatic diseases. 1995;54(12):959–64. doi: 10.1136/ard.54.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neer CS., 2nd Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. The Journal of bone and joint surgery. American volume. 1972;54(1):41–50. [PubMed] [Google Scholar]
- 4.Neer CS., 2nd Impingement lesions. Clinical orthopaedics and related research. 1983;(173):70–7. [PubMed] [Google Scholar]
- 5.Bigliani LU, Levine WN. Subacromial impingement syndrome. The Journal of bone and joint surgery. American volume. 1997;79(12):1854–68. [PubMed] [Google Scholar]
- 6.van der Heijden GJ. Shoulder disorders: a state-of-the-art review. Bailliere’s clinical rheumatology. 1999;13(2):287–309. [PubMed] [Google Scholar]
- 7.Goldberg SS, Bigliani LU. Shoulder impingement revisited: advanced concepts of pathomechanics and treatment. Instructional course lectures. 2006;55:17–27. [PubMed] [Google Scholar]
- 8.Dorrestijn O, Stevens M, Winters JC, van der Meer K, Diercks RL. Conservative or surgical treatment for subacromial impingement syndrome? A systematic review. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons … [et al.] 2009;18(4):652–60. doi: 10.1016/j.jse.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Ketola S, Lehtinen J, Arnala I, Nissinen M, Westenius H, Sintonen H, et al. Does arthroscopic acromioplasty provide any additional value in the treatment of shoulder impingement syndrome?: a two-year randomised controlled trial. The Journal of bone and joint surgery. British volume. 2009;91(10):1326–34. doi: 10.1302/0301-620X.91B10.22094. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RD, Bennett ME, Lechman TE, Stager KW, Chae J. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Archives of physical medicine and rehabilitation. 2011;92(5):837–40. doi: 10.1016/j.apmr.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu DT, Chae J, Walker ME, Kirsteins A, Elovic EP, Flanagan SR, et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Archives of physical medicine and rehabilitation. 2004;85(5):695–704. doi: 10.1016/j.apmr.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Joynt RL. The source of shoulder pain in hemiplegia. Archives of physical medicine and rehabilitation. 1992;73(5):409–13. [PubMed] [Google Scholar]
- 13.Dromerick AW, Edwards DF, Kumar A. Hemiplegic shoulder pain syndrome: frequency and characteristics during inpatient stroke rehabilitation. Archives of physical medicine and rehabilitation. 2008;89(8):1589–93. doi: 10.1016/j.apmr.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 14.Chae J, Wilson RD, Bennett ME, Lechman TE, Stager KW. Single-lead Percutaneous Peripheral Nerve Stimulation for the Treatment of Hemiplegic Shoulder Pain. A Case Series In Review. doi: 10.1111/j.1533-2500.2012.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Central hypersensitivity in chronic pain: mechanisms and clinical implications. Physical medicine and rehabilitation clinics of North America. 2006;17(2):287–302. doi: 10.1016/j.pmr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. The American journal of sports medicine. 1980;8(3):151–8. doi: 10.1177/036354658000800302. [DOI] [PubMed] [Google Scholar]
- 17.Jobe FW, Moynes DR. Delineation of diagnostic criteria and a rehabilitation program for rotator cuff injuries. The American journal of sports medicine. 1982;10(6):336–9. doi: 10.1177/036354658201000602. [DOI] [PubMed] [Google Scholar]
- 18.Yu DT, Chae J, Walker ME, Fang ZP. Percutaneous intramuscular neuromuscular electric stimulation for the treatment of shoulder subluxation and pain in patients with chronic hemiplegia: a pilot study. Archives of physical medicine and rehabilitation. 2001;82(1):20–5. doi: 10.1053/apmr.2001.18666. [DOI] [PubMed] [Google Scholar]
- 19.Hidalgo-Lozano A, Fernandez-de-las-Penas C, Alonso-Blanco C, Ge HY, Arendt-Nielsen L, Arroyo-Morales M. Muscle trigger points and pressure pain hyperalgesia in the shoulder muscles in patients with unilateral shoulder impingement: a blinded, controlled study. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2010;202(4):915–25. doi: 10.1007/s00221-010-2196-4. [DOI] [PubMed] [Google Scholar]


