Abstract
High-molecular-weight kininogen domain 5 (HK5) is an angiogenic modulator that is capable of inhibiting endothelial cell proliferation, migration, adhesion, and tube formation. Ferritin can bind to a histidine–glycine–lysine-rich region within HK5 and block its antiangiogenic effects. However, the molecular intricacies of this interaction are not well understood. Analysis of the structure of HK5 using circular dichroism and nuclear magnetic resonance [1H, 15N]-heteronuclear single quantum coherence determined that HK5 is an intrinsically unstructured protein, consistent with secondary structure predictions. Equilibrium binding studies using fluorescence anisotropy were used to study the interaction between ferritin and HK5. The interaction between the two proteins is mediated by metal ions such as Co2+, Cd2+, and Fe2+. This metal-mediated interaction works independently of the loaded ferrihydrite core of ferritin and is demonstrated to be a surface interaction. Ferritin H and L bind to HK5 with similar affinity in the presence of metals. The ferritin interaction with HK5 is the first biological function shown to occur on the surface of ferritin using its surface-bound metals.
Keywords: kininogen, ferritin, angiogenesis, metal ions, intrinsically unstructured protein, protein interaction
Introduction
Angiogenesis, the formation of new blood vessels from existing vessels, is essential for such physiological processes as wound healing and tumor growth. It is a highly regulated process and numerous endogenous modulators exist that both positively and negatively control angiogenesis. When the balance of angiogenic modulators is altered, pathologic vascularization can occur. This is often the case in situations such as tumor malignancy, where the modulator levels or “angiogenic switch”1 are tipped in the favor of promoting vessel growth.
Antiangiogenic factors can be produced from extracellular matrix components, such as tumstatin, derived from the α3 chain of type IV collagen,2 and endostatin from collagen XVIII.3 Other antiangiogenic factors are derived from plasma proteins, such as high-molecular-weight kininogen (HK), a nonenzymatic cofactor in the intrinsic blood coagulation pathway. When HK is cleaved by kallikrein, the nonapeptide bradykinin is released, leaving behind the second cleavage product HKa.4 HKa, a two-chain protein connected by a disulfide bond, has been implicated in inhibiting angiogenesis. This antiangiogenic activity has been narrowed to domain 5 of HKa (HK5), found on the light chain of HKa.5,6 Specifically, HK5 inhibits migration, proliferation, and adhesion of endothelial cells, as well as induce apoptosis7–9 both in vitro and in vivo using xenograft and chick chorioallantoic membrane assays.
HK also interacts with ferritin, an iron storage protein, through the HK5 domain.10 Once HK binds to ferritin, proteolytic cleavage into the products HKa and bradykinin is blocked, which is likely due to the steric hindrance of kallikrein by ferritin.11,12 The interaction between HK and ferritin modulates the antiangiogenic effects of HK on endothelial cells by rescuing the reduced proliferation, adhesion, migration and viability to control levels.13
Ferritin assembles as a 24 subunit icositetrahedral structure when intracellular labile iron pool (LIP) levels are high.14 The 24 monomers are a combination of heavy and light chains (ferritin H and ferritin L), which varies by systemic location. Ferroxidase sites within the four-helix bundle of ferritin H monomers (21 kDa) oxidize the excess iron atoms to a ferric state as they are shuttled from the excess LIP to the hollow core of ferritin for storage.15,16 Ferritin L monomers (19 kDa) promote incorporation or nucleation of the ferric iron atoms once they reach the ferrihydrite core,17 thereby increasing the overall stability of the protein. While iron is predominantly found in the ferrihydrite core of ferritin, the icositetrahedron does contain other metal-binding sites. The ferroxidase center within the four-helix bundle of ferritin H monomers contains two metal ion binding sites.18 Other interior sites for ruthenium and palladium coordination have been observed in the crystal structures of apoferritin through residues Asp38, Glu45, Cys48, His49, Glu53, and His173.19,20 Metal coordination sites on the exterior of ferritin include palladium coordination sites at Ser2, Gln3, and Asp40 in horse ferritin L20 as well as cadmium coordination sites at Glu92, Asp84, and Glu90 and between Asp15 residues of two human ferritin L (hFL) monomers.21 While these sites have been shown to bind metals, the biological functions of these metal-binding sites are not well understood.
HK5 exerts its antiangiogenic effects through its interaction with urokinase-type plasminogen activator receptor (uPAR), the surface-bound receptor for urokinase that is also involved in angiogenic signaling.22 The binding interface between these two proteins involves the histidine–glycine–lysine (HGK)-rich region of HK5 and domains 2 and 3 of uPAR. Ferritin binds to the HGK-rich region of HK5. However, the intricacies of the interaction between the two proteins are not well understood. In order to understand the ferritin–HK5 protein interaction, we investigated the structure of HK5 and the details of its interaction with ferritin. This information may lay a foundation for the development of potential inhibitors that can mediate the interaction and subsequently control the antiangiogenic effects of HK5 on the uPAR pathway.
Results
The secondary structure of HK5 is largely random coil
HK5 is a functional domain of high-molecular-weight kininogen that binds to multiple receptors on the endothelial cell surface. The limited structural information available for the HK protein indicates HK is a three-lobed entity whose shape changes from a linear to triangular three-lobed structure once bradykinin is cleaved from within domain 4.23 A model for the structure of HK5 had been previously proposed based on threading of the HK5 sequence onto the structures of hisactophilin,5,24 an actin-binding protein from Dicytostellium discoideum and endostatin, an antiangiogenic fragment of collagen XVIII.3,25 The models suggest that HK5 consists of predominantly β-sheets and that HK5 requires zinc to exert its antiangiogenic effects on endothelial cells. However, there is little amino acid sequence identity between the HK5 domain and endostatin or hisactophilin (15%), making structural alignments unreliable. To experimentally determine information regarding the structure of HK5, circular dichroism (CD) far-ultraviolet spectra were gathered. What was observed were CD spectra consistent with a random coil structure and minimal α-helix or β sheet, which is demonstrated by the lack of negative peaks at 222 nm for α-helices and 215 nm for β-sheets [Fig. 1(A)].26,27 This was observed in both the presence and absence of the metal ions Zn2+ and Fe2+. Consistent with the CD spectra, secondary structure prediction results from the program Jpred28 indicate that the functional domain HK5 is predominantly random coil with short stretches of predicted α-helices totaling to ∼10% of the total sequence [Fig. 1(B)]. CD spectral data of HK5 in the presence of 6 M guanidine hydrochloride show no significant change in the structure between the wavelengths 210 nm and 230 nm, supporting the idea of minimal α-helical or β-sheet structure in the protein.
Figure 1.
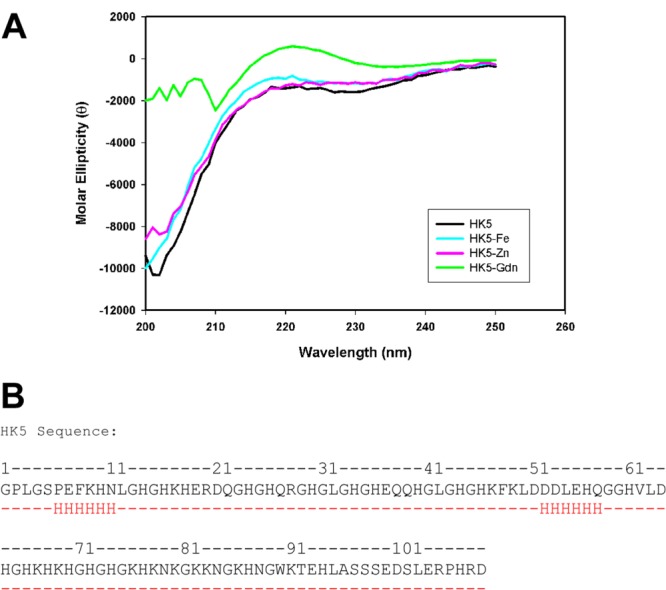
Secondary structure studies of HK5 protein. (A) Circular dichroism (CD) spectra of 0.5 mg/mL HK5 in the absence (black) or presence of 500 μM Zn(OAc)2 (pink), FeCl2 (cyan), or 6 M guanidine hydrochloride (green). These spectra are consistent with a random coil structure, and the addition of the metal ions has no observable effect on secondary structure. The spectra of HK5 in the presence of guanidinium chloride reveal the spectra between 210 nm and 230 nm is virtually unchanged, supporting the idea of minimal secondary structure. The noise in signal below 210 nm is due to the high absorbance of the guanidinium. (B) The results of JPRED secondary structure prediction program with the amino acid sequence of HK5 as the input. The results for the secondary structure prediction are shown in red, with H representing likely sites of α-helices and (-) representing sites of random coil.
To further investigate the structure of HK5, 2D [1H, 15N]-heteronuclear single quantum coherence nuclear magnetic resonance (HSQC NMR) was also utilized. HK5 was labeled with 15NH4Cl and 15N versus 1H spectra collected. HSQC spectra were collected on 15N HK5 at pH 6.5–8 at 25°C or 4°C in the presence and absence of metals. Data were also collected with HK5 in complex with unlabeled ferritin H to test the ability of metals or ferritin to induce secondary structure of HK5. This wide range of conditions was explored in part to test the potential protonation effects of the histidine rich region on HK5 secondary structure. The results suggest that under all measured conditions, HK5 has little secondary structure (Fig. 2), as exhibited by the lack of distinct peaks and the small 1H and 15N chemical shift ranges. Titration of Zn2+, Co2+, or Fe2+ up to 400 μM, did not induce any additional secondary structure. Taken together, these data suggest HK5 is likely an intrinsically unstructured protein.
Figure 2.
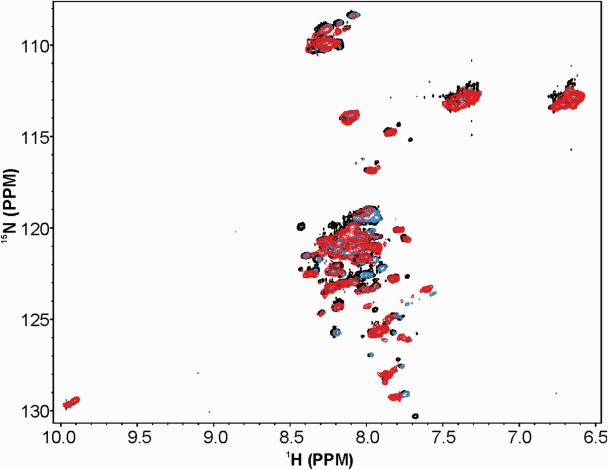
NMR HSQC spectra of HK5. The relatively collapsed spectrum of HK5 alone (black) indicates disordered structure. Titration of FeCl2 to 100 μM (blue) or 400 μM (red) did not produce distinct peaks corresponding to the protein amide backbone. Similar titrations of zinc and cobalt ions were also tested and resulted in similar spectra.
The ferritin H–HK5 interaction is enhanced in the presence of iron
Ferritin is well known as an iron storage protein, with the ability to store up to 4500 Fe3+ atoms in its ferrihydrite core.29 HK5 has also been shown to interact with metal ions such as Zn2+ for binding to endothelial cell surfaces.30 To test if metal ions play a role in the interaction between HK5 and ferritin we measured the binding affinity in the presence and absence of these metals using fluorescence anisotropy.31,32 Binding reactions containing labeled HK5 were performed with increasing ferritin H concentrations in the presence of monovalent and divalent metals. Prior to binding assays, recombinant ferritin and HK5 proteins were first treated with EDTA to remove any exogenous surface metals that might remain bound during expression/purification and could potentially have an effect on the binding interaction. Zn2+ is especially important in HKa biology as it is required for its interaction with endothelial cells. Both Ca2+ and K+ were also tested to compare the difference in affinity in the presence of monovalent and divalent ions. Fe2+ was tested due to its role in ferritin biology, and Co2+ and Cd2+ were tested as other heavy transition metals with similar coordination geometry as Fe2+. Relative anisotropy (Aobs) values were plotted as a function of ferritin H concentration (Fig. 3 and Table I). The data reveal monovalent ions such as K+ result in a slight increase in binding affinity when compared to the absence of metal with Kd values of 68 μM and 129 μM, respectively. The divalent metals Ca2+ and Zn2+ seemed to have little effect on the affinity between HK5 and ferritin H with Kd values of 112 μM for Ca2+ and 93 μM for Zn2+. Since ferritin is an iron storage protein, we rationalized that the HK5–ferritin interaction might be influenced by transition metals like Fe2+, Co2+, or Cd2+. All three metal ions were tested in the same manner and were found to significantly increase the affinity of the HK5–ferritin interaction with Kd values between 6 μM and 18 μM, which is approximately a 10-fold increase in affinity when compared to the interaction in the absence of metal (Table I).
Figure 3.
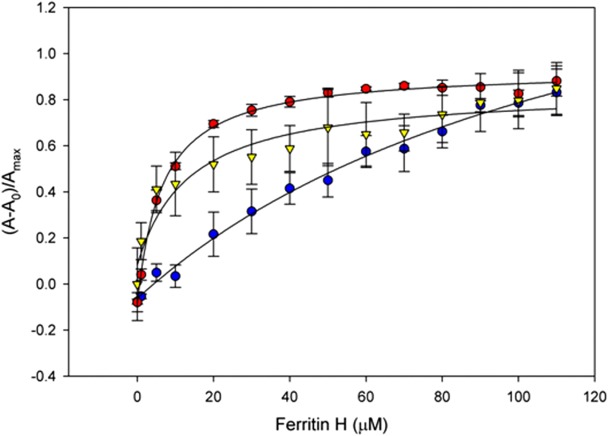
HK5–ferritin binding affinities. Representative fluorescence anisotropy binding curve of HK5–ferritin interactions in the presence of 10 μM FeCl2 (red), CdSO4 (yellow), and no metal ion added (blue). The presence of the transition metal ions iron, cobalt and cadmium resulted in significant increase in binding affinity between the two proteins. Dissociation binding constants (Kd) of HK5 and ferritin determined in the presence of metal ions is presented in Table I.
Table I.
Dissociation Binding Constants of HK5 to Ferritin H
| Metal compound | Kd (μM) ± SE |
|---|---|
| No metal | 129 ± 37 |
| ZnOAc | 93 ± 32 |
| CaCl2 | 112 ± 25 |
| KCl | 72 ± 7.3 |
| FeCl2 | 6.5 ± 0.57 |
| CoCl2 | 17.9 ± 3.2 |
| CdSO4 | 13.7 ± 5.2 |
The ferritin–HK5 interaction is not dependent on iron loading of ferritin
Ferritin H protomers contain a ferroxidase site in the middle of the four-helix bundle, which oxidizes Fe2+ to Fe3+ as excess iron atoms from the LIP are shuttled to the core. The oxidized iron ions are added to the ferrihydrite core until LIP levels decrease with the help of ferritin L monomers, which promote nucleation and core stabilization. The effects of a full ferrihydrite core on HK5 binding affinity were tested to determine if the heavy metal needed for the HK5–ferritin interaction was found on the surface of one of the proteins or in the core of ferritin. Ferritin H was loaded with iron and its affinity to HK5 was measured. Iron-loaded ferritin H does not have a significantly higher affinity for HK5 than apoferritin H in the absence of exogenous metals, with Kd values of 325 μM and 129 μM, respectively (Table II). Treatment of the ferritin H samples post-iron loading with the chelator EDTA removed any surface-bound metals from ferritin, as EDTA does not have an effect on the internal oxidized iron in the ferritin structure.18 The addition of Fe2+ increases the affinities of the HK5-apoferritin H and HK5-loaded ferritin H interactions. This demonstrates that metals located on the surface of one of the proteins are likely mediating the interaction between ferritin and HK5.
Table II.
Effects of Loaded Ferritin Core on the HK5-Ferritin H Interaction
| Loaded/unloaded ferritin H | Metal | Kd (μM) ± SE |
|---|---|---|
| Unloaded | None | 129 ± 37 |
| Unloaded | 10μM FeCl2 | 6.5 ± 0.57 |
| Loaded | None | 325 ± 175 |
| Loaded | 10μM FeCl2 | 54 ± 9.9 |
HK5 binds to both ferritin H and ferritin L
Cytoplasmic holoferritin is composed of both ferritin H and ferritin L subunits. To test whether HK5 had a preference for interacting with one of the subunits in vitro, binding affinity for HK5 and ferritin H or ferritin L were measured individually. In the absence of metal ions, HK5 has similar binding affinities to ferritin L and ferritin H, with Kd values of 247 µM and 129 µM, respectively (Table III). Similar Kd values are seen for ferritin H and ferritin L with each of the divalent metals tested, including iron, cadmium and cobalt. These data indicate that the mechanism of binding to HK5 to ferritin is similar for both human ferritin H (hFH) and hFL.
Table III.
Dissociation Binding Constants of HK5 to Ferritin H and Ferritin L
| Ferritin H/ferritin L | Metal (10μM) | Kd (μM) ± SE |
|---|---|---|
| Ferritin H | None | 129 ± 37 |
| Ferritin H | FeCl2 | 6.5 ± 0.57 |
| Ferritin L | None | 247 ± 83 |
| Ferritin L | FeCl2 | 17.6 ± 1.4 |
| Ferritin L | CoCl2 | 45.4 ± 7.7 |
| Ferritin L | CdSO4 | 18.8 ± 3.1 |
Previous structural analysis of ferritin H and ferritin L revealed metal-binding sites on the outer surface of the proteins.21,33 In the hFL structure (PDB ID: 2FFX), a cadmium ion is coordinated by Asp15 residues from two adjacent monomers. To test the role of the Asp15 residue of ferritin L in HK5 binding, we mutated the residue to an alanine. The affinity of the D15A point mutant for HK5 was measured. In the absence of metals, the affinity of the ferritin L D15A mutant for HK5 was similar to WT ferritin L, with a Kd of 247 µM for the WT ferritin L–HK5 interaction and 174 µM for the ferritin L D15A–HK5 interaction (Table IV). A difference in affinity for HK5 was observed when measuring WT ferritin L and ferritin L D15A in the presence of 10 µM Fe2+, with a nearly 2-fold higher Kd value for ferritin L D15A (29 µM) compared to WT ferritin L (17 µM).
Table IV.
Binding of HK5 to WT Ferritin and D15A Ferritin L
| WT/D15A ferritin L | Metal (10μM) | Kd (μM) ± SE |
|---|---|---|
| WT ferritin L | None | 247 ± 83 |
| WT ferritin L | FeCl2 | 17.6 ± 1.4 |
| D15A ferritin L | None | 174 ± 83 |
| D15A ferritin L | FeCl2 | 29 ± 6.6 |
Discussion
Domain 5 of HKa (HK5) is an endogenous angiogenic modulator whose effects are exerted through the uPAR receptor and subsequent Erk signaling pathway.22,34 HK5 inhibits proliferation, migration and adhesion as well as induces apoptosis of endothelial cells. The antiangiogenic effects of HK5 can be attenuated through an interaction with ferritin.13 While direct binding of HK5 to ferritin has been demonstrated,10,13 the details of the interaction between these two proteins has not been well understood. Here we demonstrate that HK5 is an intrinsically unstructured protein (IUP) and binds to ferritin in a metal-mediated interaction that occurs on the surface of ferritin and works independently of oxidized iron in its ferrihydrite core.
Previous electron microscopy data23 show that HK consists of three globular domains whose conformations change after cleavage into bradykinin and HKa from “beads on a string” to a more flexible conformation. This is likely what accounts for the ability of HKa, but not HK, to bind to endothelial cells and exert its antiangiogenic effects. The flexibility seen in the EM data is strongly indicative of the elasticity of the HK5 structure as a whole. Our CD, NMR HSQC and secondary structure prediction data support the model that HK5 is mostly random coil. The lack of structure in HK5 is consistent with its enrichment of “disorder promoting” amino acids (H, G, K) and low hydrophobicity that is also observed in other IUPs.35 At least 200 intrinsically unstructured proteins and protein domains have been identified,36–42 and are known to serve many biological purposes,43 including protein chaperoning, binding RNA and DNA molecules, aiding the assembly of multiprotein complexes, and acting as sites of posttranslational modification.44 Disordered regions are also useful in allowing one protein to bind to multiple partners due to its inherent flexibility,40 which makes these proteins especially useful in signal transduction and regulation.41,44
The intrinsically unstructured nature of HK5 likely plays an important role in its biological mechanism. HK5 binds multiple targets including cell surface receptors such as uPAR and tropomyosin, the metal ions Zn2+, Cd2+, and Fe2+, ferritin, heparin and heparan sulfate as well as platelets and neutrophils. In order to accommodate these multiple functions within a single protein, the structure must be fluid enough to conform to multiple binding sites.
Our examination of the effects of metals on the HK5–ferritin interaction reveals that the presence of metals, especially transition metals, is essential for the tightest affinity. Although HK5 has been previously shown to require the presence of Zn2+ for binding to endothelial cells,30 our data suggest that zinc exerts its effects through modulation of HK structure and does not appreciably affect the ability of HKa to interact with ferritin. The histidine/glycine rich region of HK5, which is the minimal binding region of HK5 to ferritin13 as well as its minimal antiangiogenic region,5 is likely capable of binding transition metals due to its histidine-rich nature. The mechanisms of absorption and transport of other heavy metals, such as cadmium, in humans is similar to iron which is also consistent with our observations that other metal ions may substitute for iron in facilitating the protein interactions.45–47
Ferritin is an iron-binding protein but also interacts with other metal ions such as cadmium.21 Ferritin icositetrahedrons are made of varying ratios of two isoforms, ferritin H and ferritin L. Ferritin H (21 kDa) is implicated in shuttling ferrous iron ions from the excess extracellular LIP to the ferrihydrite core, oxidizing the iron via a ferroxidase center within its four-helix bundle in the process.15,16 Ferritin L (19 kDa) promotes incorporation or nucleation of the ferric iron ions as they reach the ferrihydrite core, thereby increasing the overall stability of the protein.17 Our data show that HK5 interacts similarly with both ferritin H and ferritin L, consistent with data Tesfay et al.34 have shown for HK5 in endothelial cells. The minimal ferroxidase activity of ferritin L17,35 along with our findings that other metal ions may substitute for iron in promoting binding, further support the idea that the metal mediated interaction occurs through metal binding on the surface of HK5 and ferritin.
Structural analysis of ferritin H (PDB ID: 2FHA) and L (PDB ID: 2FFX) proteins21,33 by others has revealed that a metal-binding site exists on the surface of the ferritin icositetrahedrons. In ferritin L, this site formed by an aspartic acid residue is occupied by a Cd2+ ion, likely as a result of the cadmium in the crystallization conditions.21 This coordinated metal ion on the surface of the ferritin oligomer makes it a candidate for the metal ion that mediates the ferritin–HK5 interaction. To test this hypothesis, the point mutant D15A was made in ferritin L to prevent metal binding at the site and the affinity for HK5 measured. The results (Table IV) show that mutating the aspartic acid does decrease the affinity between HK5 and ferritin L by about half. While this mutation in ferritin supports the idea that metal-mediated binding is important for optimal HK5 binding affinity, the interaction is not solely dependent on this one specific metal coordination site. Further studies are needed to understand the larger extent of HK5 binding on the surface of ferritin, as it is possible that the HGK-rich region of HK5 needs to interact with multiple metals coordinated on the surface of ferritin or negatively charged residues on the surface of ferritin for stability. Electrostatic mapping of the ferritin H and L 24mer structures shows negatively charged regions similar in both ferritin H and L which radiate from the 4-fold axis of the structure (Fig. 4). These regions which are rich in negatively charged Glu and Asp residues could also be potential binding regions for HK5, whose minimal HGK-rich ferritin binding region has a high isoelectric point of 10.9 and overall positive charge.
Figure 4.
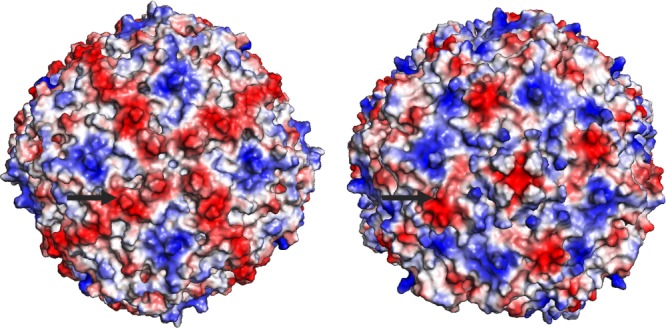
Electrostatic potential representations of ferritin H and ferritin L. Arrows point to Asp15 of the ferritin H (PDB ID: 2FHA) (left) and ferritin L (PDB ID: 2FFX) (right) structures. Red indicates electrostatic negative regions and blue electrostatic positive regions. The negatively charged regions of ferritins may serve as binding sites for the positively charged His-Gly-Lys rich region of HK5.
Metal binding sites within the ferritin monomers and in the interior of the icositetrahedron have been previously examined.18–20 However, the extent to which surface metal binding sites affect binding partners of ferritin has not been discussed. Ferritin can interact with binding partner receptors such as Scara5,48 TIM-2,49 and transferrin receptor-1 (TfR1)50 as well as α-casein,51,52 α2 macroglobulin,53,54 various immunoglobulins,53 and fibrinogen.55 Of these binding partners, only α-casein has been implicated to have a metal-mediated interaction, requiring heme for optimal binding affinity. The HK5–ferritin interaction is the first that has been determined to be occurring on the surface of ferritin and implicated by metal ions that are bound to its surface. While the Asp15 residues of ferritin monomers do have effects on metal binding and the subsequent HK5 binding affinities, it seems that other as yet determined residues are also involved, given the data from the Asp15 mutation studies.
The HK5–ferritin interaction has modulating effects on endothelial cell proliferation and angiogenesis in both in vitro and in vivo studies. This study elucidates the interaction between the two proteins as being located on the surface of ferritin and mediated by transition metals and identifies Asp15 of ferritin as an important residue. Understanding the physical parameters and mechanism of the HK5–ferritin protein interaction is important for comprehending the antiangiogenic function of HK5. Targeted disruption of the interaction could provide a means of regulating angiogenesis. These data lay a foundation for discovering potential therapeutic inhibitors to the interaction, thereby allowing increased antiangiogenic effects of HK5.
Materials and Methods
Purification of recombinant HK5
The gene encoding domain 5 of the human high-molecular-weight kininogen protein (HK5) was subcloned into the pGEX-6P-1 bacterial expression vector (GE Healthcare) in frame with the upstream gene for glutathione S-transferase (GST) and coding sequence for PreScission Protease recognition site. The pGEX-6P-1–HK5 vector was transformed into the Escherichia coli strain C41 for expression. One liter of LB (Luria-Bertani) broth supplemented with 100 μg/mL ampicillin, 250 μM ZnCl2, and 125 μM FeCl2 was inoculated with C41 pGEX-6P-1–HK5 cells, grown to an OD600 = 0.8, and induced with 1 mM isopropyl β-d−1-thiogalactopyranoside (IPTG) overnight at 16°C. Harvested cells were resuspended in 20 mM Tris–HCl pH 8.0, lysed using an EmulsiFlex C-5 cell homogenizer (Avestin), and cell debris pelleted at 41000 RCF. The supernatant was then passed over a Ni-NTA (Qiagen) column equilibrated with 50 mM Tris–HCl pH 8.0, 0.3 M NaCl. A Ni-NTA column was used because the high density of histidine residues in HK5 allows the protein to interact with the resin in the absence of a polyhistidine tag. The column was washed with 100 mL of equilibration buffer containing 50 mM imidazole. HK5 was eluted in equilibration buffer containing 500 mM imidazole. The fractions containing HK5 were pooled and treated with PreScission Protease (GE Healthcare) according to the manufacturer's protocol and dialyzed extensively against 20 mM Tris–HCl pH 8.0, 150 mM NaCl, 0.2 mM EDTA, and 2 mM dithiothreitol at 4°C. Cleaved HK5 was again passed over a Ni-NTA column following the above protocol to remove GST protein. Fractions containing HK5 were pooled and analyzed for purity via sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE). Purified protein was concentrated and flash frozen using liquid nitrogen, and stored at −80°C. The Ser5Cys point mutant of HK5 was created by QuikChange mutagenesis (Stratagene) and the mutant protein was purified and stored following the wild-type HK5 protocol.
Purification of recombinant hFH and hFL
The genes encoding hFH or hFL proteins were cloned into the pET-17 bacterial expression vector (EMD Millipore). Each vector was transformed into the E. coli strain BL21(DE3) for expression. One liter of LB broth supplemented with 100 μg/mL ampicillin was inoculated with BL21(DE3) pET-17–ferritin H or L cells, grown to an OD600 = 0.8, and induced with 1 mM IPTG overnight at 16°C. Harvested cells were resuspended in 20 mM Tris–HCl pH 7.5, 1 mM EDTA, 1 mM PMSF, and 10% sucrose, lysed using an Emulsiflex C-5 cell homogenizer, and cell debris pelleted at 41000 RCF. The supernatant was heated at 70°C for 10 min and precipitate pelleted by centrifugation at 24000 RCF for 15 min. The supernatant was passed over a Q-Sepharose (GE Healthcare) column equilibrated with 50 mM Tris–HCl pH 7.5. The column was developed with buffer containing a gradient of 0.020–2 M NaCl and ferritin eluted at approximately 500 mM NaCl. The fractions containing ferritin were pooled and loaded onto a Superdex S-200 (GE Healthcare) gel filtration column equilibrated with 50 mM Tris–HCl pH 7.5, 200 mM NaCl. The column was washed with 300 mL equilibration buffer. Fractions containing ferritin were pooled and analyzed for purity via SDS-PAGE electrophoresis. Purified protein was then concentrated to 10 mg/mL, aliquoted, flash frozen using liquid nitrogen, and stored at −80°C. Before using in equilibrium binding experiments, the ferritin samples were treated with 30 mM EDTA, and dialyzed into 50 mM Tris pH 8.0, 150 mM NaCl to remove exogenous surface-bound metals.
Expression and purification of 15N-labeled HK5
The pGEX-6P-1–HK5 vector was transformed into the E. coli strain C41 for expression. M9 minimal medium56 was prepared with 1 g/L 15NH4Cl and 100 μg/mL ampicillin. To each liter of media, 20 mL of C41 pGEX-6P-1–HK5 cells grown overnight in LB media, was added. The cells were grown to an OD600 = 0.9, and induced with 1 mM IPTG overnight at 16°C. The labeled HK5 protein was purified following the above protocol for WT HK5, concentrated, aliquoted, flash frozen with liquid nitrogen, and stored at −80°C.
CD of HK5
HK5 diluted to 0.5 mg/mL was dialyzed into PBS. To evaluate the secondary structure of HK5, triplicate CD spectra were collected from 190 nm to 250 nm at 25°C using a cuvette with 0.05 cm path length on a Jasco Model 720A spectropolarimeter in the presence or absence of FeCl2 or ZnOAc and blanked with buffer alone. Data are presented as molar ellipticity versus wavelength.
NMR HSQC spectroscopy of HK5 and HK5–ferritin complex
Two-dimensional HSQC NMR spectra57 were collected using a Bruker Avance DRX-600 NMR spectrometer at 4°C or 20°C using 15NH4Cl-labeled HK5 in the absence or presence of Fe2+ and ferritin. Standard pulse sequences were used to acquire 2D HSQC data. Data were processed using NMRPipe58 and analyzed using NMRView.59
Iron loading of ferritin H
The ferroxidase activity of ferritin was measured by monitoring ferric iron production.60 Fresh ferrous ammonium sulfate in 0.1 M HEPES, pH 7.5 was added to ferritin to a final concentration of 400 μM. The increasing OD310 of the solution was monitored at room temperature using a Cary 50 spectrophotometer (Varian) until it plateaued. Ferritin H in the absence of iron was used as a blank. To confirm that iron loading of ferritin H occurred, an aliquot of the ferritin/ferrous ammonium sulfate solution was measured at 310 nm continually for 1 h until the OD stabilized. The ferritin was then dialyzed into 50 mM Tris pH 8.0, 150 mM NaCl, 30 mM EDTA overnight, followed by gradual dialysis into 50 mM Tris pH 8.0, 150 mM NaCl.
HK5–ferritin binding assay
In order to label the HK5 protein with a single fluorescein tag we created a Ser5Cys point mutation. A cysteine is necessary for labeling with fluorescein-5-maleimide (FAM), but none are present in the WT HK5 protein. The S5C point mutant was labeled with FAM (Thermo Scientific) and quantitated following the manufacturer's protocol, with final labeling efficiency of about 15%. HK5 affinity for ferritin was measured using fluorescence anisotropy. Binding reactions (25 μL) contained 50 mM Tris pH 8.0, 150 mM NaCl, 20 nM FAM-labeled HK5 S5C plus increasing concentrations of ferritin H or ferritin L and the indicated concentration of metal ion. The HK5 protein was preincubated with metal ions and then ferritin added immediately before anisotropy measurement. When iron was used in the experiment, the time between addition of ferritin and measurement completion was less than 5 min in order to minimize oxidation of the iron by ferritin. Measurement blanks and fluorescence background samples with binding conditions minus labeled HK5 or ferritin, respectively, were used. Measurements were performed on a Safire II microplate reader (Tecan) and polarization measurements were made using excitation and emission wavelengths of 475 nm and 520 nm, respectively. Data were normalized using the equation A = (Aobs − A0)/Amax, where Aobs is the measured anisotropy at a given concentration of ferritin, A0 equals the anisotropy of HK5 in the absence of ferritin, and Amax equals the maximum anisotropy observed.61 Results are the averages of triplicate experiments. The binding data were fit to the rectangular hyperbolic equation using Sigmaplot 9.0 to calculate Kd values.
Acknowledgments
The authors would like to thank Dr. Roy Hantgan for his assistance in analysis of CD spectral data.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem. 2000;275:21340–21348. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 3.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 4.Villanueva GB, Leung L, Bradford H, Colman RW. Conformation of high molecular weight kininogen: effects of kallikrein and factor XIa cleavage. Biochem Biophys Res Commun. 1989;158:72–79. doi: 10.1016/s0006-291x(89)80178-0. [DOI] [PubMed] [Google Scholar]
- 5.Colman RW, Jameson BA, Lin Y, Johnson D, Mousa SA. Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood. 2000;95:543–550. [PubMed] [Google Scholar]
- 6.Zhang JC, Claffey K, Sakthivel R, Darzynkiewicz Z, Shaw DE, Leal J, Wang YC, Lu FM, McCrae KR. Two-chain high molecular weight kininogen induces endothelial cell apoptosis and inhibits angiogenesis: partial activity within domain 5. FASEB J. 2000;14:2589–2600. doi: 10.1096/fj.99-1025com. [DOI] [PubMed] [Google Scholar]
- 7.Guo YL, Wang S, Colman RW. Kininostatin, an angiogenic inhibitor, inhibits proliferation and induces apoptosis of human endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:1427–1433. doi: 10.1161/hq0901.095277. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Sainz IM, Wu Y, Pixley R, Espinola RG, Hassan S, Khan MM, Colman RW. The inhibition of tube formation in a collagen-fibrinogen, three-dimensional gel by cleaved kininogen (HKa) and HK domain 5 (D5) is dependent on Src family kinases. Exp Cell Res. 2008;314:774–788. doi: 10.1016/j.yexcr.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Cao DJ, Sainz IM, Guo YL, Colman RW. The inhibitory effect of HKa in endothelial cell tube formation is mediated by disrupting the uPA-uPAR complex and inhibiting its signaling and internalization. Am J Physiol Cell Physiol. 2008;295:C257–267. doi: 10.1152/ajpcell.00569.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torti SV, Torti FM. Human H-kininogen is a ferritin-binding protein. J Biol Chem. 1998;273:13630–13635. doi: 10.1074/jbc.273.22.13630. [DOI] [PubMed] [Google Scholar]
- 11.Coffman LG, Brown JC, Johnson DA, Parthasarathy N, D'Agostino RB, Jr, Lively MO, Hua X, Tilley SL, Muller-Esterl W, Willingham MC, Torti FM, Torti SV. Cleavage of high-molecular-weight kininogen by elastase and tryptase is inhibited by ferritin. Am J Physiol Lung Cell Mol Physiol. 2008;294:L505–515. doi: 10.1152/ajplung.00347.2007. [DOI] [PubMed] [Google Scholar]
- 12.Parthasarathy N, Torti SV, Torti FM. Ferritin binds to light chain of human H-kininogen and inhibits kallikrein-mediated bradykinin release. Biochem J. 2002;365:279–286. doi: 10.1042/BJ20011637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffman LG, Parsonage D, D'Agostino R, Jr, Torti FM, Torti SV. Regulatory effects of ferritin on angiogenesis. Proc Natl Acad Sci USA. 2009;106:570–575. doi: 10.1073/pnas.0812010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes. Free Radic Biol Med. 2002;33:1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- 15.Lawson DM, Treffry A, Artymiuk PJ, Harrison PM, Yewdall SJ, Luzzago A, Cesareni G, Levi S, Arosio P. Identification of the ferroxidase centre in ferritin. FEBS Lett. 1989;254:207–210. doi: 10.1016/0014-5793(89)81040-3. [DOI] [PubMed] [Google Scholar]
- 16.Levi S, Luzzago A, Cesareni G, Cozzi A, Franceschinelli F, Albertini A, Arosio P. Mechanism of ferritin iron uptake: activity of the H-chain and deletion mapping of the ferro-oxidase site. A study of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain ferritins, and of two H-chain deletion mutants. J Biol Chem. 1988;263:18086–18092. [PubMed] [Google Scholar]
- 17.Levi S, Santambrogio P, Cozzi A, Rovida E, Corsi B, Tamborini E, Spada S, Albertini A, Arosio P. The role of the L-chain in ferritin iron incorporation. Studies of homo and heteropolymers. J Mol Biol. 1994;238:649–654. doi: 10.1006/jmbi.1994.1325. [DOI] [PubMed] [Google Scholar]
- 18.Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta. 2009;1790:589–599. doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Takezawa Y, Bockmann P, Sugi N, Wang Z, Abe S, Murakami T, Hikage T, Erker G, Watanabe Y, Kitagawa S, Ueno T. Incorporation of organometallic Ru complexes into apo-ferritin cage. Dalton Trans. 2011;40:2190–2195. doi: 10.1039/c0dt00955e. [DOI] [PubMed] [Google Scholar]
- 20.Ueno T, Abe M, Hirata K, Abe S, Suzuki M, Shimizu N, Yamamoto M, Takata M, Watanabe Y. Process of accumulation of metal ions on the interior surface of apo-ferritin: crystal structures of a series of apo-ferritins containing variable quantities of Pd(II) ions. J Am Chem Soc. 2009;131:5094–5100. doi: 10.1021/ja806688s. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Li C, Ellenburg M, Soistman E, Ruble J, Wright B, Ho JX, Carter DC. Structure of human ferritin L chain. Acta Cryst. 2006;D62:800–806. doi: 10.1107/S0907444906018294. [DOI] [PubMed] [Google Scholar]
- 22.Colman RW, Pixley RA, Najamunnisa S, Yan W, Wang J, Mazar A, McCrae KR. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J Clin Invest. 1997;100:1481–1487. doi: 10.1172/JCI119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisel JW, Nagaswami C, Woodhead JL, DeLa Cadena RA, Page JD, Colman RW. The shape of high molecular weight kininogen. Organization into structural domains, changes with activation, and interactions with prekallikrein, as determined by electron microscopy. J Biol Chem. 1994;269:10100–10106. [PubMed] [Google Scholar]
- 24.Habazettl J, Gondol D, Wiltscheck R, Otlewski J, Schleicher M, Holak TA. Structure of hisactophilin is similar to interleukin-1 beta and fibroblast growth factor. Nature. 1992;359:855–858. doi: 10.1038/359855a0. [DOI] [PubMed] [Google Scholar]
- 25.Ding YH, Javaherian K, Lo KM, Chopra R, Boehm T, Lanciotti J, Harris BA, Li Y, Shapiro R, Hohenester E, Timpl R, Folkman J, Wiley DC. Zinc-dependent dimers observed in crystals of human endostatin. Proc Natl Acad Sci USA. 1998;95:10443–10448. doi: 10.1073/pnas.95.18.10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly SM, Jess TJ, Price NC. How to study proteins by circular dichroism. Biochim Biophys Acta. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Fink AL, Calciano LJ, Goto Y, Nishimura M, Swedberg SA. Characterization of the stable, acid-induced, molten globule-like state of staphylococcal nuclease. Protein Sci. 1993;2:1155–1160. doi: 10.1002/pro.5560020710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole C, Barber JD, Barton GJ. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 2008;36:W197–201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 30.van Iwaarden F, de Groot PG, Bouma BN. The binding of high molecular weight kininogen to cultured human endothelial cells. J Biol Chem. 1988;263:4698–4703. [PubMed] [Google Scholar]
- 31.Cushing PR, Fellows A, Villone D, Boisguerin P, Madden DR. The relative binding affinities of PDZ partners for CFTR: a biochemical basis for efficient endocytic recycling. Biochemistry. 2008;47:10084–10098. doi: 10.1021/bi8003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moerke NJ. Fluorescence polarization (FP) assays for monitoring peptide-protein or nucleic acid-protein binding. Curr Protoc Chem Biol. 2009;1:1–15. doi: 10.1002/9780470559277.ch090102. [DOI] [PubMed] [Google Scholar]
- 33.Lawson DM, Artymiuk PJ, Yewdall SJ, Smith JM, Livingstone JC, Treffry A, Luzzago A, Levi S, Arosio P, Cesareni G, Thomson CD, Shaw WV, Harrison PM. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature. 1991;349:541–544. doi: 10.1038/349541a0. [DOI] [PubMed] [Google Scholar]
- 34.Tesfay L, Huhn AJ, Hatcher H, Torti FM, Torti SV. Ferritin blocks inhibitory effects of two-chain high molecular weight kininogen (HKa) on adhesion and survival signaling in endothelial cells. PLoS One. 2012;7:e40030. doi: 10.1371/journal.pone.0040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levi S, Corsi B, Rovida E, Cozzi A, Santambrogio P, Albertini A, Arosio P. Construction of a ferroxidase center in human ferritin L-chain. J Biol Chem. 1994;269:30334–30339. [PubMed] [Google Scholar]
- 36.Cortese MS, Uversky VN, Dunker AK. Intrinsic disorder in scaffold proteins: getting more from less. Prog Biophys Mol Biol. 2008;98:85–106. doi: 10.1016/j.pbiomolbio.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunker AK, Brown CJ, Lawson JD, Iakoucheva LM, Obradovic Z. Intrinsic disorder and protein function. Biochemistry. 2002;41:6573–6582. doi: 10.1021/bi012159+. [DOI] [PubMed] [Google Scholar]
- 38.Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, Oldfield CJ, Campen AM, Ratliff CM, Hipps KW, Ausio J, Nissen MS, Reeves R, Kang C, Kissinger CR, Bailey RW, Griswold MD, Chiu W, Garner EC, Obradovic Z. Intrinsically disordered protein. J Mol Graph Model. 2001;19:26–59. doi: 10.1016/s1093-3263(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 39.Dunker AK, Oldfield CJ, Meng J, Romero P, Yang JY, Chen JW, Vacic V, Obradovic Z, Uversky VN. The unfoldomics decade: an update on intrinsically disordered proteins. BMC Genomics 9 Suppl. 2008;2:S1. doi: 10.1186/1471-2164-9-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunker AK, Silman I, Uversky VN, Sussman JL. Function and structure of inherently disordered proteins. Curr Opin Struct Biol. 2008;18:756–764. doi: 10.1016/j.sbi.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Iakoucheva LM, Brown CJ, Lawson JD, Obradovic Z, Dunker AK. Intrinsic disorder in cell-signaling and cancer-associated proteins. J Mol Biol. 2002;323:573–584. doi: 10.1016/s0022-2836(02)00969-5. [DOI] [PubMed] [Google Scholar]
- 42.Radivojac P, Obradovic Z, Smith DK, Zhu G, Vucetic S, Brown CJ, Lawson JD, Dunker AK. Protein flexibility and intrinsic disorder. Protein Sci. 2004;13:71–80. doi: 10.1110/ps.03128904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 44.Tompa P. The interplay between structure and function in intrinsically unstructured proteins. FEBS Lett. 2005;579:3346–3354. doi: 10.1016/j.febslet.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 45.Meltzer HM, Brantsaeter AL, Borch-Iohnsen B, Ellingsen DG, Alexander J, Thomassen Y, Stigum H, Ydersbond TA. Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ Res. 2010;110:497–504. doi: 10.1016/j.envres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Park JH, Park S, Kim Y. Iron deficiency is not associated with increased blood cadmium in infants. Ann Occup Environ Med. 2014;26:3. doi: 10.1186/2052-4374-26-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park S, Sim CS, Lee H, Kim Y. Blood manganese concentration is elevated in infants with iron deficiency. Biol Trace Elem Res. 2013;155:184–189. doi: 10.1007/s12011-013-9782-9. [DOI] [PubMed] [Google Scholar]
- 48.Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Leete T, Drexler IR, Chen X, Sanna-Cherchi S, Mohammed F, Williams D, Lin CS, Schmidt-Ott KM, Andrews NC, Barasch J. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell. 2009;16:35–46. doi: 10.1016/j.devcel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen TT, Li L, Chung DH, Allen CD, Torti SV, Torti FM, Cyster JG, Chen CY, Brodsky FM, Niemi EC, Nakamura MC, Seaman WE, Daws MR. TIM-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J Exp Med. 2005;202:955–965. doi: 10.1084/jem.20042433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L, Fang CJ, Ryan JC, Niemi EC, Lebron JA, Bjorkman PJ, Arase H, Torti FM, Torti SV, Nakamura MC, Seaman WE. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci USA. 2010;107:3505–3510. doi: 10.1073/pnas.0913192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugawara G, Inoue R, Watanabe K, Ohtsuka H, Orino K. Short communication: bovine alpha-casein is a ferritin-binding protein and inhibitory factor of milk ferritin immunoassay. J Dairy Sci. 2009;92:3810–3814. doi: 10.3168/jds.2008-1948. [DOI] [PubMed] [Google Scholar]
- 52.Usami A, Tanaka M, Yoshikawa Y, Watanabe K, Ohtsuka H, Orino K. Heme-mediated binding of alpha-casein to ferritin: evidence for preferential alpha-casein binding to ferrous iron. Biometals. 2011;24:1217–1224. doi: 10.1007/s10534-011-9470-1. [DOI] [PubMed] [Google Scholar]
- 53.Bellotti V, Arosio P, Cazzola M, Cozzi A, Levi S, Meloni F, Zoppone E. Characteristics of a ferritin-binding protein present in human serum. Br J Haematol. 1987;65:489–493. doi: 10.1111/j.1365-2141.1987.tb04156.x. [DOI] [PubMed] [Google Scholar]
- 54.Massover WH. Alpha 2-macroglobulin: a ferritin-binding protein. Ann N Y Acad Sci. 1994;737:468–471. doi: 10.1111/j.1749-6632.1994.tb44342.x. [DOI] [PubMed] [Google Scholar]
- 55.Orino K, Yamamoto S, Watanabe K. Fibrinogen as a ferritin-binding protein in horse plasma. J Vet Med Sci. 1993;55:785–787. doi: 10.1292/jvms.55.785. [DOI] [PubMed] [Google Scholar]
- 56.Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bodenhausen G, Ruben DJ. Natural abundance N-15 NMR by enhanced heteronuclear spectroscopy. Chem Phys Lett. 1980;69:185–189. [Google Scholar]
- 58.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 59.Johnson BA, Blevins RA. NMR view—a computer-program for the visualization and analysis of NMR data. J Biomol NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 60.Pagues E, Paques A, Crichton RR. A study of the mechanism of ferritin formation. The effect of pH, ionic strength and temperature, inhibition by imidazole and kinetic analysis. Eur J Biochem. 1980;107:447–453. [PubMed] [Google Scholar]
- 61.Heyduk T, Ma Y, Tang H, Ebright RH. Fluorescence anisotropy: rapid, quantitative assay for protein–DNA and protein–protein interaction. Methods Enzymol. 1996;274:492–503. doi: 10.1016/s0076-6879(96)74039-9. [DOI] [PubMed] [Google Scholar]


