Abstract
Effective regulation of nanoparticle (NP) uptake facilitates the NP-based therapeutics and diagnostics. Here, we report the use of insulin and 2-deoxyglucose (2-DG) to modulate the cellular uptake of glucose-functionalized quantum dots (Glc-QDs) in C2C12 muscle cells. The cellular uptake of Glc-QDs can be modulated up to almost two-fold under insulin stimulation while be down-regulated in the presence of 2-DG. These results demonstrate the use of secondary regulators to control the cellular uptake of NPs through membrane protein recognition in a specific and fine-tunable fashion.
Introduction
Regulating cellular uptake of nanoparticle (NP) in biological systems is critical for the development of NP-based therapeutics. NP delivery vehicles can increase delivered doses of drugs and imaging agents, while diminishing undesired off-target effects.1 NPs with tailorable structures provide a potential means of controlling therapeutic efficacy2 as well as concomitant toxicity. For example, the surface charge and hydrophobicity of NPs determine their cellular uptake, facilitating the NP-assisted intracellular delivery3 and bioimaging.4 In particular, targeting motifs on NP surface can effectively guide them to cells through membrane protein recognition, providing receptor/transporter-mediated cellular uptake5 that can be modulated by external chemicals or biomolecules.6
Glycomaterials are important nanoplatforms in biomedical applications,7 where the carbohydrate motifs allow these materials to be solubilized under physiological conditions8 and recognized by cell membrane proteins. 9 For examples, galactose-functionalized NPs can be selectively accumulated in hepatocellular carcinoma cell line HepG2 that expresses asialoglycoprotein receptor (ASGP-R).10 Also, 2-deoxyglucose-functionalized NPs can target mammary tumor cells11 and migrate across the blood brain barrier12 where glucose transporters (GLUT) are highly expressed. The use of carbohydrate-functionalized NPs for active targeting depends on the level of receptors and transporters that are in a state of flux, providing a potential strategy to control the cellular uptake of carbohydrate-functionalized NPs through modulation of ASGP13 and GLUT14 levels.
Here, we report the use of insulin to modulate the cellular uptake of glucose-functionalized quantum dots (Glc-QDs) through control of GLUT4 level on the membrane of C2C12 muscle cells (Scheme 1). The cellular uptake of Glc-QDs was investigated in both non-differentiated and differentiated muscle cells, where the Glc-QD uptake was enhanced in differentiated cells by insulin stimulation. We also demonstrated that the uptake efficiency of Glc-QDs can be inhibited in the presence of the competitive molecule 2-deoxyglucose (2-DG). Therefore, both insulin and 2-DG act as regulators to control the cellular uptake of Glc-QDs in a specific fashion, demonstrating the potential of secondary regulators for tuning the cellular uptake of NPs. Glucose-functionalized NPs can potentially be applied for GLUT4 targeting as well as controllable NP uptake in GLUT4-expressing cells such as adipocytes, skeletal and cardiac muscle cells.
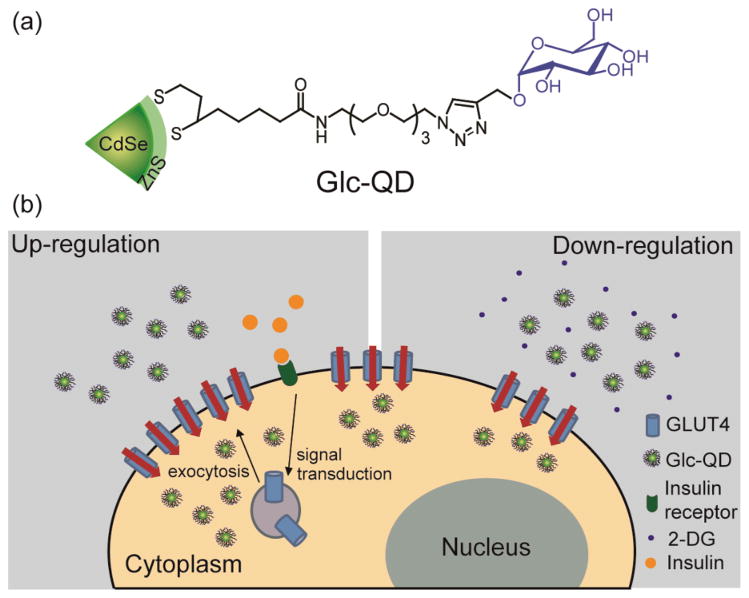
Scheme 1.
(a) Molecular structure of glucose-functionalized quantum dot (Glc-QD). (b) Schematic illustration of the cellular uptake of Glc-QDs regulated by insulin and 2-deoxyglucose (2-DG) in C2C12 muscle cells.
Results and Discussion
Glucose-conjugated ligands presenting dithiol anchoring groups were synthesized for the surface functionalization of QDs featuring 1) dihydrolipoic acid (DHLA) as a stable bidentate anchor,15 2) a tetra(ethylene glycol) (TEG) spacer to minimize non-specific interactions with proteins and cells,16 and 3) a glucose headgroup conjugated to the ligand through azide-alkyne cycloaddtion “click chemistry” (Scheme 1a). CdSe/ZnS core-shell QDs were used to prepare glucose-functionalized QDs (Glc-QDs) through a ligand exchange process. (See ESI† for the Glc-QD synthesis and characterization) The emission peak of Glc-QDs was observed at 555 nm and the dynamic light scattering (DLS) data showed that the hydrodynamic size of Glc-QDs was ca. 8 nm (Figure S2, ESI†).
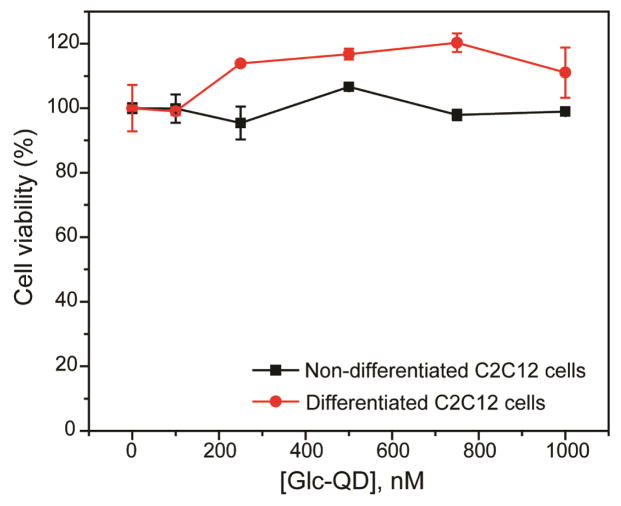
The regulation of Glc-QD uptake was investigated in C2C12 cells, a widely used skeletal muscle cell line. It is well-established that insulin stimulates the GLUT4 translocations from intracellular storage compartments to cell membrane to enhance the glucose uptake in C2C12 cells.17 Both non-differentiated and differentiated cells were cultured for Glc-QD uptake studies through choice of culture media. The morphology of C2C12 cells was changed from spindle-shaped myoblasts (Figure 1a) to fiber-shaped myotubes (Figure 1b) after differentiation. 18 The cellular uptake and intracellular distribution of Glc-QDs can be visualized using confocal microscopy. Glc-QDs were internalized and punctate fluorescence observed in the confocal images, consistent with the expected entrapment of Glc-QDs in endosomal/lysosomal compartments after cellular uptake.19 In parallel, the cytotoxicity of Glc-QDs was determined through alamarBlue® assay, where the results showed no significant toxicity was observed up to 1 μM of Glc-QDs in both non-differentiated and differentiated cells after incubation for 4 h (Figure 2).
Fig. 1.
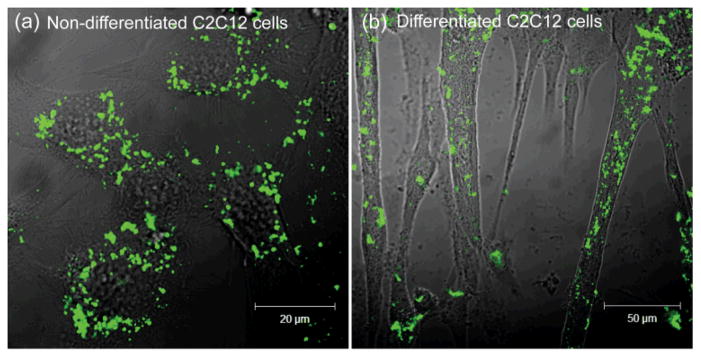
Confocal images of Glc-QDs (250 nM) in (a) non-differentiated and (b) differentiated C2C12 cells after incubation for 4 h.
Fig. 2.
Cytotoxicity studies of Glc-QDs in both non-differentiated and differentiated C2C12 cells after incubation for 4 h.
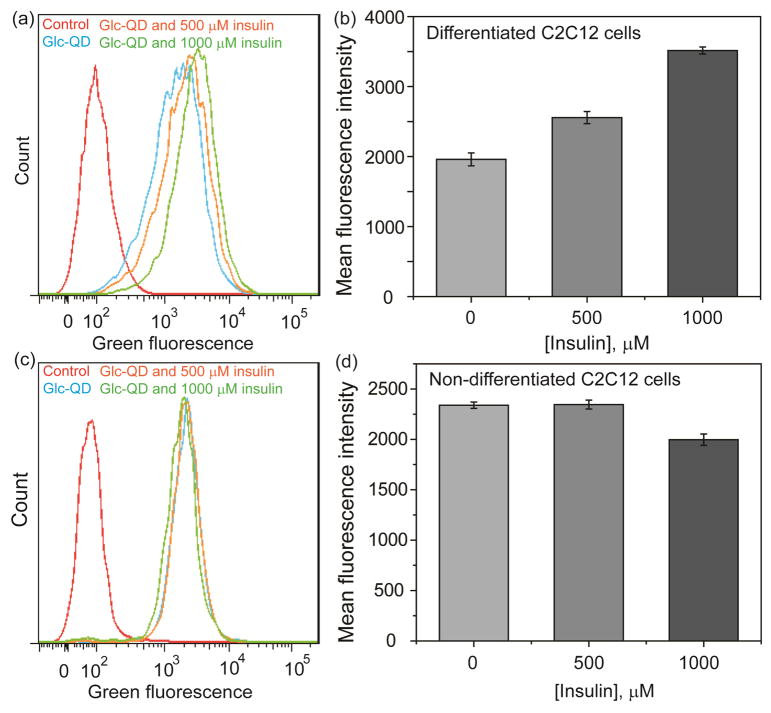
The cellular uptake efficiency of Glc-QDs was next investigated under insulin stimulation. Flow cytometry was used to quantify the uptake efficiency of Glc-QDs based on the fluorescence properties of QDs. Both non-differentiated and differentiated cells were treated with insulin for Glc-QD uptake studies. In these studies, a substantial increase of Glc-QD uptake was observed in differentiated cells in an insulin dose-dependent manner, and the cellular uptake of Glc-QDs can be over an almost two-fold range (Figure 3a and 3b). However, there was no noticeable change of Glc-QD uptake in non-differentiated cells under the insulin-stimulated conditions (Figure 3c and 3d).
Fig. 3.
Flow cytometric analysis of Glc-QD uptake in differentiated and non-differentiated C2C12 cells. Fluorescence intensity histogram and correlative mean fluorescence intensity of Glc-QDs in the presence of different amounts of insulin in (a, b) differentiated and (c, d) non-differentiated C2C12 cells. Glc-QDs (250 nM) were incubated in C2C12 cells for 4 h with the treatment of insulin.
It has been reported that GLUT4 is expressed in differentiated C2C12 cells but not in non-differentiated C2C12 cells. 20 The presence of insulin can increase the level of GLUT4 on the cell membrane of differentiated C2C12 cells to facilitate the cellular uptake of glucose.21 Therefore, the enhanced Glc-QD uptake under insulin stimulation is presumably due to the sufficient GLUT4 level in differentiated C2C12 cells. These results also indicated the glucose motifs on QD surface can be recognized by GLUT4, and the Glc-QD uptake can be fine-tuned through the control of the insulin-responsive GLUT4 level on cell membrane.
This insulin effect on regulating Glc-QD uptake was further confirmed with negative controls including QDs with different functionalities (i.e. galactose-functionalized QDs (Gal-QDs), polyethylene glycol-functionalized QDs (PEG-QDs) and trimethylammonium-functionalized QDs (TMA-QDs)) and different cell line (i.e. NIH/3T3 cells, mouse embryonic fibroblast cells). From these control studies, no noticeable change of the fluorescence distribution was observed with insulin treatment (Figure 4 and S3, ESI†), indicating the specificity of the insulin stimulation in increasing the cellular uptake of Glc-QDs in differentiated C2C12 muscle cells.
Fig. 4.
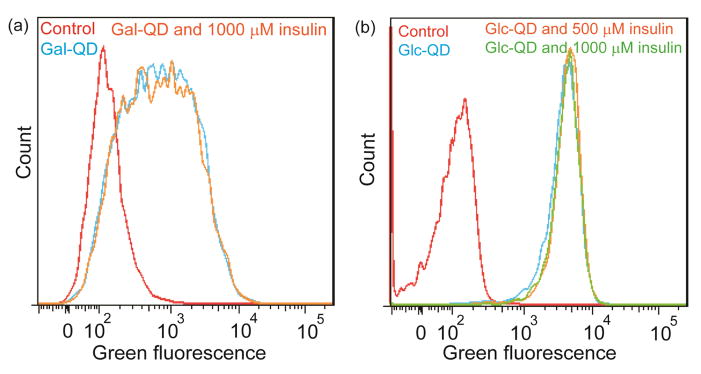
(a) Galactose functionalized QDs (Gal-QDs) were incubated in differentiated C2C12 cells for 4 h with the treatment of insulin. (b) Glc-QDs were incubated in NIH/3T3 fibroblast cells for 4 h with the treatment of insulin. The concentration of QDs used in these experiments was 250 nM.
Down-regulation of Glc-QD uptake was investigated by the treatment of 2-deoxyglucose (2-DG), a glucose analog that has been used to decrease the glucose uptake inside the cell.22 In the presence of 2-DG, greatly reduced uptake of Glc-QDs was observed in differentiated cells (Figure 5). Again, these results demonstrate that the glucose motifs on QD surface can be recognized by GLUT4 and 2-DG becomes the competing molecule to inhibit the cellular uptake of Glc-QDs.
Fig. 5.
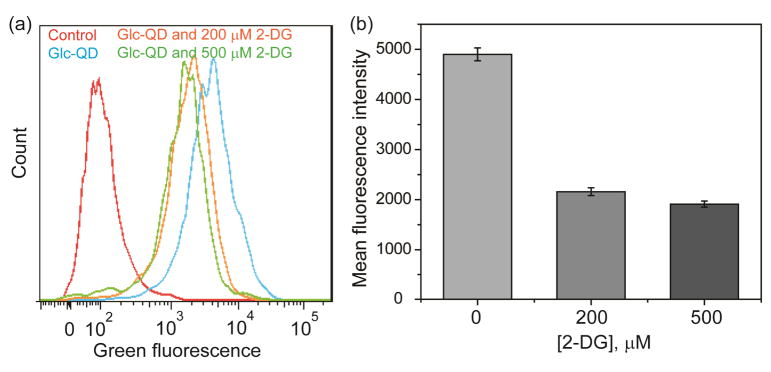
(a) Fluorescence intensity histogram and (b) correlative mean fluorescence intensity of Glc-QDs in the presence of different amounts of 2-DG in differentiated C2C12 cells.
Conclusions
We have demonstrated the glucose motifs on QD surface coupled with secondary regulators provides a means of modulating nanoparticle uptake by muscle cells. The cellular uptake of Glc-QDs can be up-regulated through insulin stimulation and down-regulated in the presence of 2-DG. These findings provide insight for the design of surface-functionalized NPs that can use cellular signalling to provide controllable uptake systems for use in the intracellular delivery and imaging applications.
Experimental Section
General
All chemicals used for the fabrication of QDs were purchased from Sigma-Aldrich, Fisher Scientific or Stream, unless otherwise specified. The chemicals were used as received. Dichloromethane (DCM) used as solvent for chemical synthesis was dried according to standard procedure. 1H NMR spectra were recorded at 400 MHz on a Bruker AVANCE 400. Fluorescence from the alamarBlue® assay was detected using a SpectraMax M5 microplate reader (Molecular Device Inc., USA). Dynamic light scattering (DLS) measurement was performed using a Malvern Zetasizer (Nano Series, Malvern Instruments Inc., USA), and the data analysis was done using Malvern PCS software. Flow cytometry analysis was performed in a BD LSR-II flow cytometer equipped with FACSDiva (BD Sciences, USA) by counting 10000 events, and the cell debris were excluded from analysis by proper dot plot gating.
Fabrication of QDs
QDs were decorated with glucose-functionalized ligands by following the reported two-step procedure.15 In the first step, trioctylphosphine oxide/trioctylphosphine (TOPO/TOP) coated QDs (10 mg) were mixed with HS-C5-TEG ligands (30 mg) in methanol (MeOH, 5 ml). The reaction mixture was stirred at 35 °C for 24 h under inert atmosphere. In this step, amphiphilic ligands replaced the native hydrophobic ligands from the surface of QDs, and the resulting amphiphilic QDs became soluble in MeOH. Next step involved the purification of QDs with hexane and addition of glucose-functionalized ligands (30 mg) to the amphiphilic QDs in MeOH. As a result, dithiol ligands slowly substituted monothiol ligands from QDs surface due to its better chelating capability. After 24 h of stirring, MeOH was evaporated and QDs were dispersed in water. Finally the aqueous QD sample was purified by dialysis. Gal-QDs were prepared by following the same procedure as Glc-QDs. The syntheses of PEG-QDs and TMA-QDs were followed the reported literatures.23,15
Cell culture
C2C12 muscle cells and NIH/3T3 fibroblast cells (American Type Culture Collection (ATCC)) were cultured at 37 °C, 5% CO2 in a humidified atmosphere. C2C12 cells were grown in the high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum and 1% antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin). NIH/3T3 cells were grown in the high glucose DMEM supplemented with 10% bovine calf serum and 1% antibiotics. Differentiation of C2C12 cells was followed the reported procedure.24 Briefly, cells were transferred to a 24-well plate (6×104 cells/well) and incubated for 48 h in growth media to about 100% confluence and then switched to differentiation media. Differentiation media was prepared in low glucose DMEM supplemented with 2% horse serum and 1% antibiotics. The differentiation media was changed when significant amount of cell death/floating or cell debris was present. Large multinucleated myotubes would be visible after C2C12 cells were cultured in differentiation media for 4 days.
Confocal microscopy studies of Glc-QDs in C2C12 cells
C2C12 cells were seeded in glass-bottomed dishes (1.8×105 cells/dish, MatTek Corporation, 14 mm microwell) 24 h prior to the confocal experiment. On the next day, the old media was removed and cells were washed with cold phosphate buffered saline (PBS). Glc-QD solution (250 nM in low glucose DMEM) was incubated with cells for 4 h. Thereafter, cells were washed with PBS three times before taking the images. Confocal microscopy images were obtained on a Zeiss LSM 510 Meta microscope by using a 63× objective. Settings of the confocal microscope: green channel of QD: λex= 488 nm and λem= BP 505–550 nm (BP: band pass).
Cytotoxicity study
Cytotoxicity was determined by an alamarBlue® assay (Invitrogen Biosource, USA), which is based on the conversion of resazurin to resorufin via a reduction reaction dependent on cellular metabolic activity.25 Non-differentiated and differentiated cells were cultured in a 24-well plate (6×104 cells/well). The cells were incubated with Glc-QD solutions (various concentrations in low glucose DMEM) for 4 h. After incubation, cells were washed with PBS and treated with 10% alamarBlue® dye in low glucose DMEM with 10% fetal bovine serum (500 μL/well) for 2 h. The fluorescence intensity was recorded using a SpectroMax M5 microplate reader to determine cell viability. Settings of the microplate reader: λex= 560 nm, λem= 590 nm.
Insulin and 2-DG treatment
Non-differentiated and differentiated cells were cultured in a 24-well plate (6×104 cells/well). The cells were incubated with low glucose DMEM for 1 h as serum starvation process. Different amounts of insulin or 2-DG were prepared in glucose-free DMEM to be incubated with cells for 30 min. Glc-QDs (250 nM) were prepared along with different amounts of insulin or 2-DG in low glucose DMEM and incubated with cells for 4 h before the flow cytometry measurement.
Supplementary Material
Acknowledgments
This research was supported by the NIH (EB014277) and the NSF (CHE-1307021). We thank Professor Lawrence M. Schwartz for providing C2C12 cell line.
Footnotes
Electronic Supplementary Information (ESI) available: synthesis of glucose-terminated ligand, characterization of Glc-QDs (i.e. mass spectrum, emission spectrum and DLS data) and negative controls (i.e. PEG-QDs and TMA-QDs) for the insulin effect on regulating QD uptake in differentiated C2C12 cells. See DOI: 10.1039/c000000x/
Notes and References
- 1.Caballero-Diaz E, Pfeiffer C, Kastl L, Rivera-Gil P, Simonet B, Valcarcel M, Jimenez-Lamana J, Laborda F, Parak WJ. Part Part Syst Char. 2013;30:1079. [Google Scholar]
- 2.(a) Liu X, Chen Y, Li H, Huang N, Jin Q, Ren K, Ji J. ACS Nano. 2013;7:6244. doi: 10.1021/nn402201w. [DOI] [PubMed] [Google Scholar]; (b) Ahrens ET, Feili-Hariri M, Xu H, Genove G, Morel PA. Magn Reson Med. 2003;49:1006. doi: 10.1002/mrm.10465. [DOI] [PubMed] [Google Scholar]
- 3.(a) Yezhelyev MV, Qi LF, O’Regan RM, Nie S, Gao XH. J Am Chem Soc. 2008;130:9006. doi: 10.1021/ja800086u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee J, Choi Y, Kim J, Park E, Song R. Chemphyschem. 2009;10:806. doi: 10.1002/cphc.200800504. [DOI] [PubMed] [Google Scholar]; (c) Kim ST, Chompoosor A, Yeh YC, Agasti SS, Solfiell DJ, Rotello VM. Small. 2012;8:3253. doi: 10.1002/smll.201201141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J, Kim J, Park E, Jo S, Song R. Phys Chem Chem Phys. 2008;10:1739. doi: 10.1039/b801317a. [DOI] [PubMed] [Google Scholar]
- 5.(a) Farokhzad OC, Cheng JJ, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Proc Natl Acad Sci U S A. 2006;103:6315. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, Freichels H, Jerome C, Marchand-Brynaert J, Feron O, Preat V. J Control Release. 2009;140:166. doi: 10.1016/j.jconrel.2009.08.011. [DOI] [PubMed] [Google Scholar]; (c) Kukowska-Latallo JF, Candido KA, Cao ZY, Nigavekar SS, Majoros IJ, Thomas TP, Balogh LP, Khan MK, Baker JR. Cancer Res. 2005;65:5317. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]; (d) Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. ACS Nano. 2008;2:889. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Tuma P, Hubbard AL. Physiol Rev. 2003;83:871. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 6.Zhang MZ, Yu Y, Yu RN, Wan M, Zhang RY, Zhao YD. Small. 2013;9:4183. doi: 10.1002/smll.201300994. [DOI] [PubMed] [Google Scholar]
- 7.(a) Wang X, Ramström O, Yan M. Adv Mater. 2010;22:1946. doi: 10.1002/adma.200903908. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) El-Boubbou K, Huang X. Curr Med Chem. 2011;18:2060. doi: 10.2174/092986711795656144. [DOI] [PubMed] [Google Scholar]
- 8.(a) Basiruddin SK, Maity AR, Jana NR. RSC Adv. 2012;2:11915. [Google Scholar]; (b) Earhart C, Jana NR, Erathodiyil N, Ying JY. Langmuir. 2008;24:6215. doi: 10.1021/la800066g. [DOI] [PubMed] [Google Scholar]
- 9.(a) Benito-Alifonso D, Tremel S, Hou B, Lockyear H, Mantell J, Fermin DJ, Verkade P, Berry M, Galan MC. Angew Chem Int Ed. 2014;53:810. doi: 10.1002/anie.201307232. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sur I, Cam D, Kahraman M, Baysal A, Culha M. Nanotechnology. 2010;21(175104):1–10. doi: 10.1088/0957-4484/21/17/175104. [DOI] [PubMed] [Google Scholar]; (c) Basuki JS, Esser L, Duong HTT, Zhang Q, Wilson P, Whittaker MR, Haddleton DM, Boyer C, Davis TP. Chem Sci. 2014;5:715. [Google Scholar]; (d) Chen CT, Munot YS, Salunke SB, Wang YC, Lin RK, Lin CC, Chen CC, Liu YH. Adv Funct Mater. 2008;18:527. [Google Scholar]; (e) Geng F, Song K, Xing JZ, Yuan C, Yan S, Yang Q, Chen J, Kong B. Nanotechnology. 2011;22:285101. doi: 10.1088/0957-4484/22/28/285101. [DOI] [PubMed] [Google Scholar]; (f) Rojo J, Diaz V, de la Fuente JM, Segura I, Barrientos AG, Riese HH, Bernade A, Penades S. Chembiochem. 2004;5:291. doi: 10.1002/cbic.200300726. [DOI] [PubMed] [Google Scholar]; (g) De la Fuente JM, Penades S. Biochim Biophys Acta. 2006;1760:636. doi: 10.1016/j.bbagen.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 10.(a) Kikkeri R, Lepenies B, Adibekian A, Laurino P, Seeberger PH. J Am Chem Soc. 2009;131:2110. doi: 10.1021/ja807711w. [DOI] [PubMed] [Google Scholar]; (b) Lai CH, Lin CY, Wu HT, Chan HS, Chuang YJ, Chen CT, Lin CC. Adv Funct Mater. 2010;20:3948. [Google Scholar]
- 11.(a) Shan XH, Hu H, Xiong F, Gu N, Geng XD, Zhu W, Lin J, Wang YF. Eur J Radiol. 2012;81:95. doi: 10.1016/j.ejrad.2011.03.013. [DOI] [PubMed] [Google Scholar]; (b) Xiong F, Zhu ZY, Xiong C, Hua XQ, Shan XH, Zhang Y, Gu N. Pharm Res. 2012;29:1087. doi: 10.1007/s11095-011-0653-9. [DOI] [PubMed] [Google Scholar]
- 12.(a) Jiang X, Xin H, Ren Q, Gu J, Zhu L, Du F, Feng C, Xie Y, Sha X, Fang X. Biomaterials. 2014;35:518. doi: 10.1016/j.biomaterials.2013.09.094. [DOI] [PubMed] [Google Scholar]; (b) Gromnicova R, Davies HA, Sreekanthreddy P, Romero IA, Lund T, Roitt IM, Phillips JB, Male DK. PLoS One. 2013;8:e81043. doi: 10.1371/journal.pone.0081043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) De la Vega LA, Stockert RJ. Am J Physiol Cell Physiol. 2000;279:C2037. doi: 10.1152/ajpcell.2000.279.6.C2037. [DOI] [PubMed] [Google Scholar]; (b) Yang J, Bo XC, Ding XR, Dai JM, Zhang ML, Wang XH, Wang SQ. J Viral Hepat. 2006;13:158. doi: 10.1111/j.1365-2893.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 14.(a) Bryant NJ, Govers R, James DE. Nat Rev Mol Cell Biol. 2002;3:267. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]; (b) Bose A, Guilherme A, Robida SI, Nicoloro SM, Zhou QL, Jiang ZY, Pomerleau DP, Czech MP. Nature. 2002;420:821. doi: 10.1038/nature01246. [DOI] [PubMed] [Google Scholar]; (c) Ralphe JC, Nau PN, Mascio CE, Segar JL, Scholz TD. Pediatr Res. 2005;58:713. doi: 10.1203/01.PDR.0000180546.42475.69. [DOI] [PubMed] [Google Scholar]; (d) Thorens B, Mueckler M. Am J Physiol Endocrinol Metab. 2010;298:E141. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh YC, Patra D, Yan B, Saha K, Miranda OR, Kim CK, Rotello VM. Chem Commun. 2011;47:3069. doi: 10.1039/c0cc04975a. [DOI] [PubMed] [Google Scholar]
- 16.(a) Hong R, Fischer NO, Verma A, Goodman CM, Emrick TS, Rotello VM. J Am Chem Soc. 2004;126:739. doi: 10.1021/ja037470o. [DOI] [PubMed] [Google Scholar]; (b) You CC, De M, Rotello VM. Org Lett. 2005;7:5685. doi: 10.1021/ol052367k. [DOI] [PubMed] [Google Scholar]; (c) Liu W, Howarth M, Greytak AB, Zheng Y, Nocera DG, Ting AY, Bawendi MG. J Am Chem Soc. 2008;130:1274. doi: 10.1021/ja076069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nedachi T, Kanzaki M. Am J Physiol Endocrinol Metab. 2006;291:E817. doi: 10.1152/ajpendo.00194.2006. [DOI] [PubMed] [Google Scholar]
- 18.Manabe Y, Miyatake S, Takagi M, Nakamura M, Okeda A, Nakano T, Hirshman MF, Goodyear LJ, Fujii NL. PLoS One. 2012;7:e52592. doi: 10.1371/journal.pone.0052592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moros M, Hernaez B, Garet E, Dias JT, Saez B, Grazu V. ACS Nano. 2012;6:1565. doi: 10.1021/nn204543c. [DOI] [PubMed] [Google Scholar]
- 20.(a) Guillet-Deniau I, Leturque A, Girard J. J Cell Sci. 1994;107:487. [PubMed] [Google Scholar]; (b) Yamamoto DL, Csikasz RI, Li Y, Sharma G, Hjort K, Karlsson R, Bengtsson T. J Histochem Cytochem. 2008;56:881. doi: 10.1369/jhc.2008.951228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Rodnick KJ, Slot JW, Studelska DR, Hanpeter DE, Robinson LJ, Geuze HJ, James DE. J Biol Chem. 1992;267:6278. [PubMed] [Google Scholar]; (b) Ralston E, Ploug T. J Cell Sci. 1996;109:2967. doi: 10.1242/jcs.109.13.2967. [DOI] [PubMed] [Google Scholar]; (c) Ariga M, Nedachi T, Katagiri H, Kanzaki M. J Biol Chem. 2008;283:10208. doi: 10.1074/jbc.M710604200. [DOI] [PubMed] [Google Scholar]
- 22.Litta Modignani R, Foa PP. Proc SOc Exp Blol and Med. 1963;112:106. doi: 10.3181/00379727-112-28099. [DOI] [PubMed] [Google Scholar]
- 23.Uyeda HT, Medintz IL, Jaiswal JK, Simon SM, Mattoussi H. J Am Chem Soc. 2005;127:3870. doi: 10.1021/ja044031w. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz LM, Gao Z, Brown C, Parelkar SS, Glenn H. Methods Mol Biol. 2009;559:313. doi: 10.1007/978-1-60327-017-5_22. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien J, Wilson I, Orton T, Pognan F. Eur J Biochem. 2000;267:5421. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.