Abstract
Tendinopathy is a debilitating musculoskeletal condition which can cause significant pain and lead to complete rupture of the tendon, which often requires surgical repair. Due in part to the large spectrum of tendon pathologies, these disorders continue to be a clinical challenge. Animal models are often used in this field of research as they offer an attractive framework to examine the cascade of processes that occur throughout both tendon pathology and repair. This review discusses the structural, mechanical, and biological changes that occur throughout tendon pathology in animal models, as well as strategies for the improvement of tendon healing.
Cite this article: Bone Joint Res 2014;3:193–202.
Keywords: Tendon, Tendinopathy, Animal Model, Literature Review
Introduction
Musculoskeletal pathologies account for more than half of chronic conditions for populations over the age of 50 in developed countries1 and for 30% to 50% of all sports-related injuries.2 In 2006, 15.6% of individuals surveyed in the United Kingdom reported the prevalence of a longstanding musculoskeletal disorders.3 In the same year in the United States, musculoskeletal diseases and injuries resulted in direct healthcare costs and lost wages adding up to $950 billion.1 Pathologies of the tendon, or tendinopathies, account for a substantial portion of musculoskeletal disorders. Their severity ranges from transient pain and inflammation, to chronic conditions involving partial or total ruptures of the tendon.
Tendons are soft tissues that transfer forces created by muscle to bones. Understanding the basics of mechanical function and structure of a healthy tendon is essential to re-establishing these attributes in the injured tendon. Damage to tendons often results in pain that impairs a person’s ability to move in a smooth and coordinated manner. These changes have a cascading effect, leading to altered joint-loading patterns, which can ultimately result in mechanical degradation of joint integrity.4
A healthy mature tendon consists of a hierarchy of structured collagen intermingled with tenocytes and embedded in an extracellular matrix (Fig. 1).5 At the macroscopic scale (1 mm to 10 mm), a tendon consists of bundles of fascicles that are covered by connective tissues known as the epitenon and endotenon, respectively. These connective tissues contain the neurovascular structures supplying the tendon. Tendon fascicles (50 µm to 300 µm) consist of bundles of collagen fibres with tenocytes between fascicles. The next level of tendon structure consists of parallel collagen fibrils (50 nm to 500 nm) that have a ‘crimped’ appearance in the absence of tensile load directed along the length of the tissue. At the smallest levels are microfibrils and tropocollagen molecules, which are around 1.5 nm in diameter.
Fig. 1.

Schematic representation of the hierarchical structure of a tendon. Reprinted from Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg 2012;21:228-2375 with permission from Elsevier.
Tendinopathy can be identified with a variety of assays. Through histology, tendinopathy can be identified by some or all of these characteristics: small tears and disorganisation of the collagen fibres, changes in cell number and shape, variations in vascularity, and varying glycosaminoglycan levels.6 Biochemical tests can also identify tendinopathy by identifying the regulation of matrix metalloproteinases and their inhibitors.7 Tendinopathy also leads to altered mechanical properties prior to tissue failure.8 Due in part to the large spectrum of tendon pathologies, tendinopathic disorders continue to be a challenge to address clinically. The efficacious and long-lasting repair of tendons continues to challenge surgeons. For example, poor surgical outcomes and re-tear rates of large rotator cuff tears have been reported to be between 76% and 94%.9,10
Animal models offer an attractive framework to investigate the etiology of tendinopathy. Unlike human tissue, which only can be examined during end-stage chronic pathology, animal models provide the opportunity to obtain tissue during all stages of tendinopathy. Additionally, animal models provide the ability to reproduce consistent and repeatable injuries that can be treated in a controlled and quantifiable manner and also allow the evaluation of invasive treatments and assessments that would be unethical with human subjects. Another unique advantage of animal models is the capability of modifying the genome, particularly in the murine model. This technology allows for comparison of tendon properties in mice with and without the ability to express a particular gene globally, in a particular tissue, or at a particular time. For example, Scleraxis-knockout mice demonstrate an inferior ability to generate healthy tendons at birth compared with controls, suggesting the important role of this molecule in tendon development.11-13 Similar studies have been conducted to investigate the role of decorin (DCN), byglycan (BGN), mohawk (MKX), collagen V (COL V), collagen XI (COL XI), interleukin-4 (IL-4) and interleukin-6 (IL-6) to name a few.14-21
However, animal models of tendinopathy cannot truly replicate the human condition. Many lab animals are quadrupeds and subject their tendons to different magnitudes of load than their human counterparts, making it difficult to replicate the pathology seen clinically. Additionally, molecular differences between animals and humans further confound the ability to make direct comparisons between species. For example, the rat rotator cuff model does not fully represent the anatomy, movement kinematics, or kinetics that exist in the human shoulder22 and rodents do not possess a homologue of the human MMP1 gene.23 Despite these limitations, the rat model is still widely used, as it is considered a good choice given the practical considerations.22 Overall, it is important to understand that while translational research is the goal, animal models allow researchers to understand cellular and tissue-level principles in the context of a living organism.24,25
This manuscript will review and evaluate animal models that have been developed to understand the aetiology and pathology of tendinopathy as well as some of their translational implications. To compile the list of the most relevant literature, the search term ‘tendon animal model’ was used in PubMed (1525 articles), in order to prevent inadvertent exclusion of articles of interest. These were then restricted to those from the last three years (423 articles). The three-year window of time was selected as our primary goal in order to provide a summary of the most recent literature. We excluded articles that did not use animal models and those that focused on ligaments. A small number of often referenced and highly regarded previous publications were included. Several review articles or book chapters were also included because they provide comprehensive overviews prior to the most recent literature, which is the focus of this article. This review is organised according to the four major tendon groups that are commonly studied with animal models: rotator cuff; flexor; achilles and patellar tendons.
Rotator cuff
Rotator cuff tendon tears are common shoulder injuries that often require surgical repair. Despite the advanced approaches to rotator cuff repairs and post-surgical rehabilitation, the rate of both failures and re-tears have been estimated to be as high as between 76% and 94%.9,10 Animal models have been used extensively to investigate rotator cuff tendon repair, and a careful examination of over 30 species of animal concluded that the rat shoulder possesses an anatomic architecture that most resembles the human shoulder.22 (Fig. 2) For this reason, the rat has been the most commonly used animal model in rotator cuff research, with more than 100 full-length, peer-reviewed publications to date. Nevertheless, a variety of other valuable animal models also continue to be used to replicate aspects of rotator cuff injury and repair, including murine,26-28 rabbit,29,30 ovine,31-37 canine,38,39 bovine,40-43 and primate.44,45
Fig. 2.
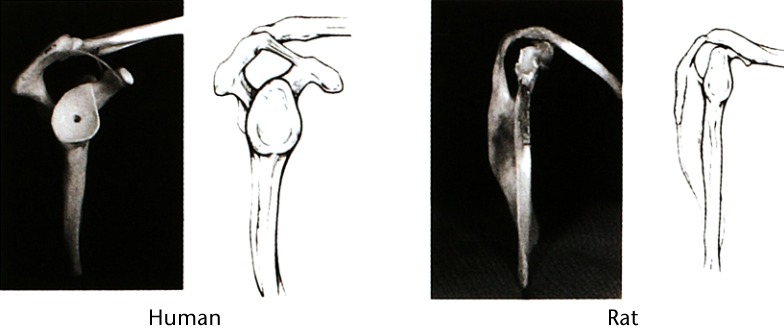
Photographs and schematic representations showing the similiarities of human and rat shoulders from a lateral view. In both anatomies, the supraspinatus tendon passes through the enclosed arch of the acromion. Reprinted from Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg 1996;5:383-9222 with permission from Elsevier.
Because of the high failure rates of rotator cuff repairs, the methods and materials employed in the surgical repair of rotator cuff tears continues to be an active field of research. Studies in this area often use in vitro ovine or bovine models, and focus on surgical variables during repair such as type of suture material41; number of sutures or rows of suture29,32-39,42; type of knot36,37,42 and type and/or number of anchors37,42,43 used in the repair. Several of these studies indicate that knot slipping may be the cause of repair failures, as this phenomenon occurs at substantially lower loads than anchor pull-out.36,43 Additionally, it has been suggested that increases in the number of stitches used during the repair is the determining factor in the failure load.32 On the other hand, it has been suggested that the contact area and failure strength is dependent upon the number of rows used in the repair.33,35,37 In general, although each study makes a compelling argument for the preferred protocol, there is still no clear consensus on the best materials and methods to employ.
The rat model is used to investigate rotator cuff pathology in animals and to better understand the cascade of biological processes that occur in the shoulder joint after a rotator cuff tear. To create a model, the most common practice is a surgical transection of one or several tendons in the shoulder.46-49 To replicate the chronic effects of tendinopathy and rotator cuff tears more closely, a tendinopathic condition can be created by overuse, in which rats are run on downward-sloping treadmills to impose eccentric forces on the tendon.50 Alternatively, a combination of overuse followed by acute injury has also been used to model rotator cuff tendinopathy.51,52 Use of these paradigms has led to the conclusion that damage to rotator cuff tendons leads to an increase in atrophy and/or fatty infiltration of the muscle.46-48 It has also been discovered that acute rupture of the tendon leads to decreased regulation of the signaling pathway that maintains muscle mass in response to mechanical loading (Akt/mTOR), but denervation without transection of the tendon leads to upregulation in this pathway.47 Interestingly, rotator cuff tears can have a direct effect on neighbouring intact tendons, such as the biceps, and can include decreased collagen organisation, more rounded cell shape, increased Aggrecan expression and decreased modulus.53 Finally, it has also been shown that the glenoid cartilage is also altered by a rotator cuff tear, as significant decreases in mechanical properties and thickness have been measured regionally in the glenoid.54
To date, the synergistic effects of repair techniques and various rehabilitative protocols on rotator cuff healing is still a debated topic. Current research has not clearly elucidated the role of mechanical loading in the pathological shoulder joint, but also the benefits/drawbacks of post-operative immobilisation continue to be confounding.5 Methods such as casting immobilisation,49,55 botulinum toxin injections,56,57 and overuse activity51,52 have been used to alter the mechanical loads imparted upon the shoulder joint before and after surgical intervention. Results suggest that pre-operative immobilisation may have beneficial effects on long-term healing because this approach has resulted in improved cellularity and collagen organisation, while simultaneously increasing the Collagen I:Collagen III ratio, which is indicative of the end stages of tendon healing.57 Decreased post-operative loading has been shown to result in increased organisation of collagen, decreased cellularity and a more elongated cell shape.49 While the benefit of post-operative immobilisation of the shoulder seems to be beneficial, the effectiveness of such a strategy is limited to discrete time frames.55 It has also been suggested that myogenic and adipogenic genes are influenced by mechanical loads, as they are upregulated in muscle when unloaded, but tendon-specific genes are more influenced by the presence of the injury.57 Overuse of the shoulder following a rotator cuff repair also causes significant changes in transcriptional regulation of chondrogenic genes, while also resulting in deleterious changes to the mechanical integrity of the tendons and cartilage within the shoulder. Surprisingly, joint function is not affected by these changes.51,52
Animal systems have been used routinely to investigate the use of potential regenerative agents (i.e., growth factors,40,58 platelet-rich plasma (PRP),30,59,60 hormones,61,62 bone morphogenetic protein,63 autologous cell seeding,64 and stem cells65-68)while the design, implementation, and translational viability of engineered tissue constructs have been concurrently developed. These studies simulated an acute rupture of the rotator cuff tendon(s) of rats,69 rabbits,30 canines,39 or primates45 and regenerative agent(s) were subsequently applied directly to the injury site,30 injected into the joint space,59 delivered via subcutaneous injection,61 osmotic pump,58 or scaffolds/ grafts.40,45,60,63,64,70 Scaffold designs are not limited to the purpose of delivering agents in a controlled manner, as some have also been proven to improve mechanical strength at the repair site39 and remodel to tendon-like architecture while integrating bone and tendon.45 The scope of this review does not permit a full exploration of the effects of all of the regenerative agents used in tendon research, so we will only briefly review a few studies examining the use of PRP and stem cells, which have recently garnered considerable interest as potential therapy modalities. Research investigating the effects of PRP has yielded mixed results. In some cases, it has been shown that the addition of PRP has decreased inflammation, improved tendon thickness and continuity, and increased biomechanical strength,30,59 but other studies have shown that the presence of PRP did not have any substantial phsyiological effects and the failure load of rotator cuff repairs was not altered by PRP augmentation.60 The healing potential of mesenchymal and adipose-derived stem cells has recently been tested with rabbit and rat models65-68,70 and results of such experiments have provided more consistent results than PRP-based studies. Results suggest that stem cell-based therapeutic modalities have the potential to decrease fatty infiltration after cuff repair,66 offer improvement in tendon-to-bone healing,65,70 increase generation of collagen I67,68 and improve the tendon’s mechanical properties.68 For a more thorough review of the use of engineered regenerative agents in the rotator cuff, the reader is referred to Isaac et al.71
Achilles
The Achilles tendon is the largest and strongest tendon in the human body, routinely experiencing loads up to 12.5 times the weight of the individual.72 This, along with other factors, likely contributes to substantial Achilles tendon pathology and highlights the need for both surgical and conservative Achilles tendon research.
Rats have been used frequently to model Achilles tendon rupture and tendinopathy, using primarily one of two methods of inducing injury; mechanical or chemical. Mechanical induction of tendinopathy has proven to be dependent on activity level. For example, rats that ran on a 10° incline at 17 m/min to 20 m/min for 60 min/day showed only slight adaptive changes in their Achilles tendons,73 however, a slight increase in speed and duration resulted in signs of tendinosis such as fibrillar mirotearing, hypercellularity and increased GAG deposition.74 Alternatively, tendinopathy has been generated by having rats run in a bipedal position,75 or with repetitive electrically-induced eccentric contraction of the calf.76 Alternatively, chemically-induced models of tendinopathy are attractive because they require less time and resources. Although the collagenase-induced Achilles tendinopathy model has been the most widely used approach, several new methods have recently been proposed. For example, after an intratendinous injection of TGF-β1, Achilles tendons show both attenuated material properties and a gene expression profile consistent with chronic tendinopathy, with a reverse seen to both changes after exercise.77 Another novel approach to chemical induction of tendinopathy consists of using injections of Substance P, a well-known neuropeptide and modulator of pain that encourages tenocyte proliferation and neovascularisation. Although the presence of Substance P seems like it would be beneficial to tendon healing, it has been shown that repeated injections of Substance P followed by exercise elicits an exacerbated inflammation-repair response, which leads to a tendinopathic condition.78 The role of Subtance P in tendinopathy is therefore particularly intriguing as it can be effectively added or blocked,78 potentially leading to clinical applications.
Similar to the rotator cuff, animal models have been used extensively to improve Achilles tendon repair methods through improvements in material and techniques. For example, impregnating a suture with Butyric acid has shown improved biomechanical and histological properties in a rabbit model,79 while coating a suture with mesenchymal stem cells appears to improve repair strength in the period when a repair is typically the weakest (7 to 10 days).80 Demonstrating the importance of suture technique, a bovine model showed that the triple-strand technique provided greater mean peak load to failure, and greater resistance to gapping when compared with Dresden, Krackow, and modified oblique Dresden techniques (Fig. 3).81 This result likely reiterates the well-known tenet of tendon repair – repair strength correlates to the number of sutures crossing the repair site.
Fig. 3.
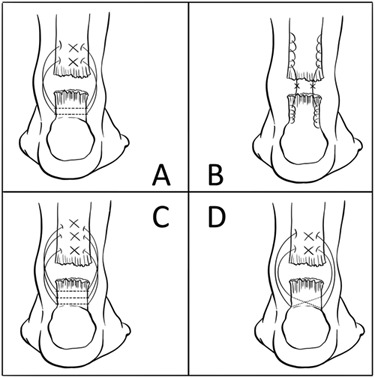
Diagram showing the suture configurations of the a) Dresden, b) Krackow, c) triple and d) oblique technique (figure modified from original with permission).81
There is a variety of rehabilitation protocols for Achilles tendon repair, and animal models have been valuable in defining areas for human study. Examination of the biological processes that occur in the rabbit under various rehabilitation protocols has led to the understanding that protein expression profiles in the Achilles tendon are significantly affected by early movement when compared with immobilisation.82 Moreover, the effect of a single loading episode on healing Achilles tendon results in significant, yet short-lived changes in the expression of inflammatory, healing and coagulation markers in a rat model.83 In addition, it appears that the magnitude of the loading episode after repair may play a role in determining tissue quality and callus formation, which define the mechanical integrity of the healing Achilles tendon.84 These studies suggest that frequent, short, early loading after an Achilles tendon injury is important in improving and expediting tendon healing.85
The use of imaging modalities varies depending on the pathology, but the superficial location of the Achilles tendon makes it a particularly attractive tendon for examination with ultrasound. Ultrasound has the potential to be a cost-effective, non-invasive method of determining degree and location of Achilles tendon disease. Sonoelastography is an ultrasound based imagine technique which has demonstrated an ability to track tendon elasticity - a possible surrogate for healing.86 As another example, chronic local hypervascularity has been linked to the pain associated with tendinopathy, and contrast-enhanced sonography is proving efficacious in grading vascularity after induced tendinopathy.87
Tissue engineering approaches using the rabbit model have been commonly used to address sequelae of Achilles pathology such as adhesions88 and tendon defects.89 For example, a recently developed electrospun silk wrap has been shown to be effective in providing significant reduction in adhesion formation in rabbit Achilles tendons, while also improving the biomechanical properties of the repaired tendon.88 Conversely, a new equine collagen membrane has shown histological signs of integrating into a rabbit Achilles tendon defect; however, the graft’s effects on resultant material properties or healing remains unknown.89 As Achilles tendon ruptures appear to be on the rise,90,91 this work could prove instrumental in improving both the strength and quality of operative tendon repair.
A more thorough understanding of the biological interplay between tendinopathy and other disease states remains elusive. For example, the effects of hypercholesterolemia on the rotator cuff and patellar tendons have been studied in several animals44,92,93 In regards to the Achilles tendon, a rat model has been used to elucidate the deleterious effect of diabetes on tendons and tendinopathy. In a diabetic state, activity improves material properties of the Achilles tendon,94 while that same state attenuates expression of several healing markers after an acute injury.95 This work points to the importance of moderate exercise in diabetic patients, while also suggesting that the healing response of tendons in such patients is indeed impaired.
Flexor
The full recovery of digit function following a flexor tendon injury remains a clinical challenge, with suboptimal repair rates ranging up to 31%.96,97 The most common complication is caused by deformation or gapping between tendon stumps, which leads to decreased mechanical properties and increased potential for rupture of the repaired tendon.98 Even if gapping does not occur, curbing the formation of peritendinous adhesions following repair remains difficult. These adhesions inhibit the smooth gliding of the tendon past surrounding tissues, which leads to reduced mobility, pain, and the inability to perform activities of daily living.99 In the past, the canine model was relied upon to perform research on flexor tendons.98-100 More recently, a wider variety of animals have been used to characterise flexor tendon injury and repair, including chicken,101 canine,102-104 ovine,105 porcine,106,107 and rabbit105-110 models. The best choice of model system will depend on the essential characteristics that must be mimicked for a particular research question.
Many flexor tendon studies have focused on improving the fixation methods and rehabilitation protocols used to prevent gapping and subsequent formation of an adhesion. Recently, an ex vivo uniaxial test demonstrated that the Yotsumoto-Dona technique, a side-locking loop structure paired with a horizontal mattress peripheral suture, performed significantly better in 2 mm gap force, yield force, ultimate force, stiffness, energy to yield, and energy to failure tests when compared with the often-used modified Kessler technique, which consists of a grasping type structure, paired with a running peripheral suture.106 In a separate ex vivo study, pulley-wrapped tension was applied to the tendon to better simulate gliding over bony surfaces.107 In this case, the interrupted horizontal mattress technique proved to be significantly more effective in regards to ultimate tensile strength and resistance to gapping. An in vivo approach to this problem provides a more comprehensive perspective on this issue, as biological healing factors and cyclic loading are taken into account. It was recently demonstrated that extending the core suture purchase and deepening the epitendinous suture repair was critical in improving repairs, as this strategy significantly reduced the incidence of gap formation and tendon rupture in a canine model. As far as rehabilitation protocols are concerned, several studies have suggested that early active mobilisation leads to more effective tendon gliding, less adhesion formation and more joint mobility108,109; however, this type of approach may lead to unacceptably high rupture rates.99 It has been suggested more recently that a well-controlled rehabilitation protocol may result in a somewhat limited range of movement, but this measured approach to remobilisation lowered the risk of rupture, perhaps making this option preferable.101
Similar to other tendons, the role of regenerative agents such as BMP-2, MSCs, and PRP have been examined to determine if they have the ability to augment scar formation in flexor tendon, but these approaches have been met with very limited success.103,105,110 On the other hand, although it is not typically used in the treatment of other tendons, the paired use of sodium hyaluronate (NaH) and human recombinant basic fibroblast growth factor has recently provided intriguing results regarding repair of a flexor tendon. Continued subcutaneous injections of these agents on a rabbit flexor repair site significantly reduced tendon diameter, increased ultimate tensile strength and yield strain, enhanced the maturation rate of the tenoblasts, and increased the diameter and density of the collagen fibrils.111-113 Additionally, a one-time direct application of NaH with Lactoferrin Peptide (PXL01) dissolved into solution curbed the need for repeated subcutaneous injections, and significantly increased the mobility of the rabbit paw, while having no adverse effects on the mechanical strength of the tendon.114
The use of tissue engineering applications has been investigated in flexor tendons, as the release of growth agents over extended periods of time may be beneficial in preventing gapping and rupture. Soft or absorbable constructs, such as calcium phosphate matrices, collagen sponges, and bioabsorbable membranes, have been developed to introduce growth factors to repaired flexor tendon sites.103,115 Such approaches have provided limited positive results, but can be easily adapted to accommodate different choices of regenerative agents. Rigid scaffolds offer a similar ability to reliably deliver growth factors and cells in a controlled manner, but they are also able to maintain a rigid form suitable for tendon repair surgery and long-term mechanical strength to prevent gapping (Fig. 4).104 Overall, these approaches are still in developmental phases, but the use of such technologies is promising.
Fig. 4.
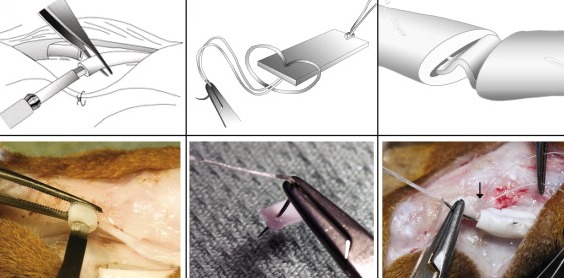
Schematic and photographic representations of the surgical protocol used to secure a mechanically rigid scaffold into the core of a flexor tendon. Reprinted from Manning CN, Schwartz AG, Liu W, et al. Controlled delivery of mesenchymal stem cells and growth factors using a nanofiber scaffold for tendon repair. Acta Biomater 2013;9:6905-14104 with permission from Elsevier.
Patella
The human patellar tendon is also susceptible to tendinopathy. However, the patellar tendon is of particular interest not only because it regularly experiences high-force cyclical loading, but also because portions of the tendon are commonly harvested during anterior cruciate ligament repairs. The patellar tendon is amenable to study with animal models because the tendon readily undergoes experimental cyclical loading, is easily dissected, and portions of the tendon are easily harvested to replicate a clinically relevant injury.
Because of the emerging evidence pointing to the importance of tendon fatigue as a precursor to tendinopathy and possibly tendon rupture, the patellar tendon of rats and mice has been used in the development of an in vivo fatigue model116,117 (Fig. 5). Results from this model have suggested that the initial accumulation of sub-rupture damage caused by in vivo cyclic loading leads to subsequent changes in mechanical function.116 Furthermore, like in the Achilles tendon, the upregulation of genes such as collagen I (Col I), collagen XII (Col XII), matrix metalloproteinase 2 (MMP2), and tissue inhibitor of metalloproteinase (TIMP3) shows an initially adaptive response to cyclic loading that is attenuated after a certain amount of damage.118 However, while a fatigue model evokes a different molecular response than an acute rupture, structural restoration of an overly fatigued tendon may never be complete.117,119 This points to the importance of developing better tools for recognising tendon fatigue in the clinical setting, as irreversible tendon damage may occur earlier than the consensus suggests.
Fig. 5.
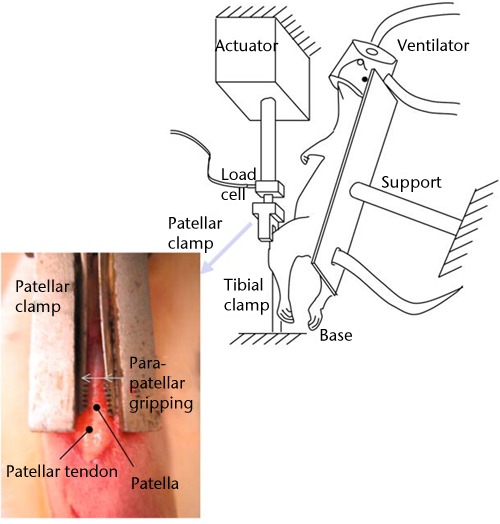
Image demonstrating the experimental set-up for in vivo fatigue testing of the patellar tendon. This allowed in vivo tendon loading without interfering with the movement of the tendon. Reprinted from Fung DT, Wang VM, Andarawis-Puri N, et al. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech 2010;43:274-9117 with permission from Elsevier.
Adult tendons do not heal through a regenerative process, but rather a scarring process120; therefore, understanding the biological underpinnings of tendon healing has become a significant vein of research, commonly employing the patellar tendon as a model. In comparison with tendinopathy of the patellar tendon, acute defects of the patellar tendon are commonly iatrogenic when the tendon is harvested for the purposes of anterior cruciate ligament reconstruction surgery. Therefore, those studying the reasons for inefficient tendon healing have employed a similar approach, where the central third of the patellar tendon is removed, known as the ‘window defect’ model. As sometimes observed in patients,121 rat tendons exhibit ectopic chondrogenesis and ossification following this injury as well, while expression of biglycan increases and levels of aggrecan and decorin decrease.122 Although the function of these proteoglycans is not fully understood, these changes may, in part, explain the poor tissue quality that is often observed after this injury. Indeed, despite some histological and molecular indications of healing, ultimate load and stiffness only reach 48% and 63% of baseline respectively.123 Thus, efforts have been made to recoup the mechanical deficiencies that occur as a result of harvest of the patellar tendon. For example, after the introduction of tendon-derived stem cells to a window defect in a rat model, tendons exhibited increases in collagen production and improvement in resultant alignment and material properties.124
Physical therapy is currently a mainstay of non-operative treatment for patellar tendinopathy. Specifically, eccentric training has shown significant increases in failure load, failure stress, and vascularisation, while concentric training only significantly improved failure stress.125 Nevertheless, alternatives to physical therapy have recently been studied as viable options for tendon rehabilitation. Laser, light emitting diode, radiofrequency ablation, hyperbaric oxygen and autologous tenocyte therapies have all shown some promise with respect to improving the mechanical or histological properties of healing tendon,126-128 while the beneficial effects of high-energy extracorporeal shockwave therapy have not shown as much benefit in tendon repair as in the treatment of other musculoskeletal disorders.129-131 In general, the goal is to improve the quality of tendon tissue after injury and allow earlier and more aggressive rehabilitation protocols to speed recovery.
The effects of age on tendon mechanics and metabolism continue to be elucidated through animal models. The Achilles tendon has been used to understand better prenatal tendon properties, which have the ability to undergo scarless repair, forming a structurally uninjured tendon. Not surprisingly, as a neonatal mouse Achilles tendon matures, collagen content increases, fibril diameter increases, and the tendon becomes stronger.132 On the other end of the age spectrum, a study of both the murine patellar tendon and in vivo rat Achilles tendon has suggested that an aged tendon has inferior mechanical and histological properties.14 Specifically, it is the maladaptive changes in passive biomechanical properties of an aged tendon, such as increased stiffness, increased peak tension and increased estimated modulus that are most interesting, as they are postulated to be part of the reason why the incidence of Achilles tendon ruptures is more common in middle-age. Thus, the ability to predict the viscoelastic behavior of a tendon could have clinical applications. This highlights the importance of a recently developed empirical model which uses in vivo measurements to accurately predict the viscoelastic properties of damaged or aged murine patellar tendons based on a single stress measurement.133
Conclusion
Tendinopathy can result in significant pain and disability, which has driven the need for research dedicated to tendon repair and healing. In comparison to other fields, tendon research is still in its infancy, and the complex nature of the tissue continues to provide intriguing answers to pointed research questions. This review discusses the role of animal models in regards to the current understanding of the mechanical, structural and biological changes that occur during tendon repair and healing. The unique benefits of animal modeling techniques will continue to be used in the future to promote experimental endeavours in this field of study. Through the use of such models, it is expected that translation to the human tendon will be successful and that therapeutics, diagnostics and clinical outcomes will continue to improve.
Funding Statement
L. J. Soslowsky reports that his institution, the University of Pennsylvania, has received grants from DJO and Amniox, neither of which is related to this article.
Footnotes
Author contributions:M. W. Hast: Literature review, Writing the paper
A. Zuskov: Literature review, Writing the paper
L. J. Soslowsky: Oversaw the review and writing process
ICMJE Conflict of Interest:None declared
References
- 1.No authors listed. Bone and Joint Initiative USA: The Burden of Musculoskeletal Diseases in the United States. Second ed. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2011.
- 2.Kannus P. Tendons--a source of major concern in competitive and recreational athletes. Scand J Med Sci Sports 1997;7:53–54 [PubMed] [Google Scholar]
- 3.No authors listed. Office for National Statistics. General Household Survey 2006 http://www.statistics.gov.uk (date last accessed 6 June 2014).
- 4.Woo SL-Y, Thay TQ, Abramowitch SD, Gilbert T. Structure and function of ligaments and tendons. In Basic orthopaedic Biomechanics & Mechanobiology, Ed. Mow VC, Hayes WC. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins, 2005:301-342.
- 5.Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg 2012;21:228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rio E, Moseley L, Purdam C, et al. The pain of tendinopathy: physiological or pathophysiological? Sports Med 2014;44:9–23 [DOI] [PubMed] [Google Scholar]
- 7.Del Buono A, Oliva F, Longo UG, et al. Metalloproteases and rotator cuff disease. J Shoulder Elbow Surg 2012;21:200–208 [DOI] [PubMed] [Google Scholar]
- 8.Wang JH, Guo Q, Li B. Tendon biomechanics and mechanobiology--a minireview of basic concepts and recent advancements. J Hand Ther 2012;25:133-40; quiz 141. [DOI] [PMC free article] [PubMed]
- 9.Bishop J, Klepps S, Lo IK, et al. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg 2006;15:290–299 [DOI] [PubMed] [Google Scholar]
- 10.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg [Am] 2004;86-A:219–224 [DOI] [PubMed] [Google Scholar]
- 11.Murchison ND, Price BA, Conner DA, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 2007;134:2697–2708 [DOI] [PubMed] [Google Scholar]
- 12.Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001;128:3855–3866 [DOI] [PubMed] [Google Scholar]
- 13.Scott A, Sampaio A, Abraham T, Duronio C, Underhill TM. Scleraxis expression is coordinately regulated in a murine model of patellar tendon injury. J Orthop Res 2011;29:289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunkman AA, Buckley MR, Mienaltowski MJ, et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol 2013;32:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danielson KG, Baribault H, Holmes DF, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 1997;136:729–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dourte LM, Pathmanathan L, Mienaltowski MJ, et al. Mechanical, compositional, and structural properties of the mouse patellar tendon with changes in biglycan gene expression. J Orthop Res 2013;31:1430–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthet E, Chen C, Butcher K, et al. Smad3 binds Scleraxis and Mohawk and regulates tendon matrix organization. J Orthop Res 2013;31:1475–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito Y, Toriuchi N, Yoshitaka T, et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc Natl Acad Sci U S A 2010;107:10538–10542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SM, Zhang G, Birk DE. Collagen V localizes to pericellular sites during tendon collagen fibrillogenesis. Matrix Biol 2014;33:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenstrup RJ, Smith SM, Florer JB, et al. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. J Biol Chem 2011;286:20455–20465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech 2006;39:61–69 [DOI] [PubMed] [Google Scholar]
- 22.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg 1996;5:383–392 [DOI] [PubMed] [Google Scholar]
- 23.Schorpp M, Mattei MG, Herr I, et al. Structural organization and chromosomal localization of the mouse collagenase type I gene. Biochem J 1995. May 15;308:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lui PPY, Maffulli N, Rolf C, Smith RKW. What are the validated animal models for tendinopathy? Scand J Med Sci Sports 2011;21:3–17 [DOI] [PubMed] [Google Scholar]
- 25.McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol 2014;87:162–171 [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Laron D, Natsuhara K, et al. A mouse model of massive rotator cuff tears. J Bone Joint Surg [Am] 2012;94-A:41. [DOI] [PubMed] [Google Scholar]
- 27.Beason DP, Kuntz AF, Hsu JE, Miller KS, Soslowsky LJ. Development and evaluation of multiple tendon injury models in the mouse. J Biomech 2012;45:1550–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samagh SP, Kramer EJ, Melkus G, et al. MRI quantification of fatty infiltration and muscle atrophy in a mouse model of rotator cuff tears. J Orthop Res 2013;31:421–426 [DOI] [PubMed] [Google Scholar]
- 29.Quigley RJ, Gupta A, Oh JH, et al. Biomechanical comparison of single-row, double-row, and transosseous-equivalent repair techniques after healing in an animal rotator cuff tear model. J Orthop Res 2013;31:1254–1260 [DOI] [PubMed] [Google Scholar]
- 30.Chung SW, Song BW, Kim YH, Park KU, Oh JH. Effect of platelet-rich plasma and porcine dermal collagen graft augmentation for rotator cuff healing in a rabbit model. Am J Sports Med 2013;41:2909–2918 [DOI] [PubMed] [Google Scholar]
- 31.Lovric V, Ledger M, Goldberg J, et al. The effects of low-intensity pulsed ultrasound on tendon-bone healing in a transosseous-equivalent sheep rotator cuff model. Knee Surg Sports Traumatol Arthrosc 2013;21:466–475 [DOI] [PubMed] [Google Scholar]
- 32.Jost PW, Khair MM, Chen DX, et al. Suture number determines strength of rotator cuff repair. J Bone Joint Surg [Am] 2012;94:100. [DOI] [PubMed] [Google Scholar]
- 33.Baums MH, Spahn G, Buchhorn GH, et al. Biomechanical and magnetic resonance imaging evaluation of a single- and double-row rotator cuff repair in an in vivo sheep model. Arthroscopy 2012;28:769–777 [DOI] [PubMed] [Google Scholar]
- 34.Onay U, Akpınar S, Akgün RC, Balçık C, Tuncay IC. Comparison of repair techniques in small and medium-sized rotator cuff tears in cadaveric sheep shoulders. Acta Orthop Traumatol Turc 2013;47:179–183 [DOI] [PubMed] [Google Scholar]
- 35.Ostrander RV 3rd, McKinney BI. Evaluation of footprint contact area and pressure using a triple-row modification of the suture-bridge technique for rotator cuff repair. J Shoulder Elbow Surg 2012;21:1406–1412 [DOI] [PubMed] [Google Scholar]
- 36.Savage AJ, Spruiell MD, Schwertz JM, et al. The effect of sliding knots on the suture-tendon interface strength: a biomechanical analysis comparing sliding and static arthroscopic knots. Am J Sports Med 2013;41:296–301 [DOI] [PubMed] [Google Scholar]
- 37.Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc 2011;19:1582–1587 [DOI] [PubMed] [Google Scholar]
- 38.Bey MJ, Kline SK, Baker AR, et al. Estimation of dynamic, in vivo soft-tissue deformation: experimental technique and application in a canine model of tendon injury and repair. J Orthop Res 2011;29:822–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker AR, McCarron JA, Tan CD, Iannotti JP, Derwin KA. Does augmentation with a reinforced fascia patch improve rotator cuff repair outcomes? Clin Orthop Relat Res 2012;470:2513–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hee CK, Dines JS, Dines DM, et al. Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model. Am J Sports Med 2011;39:1630–1639 [DOI] [PubMed] [Google Scholar]
- 41.De Carli A, Lanzetti RM, Monaco E, et al. The failure mode of two reabsorbable fixation systems: Swivelock with Fibertape versus Bio-Corkscrew with Fiberwire in bovine rotator cuff. J Orthop Sci 2012;17:789–795 [DOI] [PubMed] [Google Scholar]
- 42.Anderl W, Heuberer PR, Laky B, et al. Superiority of bridging techniques with medial fixation on initial strength. Knee Surg Sports Traumatol Arthrosc 2012;20:2559–2566 [DOI] [PubMed] [Google Scholar]
- 43.Wieser K, Farshad M, Vlachopoulos L, et al. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy 2012;28:1622–1627 [DOI] [PubMed] [Google Scholar]
- 44.Beason DP, Hsu JE, Marshall SM, et al. Hypercholesterolemia increases supraspinatus tendon stiffness and elastic modulus across multiple species. J Shoulder Elbow Surg 2013;22:681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu H, Sandor M, Qi S, et al. Implantation of a porcine acellular dermal graft in a primate model of rotator cuff repair. J Shoulder Elbow Surg 2012;21:580–588 [DOI] [PubMed] [Google Scholar]
- 46.Palumbo C, Rovesta C, Ferretti M. Striated muscle fiber apoptosis after experimental tendon lesion in a rat model. J Anat 2012;221:358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Joshi SK, Samagh SP, et al. Evaluation of Akt/mTOR activity in muscle atrophy after rotator cuff tears in a rat model. J Orthop Res 2012;30:1440–1446 [DOI] [PubMed] [Google Scholar]
- 48.Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg 2012;21:847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peltz CD, Hsu JE, Zgonis MH, et al. Decreased loading after rotator cuff tears leads to improved biceps tendon properties in a rat model. J Shoulder Elbow Surg 2011;20:698–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Castro Pochini A, Ejnisman B, de Seixas Alves MT, et al. Overuse of training increases mechanoreceptors in supraspinatus tendon of rats SHR. J Orthop Res 2011;29:1771–1774 [DOI] [PubMed] [Google Scholar]
- 51.Reuther KE, Thomas SJ, Sarver JJ, et al. Effect of return to overuse activity following an isolated supraspinatus tendon tear on adjacent intact tendons and glenoid cartilage in a rat model. J Orthop Res 2013;31:710–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reuther KE, Thomas SJ, Evans EF, et al. Returning to overuse activity following a supraspinatus and infraspinatus tear leads to joint damage in a rat model. J Biomech 2013;46:1818–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peltz CD, Hsu JE, Zgonis MH, et al. Intra-articular changes precede extra-articular changes in the biceps tendon after rotator cuff tears in a rat model. J Shoulder Elbow Surg 2012;21:873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reuther KE, Sarver JJ, Schultz SM, et al. Glenoid cartilage mechanical properties decrease after rotator cuff tears in a rat model. J Orthop Res 2012;30:1435–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uezono K, Ide J, Tokunaga T, et al. Effect of immobilization on rotator cuff reconstruction with acellular dermal matrix grafts in an animal model. J Shoulder Elbow Surg 2013;22:1290–1297 [DOI] [PubMed] [Google Scholar]
- 56.Killian ML, Lim CT, Thomopoulos S, et al. The effect of unloading on gene expression of healthy and injured rotator cuffs. J Orthop Res 2013;31:1240–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ficklscherer A, Hartl TK, Scharf M, et al. Effects of selective paralysis of the supraspinatus muscle using botulinum neurotoxin a in rotator cuff healing in rats. J Orthop Res 2013;31:716–723 [DOI] [PubMed] [Google Scholar]
- 58.Buchmann S, Sandmann GH, Walz L, et al. Refixation of the supraspinatus tendon in a rat model--influence of continuous growth factor application on tendon structure. J Orthop Res 2013;31:300–305 [DOI] [PubMed] [Google Scholar]
- 59.Hapa O, Cakıcı H, Kükner A, et al. Effect of platelet-rich plasma on tendon-to-bone healing after rotator cuff repair in rats: an in vivo experimental study. Acta Orthop Traumatol Turc 2012;46:301–307 [DOI] [PubMed] [Google Scholar]
- 60.Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med 2012;40:2037–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baumgarten KM, Oliver HA, Foley J, et al. Human growth hormone may be detrimental when used to accelerate recovery from acute tendon-bone interface injuries. J Bone Joint Surg [Am] 2013;95-A:783–789 [DOI] [PubMed] [Google Scholar]
- 62.Hettrich CM, Beamer BS, Bedi A, et al. The effect of rhPTH on the healing of tendon to bone in a rat model. J Orthop Res 2012;30:769–774 [DOI] [PubMed] [Google Scholar]
- 63.Coen MJ, Chen ST, Rundle CH, Wergedal JE, Lau KH. Lentiviral-based BMP4 in vivo gene transfer strategy increases pull-out tensile strength without an improvement in the osteointegration of the tendon graft in a rat model of biceps tenodesis. J Gene Med 2011;13:511–521 [DOI] [PubMed] [Google Scholar]
- 64.Loeffler BJ, Scannell BP, Peindl RD, et al. Cell-based tissue engineering augments tendon-to-bone healing in a rat supraspinatus model. J Orthop Res 2013;31:407–412 [DOI] [PubMed] [Google Scholar]
- 65.Kida Y, Morihara T, Matsuda K, et al. Bone marrow-derived cells from the footprint infiltrate into the repaired rotator cuff. J Shoulder Elbow Surg 2013;22:197–205 [DOI] [PubMed] [Google Scholar]
- 66.Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elbow Surg 2014;23:445–455 [DOI] [PubMed] [Google Scholar]
- 67.Kim YS, Lee HJ, Ok JH, Park JS, Kim DW. Survivorship of implanted bone marrow-derived mesenchymal stem cells in acute rotator cuff tear. J Shoulder Elbow Surg 2013;22:1037–1045 [DOI] [PubMed] [Google Scholar]
- 68.Yokoya S, Mochizuki Y, Natsu K, et al. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med 2012;40:1259–1268 [DOI] [PubMed] [Google Scholar]
- 69.Beason DP, Connizzo BK, Dourte LM, et al. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. J Shoulder Elbow Surg 2012;21:245–250 [DOI] [PubMed] [Google Scholar]
- 70.Levy DM, Saifi C, Perri JL, et al. Rotator cuff repair augmentation with local autogenous bone marrow via humeral cannulation in a rat model. J Shoulder Elbow Surg 2013;22:1256–1264 [DOI] [PubMed] [Google Scholar]
- 71.Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg 2012;21:181–190 [DOI] [PubMed] [Google Scholar]
- 72.Komi PV. Relevance of in vivo force measurements to human biomechanics. J Biomech 1990;23(Suppl1):23–34 [DOI] [PubMed] [Google Scholar]
- 73.Heinemeier KM, Skovgaard D, Bayer ML, et al. Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes. J Appl Physiol (1985) 2012;113:827–836 [DOI] [PubMed] [Google Scholar]
- 74.Silva RD, Glazebrook MA, Campos VC, Vasconcelos AC. Achilles tendinosis: a morphometrical study in a rat model. Int J Clin Exp Pathol 2011;4:683–691 [PMC free article] [PubMed] [Google Scholar]
- 75.Ng GY, Chung PY, Wang JS, Cheung RT. Enforced bipedal downhill running induces Achilles tendinosis in rats. Connect Tissue Res 2011;52:466–471 [DOI] [PubMed] [Google Scholar]
- 76.Cho NS, Hwang JH, Lee YT, Chae SW. Tendinosis-like histologic and molecular changes of the Achilles tendon to repetitive stress: a pilot study in rats. Clin Orthop Relat Res 2011;469:3172–3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bell R, Li J, Gorski DJ, et al. Controlled treadmill exercise eliminates chondroid deposits and restores tensile properties in a new murine tendinopathy model. J Biomech 2013;46:498–505 [DOI] [PubMed] [Google Scholar]
- 78.Andersson G, Backman LJ, Scott A, et al. Substance P accelerates hypercellularity and angiogenesis in tendon tissue and enhances paratendinitis in response to Achilles tendon overuse in a tendinopathy model. Br J Sports Med 2011;45:1017–1022 [DOI] [PubMed] [Google Scholar]
- 79.Leek BT, Tasto JP, Tibor LM, et al. Augmentation of tendon healing with butyric acid-impregnated sutures: biomechanical evaluation in a rabbit model. Am J Sports Med 2012;40:1762–1771 [DOI] [PubMed] [Google Scholar]
- 80.Yao J, Woon CY, Behn A, et al. The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg Am 2012;37:1639–1645 [DOI] [PubMed] [Google Scholar]
- 81.Ortiz C, Wagner E, Mocoçain P, et al. Biomechanical comparison of four methods of repair of the Achilles tendon: a laboratory study with bovine tendons. J Bone Joint Surg [Br] 2012;94-B:663–667 [DOI] [PubMed] [Google Scholar]
- 82.Jielile J, Jialili A, Sabirhazi G, et al. Proteomic analysis of differential protein expression of achilles tendon in a rabbit model by two-dimensional polyacrylamide gel electrophoresis at 21 days postoperation. Appl Biochem Biotechnol 2011;165:1092–1106 [DOI] [PubMed] [Google Scholar]
- 83.Eliasson P, Andersson T, Aspenberg P. Influence of a single loading episode on gene expression in healing rat Achilles tendons. J Appl Physiol (1985) 2012;112:279–288 [DOI] [PubMed] [Google Scholar]
- 84.Andersson T, Eliasson P, Hammerman M, Sandberg O, Aspenberg P. Low-level mechanical stimulation is sufficient to improve tendon healing in rats. J Appl Physiol (1985) 2012;113:1398–1402 [DOI] [PubMed] [Google Scholar]
- 85.Eliasson P, Andersson T, Aspenberg P. Achilles tendon healing in rats is improved by intermittent mechanical loading during the inflammatory phase. J Orthop Res 2012;30:274–279 [DOI] [PubMed] [Google Scholar]
- 86.Gehmert S, Jung EM, Kügler T, et al. Sonoelastography can be used to monitor the restoration of Achilles tendon elasticity after injury. Ultraschall Med 2012;33:581–586 [DOI] [PubMed] [Google Scholar]
- 87.Chang KV, Wu CH, Ding YH, et al. Application of contrast-enhanced sonography with time-intensity curve analysis to explore hypervascularity in Achilles tendinopathy by using a rabbit model. J Ultrasound Med 2012;31:737–746 [DOI] [PubMed] [Google Scholar]
- 88.Ni T, Senthil-Kumar P, Dubbin K, et al. A photoactivated nanofiber graft material for augmented Achilles tendon repair. Lasers Surg Med 2012;44:645–652 [DOI] [PubMed] [Google Scholar]
- 89.Gigante A, Busilacchi A, Lonzi B, et al. Purified collagen I oriented membrane for tendon repair: an ex vivo morphological study. J Orthop Res 2013;31:738–745 [DOI] [PubMed] [Google Scholar]
- 90.Nyyssönen T, Lüthje P, Kröger H. The increasing incidence and difference in sex distribution of Achilles tendon rupture in Finland in 1987-1999. Scand J Surg 2008;97:272–275 [DOI] [PubMed] [Google Scholar]
- 91.Ufberg J, Harrigan RA, Cruz T, Perron AD. Orthopedic pitfalls in the ED: Achilles tendon rupture. Am J Emerg Med 2004;22:596–600 [DOI] [PubMed] [Google Scholar]
- 92.Beason DP, Abboud JA, Kuntz AF, Bassora R, Soslowsky LJ. Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J Orthop Res 2011;29:380–383 [DOI] [PubMed] [Google Scholar]
- 93.Beason DP, Tucker JJ, Lee CS, et al. Rat rotator cuff tendon-to-bone healing properties are adversely affected by hypercholesterolemia. J Shoulder Elbow Surg 2014;23:867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Oliveira RR, Bezerra MA, de Lira KD, et al. Aerobic physical training restores biomechanical properties of Achilles tendon in rats chemically induced to diabetes mellitus. J Diabetes Complications 2012;26:163–168 [DOI] [PubMed] [Google Scholar]
- 95.Ahmed AS, Schizas N, Li J, et al. Type 2 diabetes impairs tendon repair after injury in a rat model. J Appl Physiol (1985) 2012;113:1784–1791 [DOI] [PubMed] [Google Scholar]
- 96.Peck FH, Bücher CA, Watson JS, Roe A. A comparative study of two methods of controlled mobilization of flexor tendon repairs in zone 2. J Hand Surg Br 1998;23:41–45 [DOI] [PubMed] [Google Scholar]
- 97.Kitsis CK, Wade PJ, Krikler SJ, Parsons NK, Nicholls LK. Controlled active motion following primary flexor tendon repair: a prospective study over 9 years. J Hand Surg Br 1998;23:344–349 [DOI] [PubMed] [Google Scholar]
- 98.Gelberman RH, Boyer MI, Brodt MD, Winters SC, Silva MJ. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg [Am] 1999;81-A:975–982 [DOI] [PubMed] [Google Scholar]
- 99.Gelberman RH, Vandeberg JS, Manske PR, Akeson WH. The early stages of flexor tendon healing: a morphologic study of the first fourteen days. J Hand Surg Am 1985;10:776–784 [DOI] [PubMed] [Google Scholar]
- 100.Gelberman RH, Manske PR, Akeson WH, et al. Flexor tendon repair. J Orthop Res 1986;4:119–128 [DOI] [PubMed] [Google Scholar]
- 101.Lee H, Hou Z, Liu P, et al. An experimental study comparing active mobilization to passive flexion-active extension-active flexion after flexor tendon repair in zone 2. J Hand Surg Am 2013;38:672–676 [DOI] [PubMed] [Google Scholar]
- 102.Fufa DT, Osei DA, Calfee RP, et al. The effect of core and epitendinous suture modifications on repair of intrasynovial flexor tendons in an in vivo canine model. J Hand Surg Am 2012;37:2526–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomopoulos S, Kim HM, Silva MJ, et al. Effect of bone morphogenetic protein 2 on tendon-to-bone healing in a canine flexor tendon model. J Orthop Res 2012;30:1702–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manning CN, Schwartz AG, Liu W, et al. Controlled delivery of mesenchymal stem cells and growth factors using a nanofiber scaffold for tendon repair. Acta Biomater 2013;9:6905–6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinello T, Bronzini I, Perazzi A, et al. Effects of in vivo applications of peripheral blood-derived mesenchymal stromal cells (PB-MSCs) and platlet-rich plasma (PRP) on experimentally injured deep digital flexor tendons of sheep. J Orthop Res 2013;31:306–314 [DOI] [PubMed] [Google Scholar]
- 106.Rigó IZ, Haugstvedt JR, Ludvigsen P, Røkkum M. Comparison of modified Kessler and Yotsumoto-Dona suture: a biomechanical study on porcine tendons. J Plast Surg Hand Surg 2012;46:313–317 [DOI] [PubMed] [Google Scholar]
- 107.Henderson J, Sutcliffe M, Gillespie P. Epitendinous suture techniques in extensor tendon repairs--an experimental evaluation. J Hand Surg Am 2011;36:1968–1973 [DOI] [PubMed] [Google Scholar]
- 108.Silfverskiöld KL, May EJ. Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion. J Hand Surg Am 1994;19:53–60 [DOI] [PubMed] [Google Scholar]
- 109.Becker H, Orak F, Duponselle E. Early active motion following a beveled technique of flexor tendon repair: report on fifty cases. J Hand Surg Am 1979;4:454–460 [DOI] [PubMed] [Google Scholar]
- 110.Derby BM, Reichensperger J, Chambers C, et al. Early growth response factor-1: expression in a rabbit flexor tendon scar model. Plast Reconstr Surg 2012;129:435–442 [DOI] [PubMed] [Google Scholar]
- 111.Oryan A, Moshiri A, Meimandi Parizi AH, Raayat Jahromi A. Repeated administration of exogenous Sodium-hyaluronate improved tendon healing in an in vivo transection model. J Tissue Viability 2012;21:88–102 [DOI] [PubMed] [Google Scholar]
- 112.Oryan A, Moshiri A. A long term study on the role of exogenous human recombinant basic fibroblast growth factor on the superficial digital flexor tendon healing in rabbits. J Musculoskelet Neuronal Interact 2011;11:185–195 [PubMed] [Google Scholar]
- 113.Moshiri A, Oryan A. Structural and functional modulation of early healing of full-thickness superficial digital flexor tendon rupture in rabbits by repeated subcutaneous administration of exogenous human recombinant basic fibroblast growth factor. J Foot Ankle Surg 2011;50:654–662 [DOI] [PubMed] [Google Scholar]
- 114.Håkansson J, Mahlapuu M, Ekström L, Olmarker K, Wiig M. Effect of lactoferrin peptide (PXL01) on rabbit digit mobility after flexor tendon repair. J Hand Surg Am 2012;37:2519–2525 [DOI] [PubMed] [Google Scholar]
- 115.Sato T, Shimizu H, Beppu M, Takagi M. Effects on bone union and prevention of tendon adhesion by new porous anti-adhesive poly L-lactide-co-ε-caprolactone membrane in a rabbit model. Hand Surg 2013;18:1–10 [DOI] [PubMed] [Google Scholar]
- 116.Sereysky JB, Andarawis-Puri N, Jepsen KJ, Flatow EL. Structural and mechanical effects of in vivo fatigue damage induction on murine tendon. J Orthop Res 2012;30:965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fung DT, Wang VM, Andarawis-Puri N, et al. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech 2010;43:274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Andarawis-Puri N, Sereysky JB, Sun HB, Jepsen KJ, Flatow EL. Molecular response of the patellar tendon to fatigue loading explained in the context of the initial induced damage and number of fatigue loading cycles. J Orthop Res 2012;30:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andarawis-Puri N, Sereysky JB, Jepsen KJ, Flatow EL. The relationships between cyclic fatigue loading, changes in initial mechanical properties, and the in vivo temporal mechanical response of the rat patellar tendon. J Biomech 2012;45:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng 2012;14:47–71 [DOI] [PubMed] [Google Scholar]
- 121.Rui YF, Lui PP, Rolf CG, et al. Expression of chondro-osteogenic BMPs in clinical samples of patellar tendinopathy. Knee Surg Sports Traumatol Arthrosc 2012;20:1409–1417 [DOI] [PubMed] [Google Scholar]
- 122.Lui PP, Cheuk YC, Lee YW, Chan KM. Ectopic chondro-ossification and erroneous extracellular matrix deposition in a tendon window injury model. J Orthop Res 2012;30:37–46 [DOI] [PubMed] [Google Scholar]
- 123.Dyment NA, Kazemi N, Aschbacher-Smith LE, et al. The relationships among spatiotemporal collagen gene expression, histology, and biomechanics following full-length injury in the murine patellar tendon. J Orthop Res 2012;30:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ni M, Lui PP, Rui YF, et al. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res 2012;30:613–619 [DOI] [PubMed] [Google Scholar]
- 125.Kaux JF, Drion P, Libertiaux V, et al. Eccentric training improves tendon biomechanical properties: a rat model. J Orthop Res 2013;31:119–124 [DOI] [PubMed] [Google Scholar]
- 126.Ng GY, Chung PY. Effects of a therapeutic laser and passive stretching program for treating tendon overuse. Photomed Laser Surg 2012;30:155–159 [DOI] [PubMed] [Google Scholar]
- 127.Xavier M, David DR, de Souza RA, et al. Anti-inflammatory effects of low-level light emitting diode therapy on Achilles tendinitis in rats. Lasers Surg Med 2010;42:553–558 [DOI] [PubMed] [Google Scholar]
- 128.Tibor LM, Leek BT, Chase DC, et al. A biomechanical assessment of tendon repair after radiofrequency treatment. Am J Orthop (Belle Mead NJ) 2012;41:E115–E121 [PubMed] [Google Scholar]
- 129.Penteado FT, Faloppa F, Giusti G, et al. High-energy extracorporeal shockwave therapy in a patellar tendon animal model: a vascularization focused study. Clinics (Sao Paulo) 2011;66:1611–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Çınar BM, Çirci E, Balçık C, et al. The effects of extracorporeal shock waves on carrageenan-induced Achilles tendinitis in rats: a biomechanical and histological analysis. Acta Orthop Traumatol Turc 2013;47:266–272 [DOI] [PubMed] [Google Scholar]
- 131.Yoo SD, Choi S, Lee GJ, et al. Effects of extracorporeal shockwave therapy on nanostructural and biomechanical responses in the collagenase-induced Achilles tendinitis animal model. Lasers Med Sci 2012;27:1195–1204 [DOI] [PubMed] [Google Scholar]
- 132.Ansorge HL, Adams S, Birk DE, Soslowsky LJ. Mechanical, compositional, and structural properties of the post-natal mouse Achilles tendon. Ann Biomed Eng 2011;39:1904–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buckley MR, Dunkman AA, Reuther KE, et al. Validation of an empirical damage model for aging and in vivo injury of the murine patellar tendon. J Biomech Eng 2013;135:041005. [DOI] [PMC free article] [PubMed]


