Abstract
Background
N-acetylcysteine (NAC) has been suggested as a beneficial treatment for idiopathic pulmonary fibrosis (IPF). A placebo-controlled study of this agent administrated orally alone in an IPF population has not been conducted.
Methods
An initially designed three-arm randomized, double-blind, placebo-controlled trial of prednisone plus azathioprine plus NAC (three-drug regimen) versus NAC versus placebo in IPF patients with mild-moderate impairment in pulmonary function was interrupted due to safety concerns associated with the three-drug regimen. The trial continued as a two-arm design (NAC vs. placebo) without other changes and enrolled 133 and 131 patients in the NAC and placebo arms, respectively. The primary outcome measure was the change in forced vital capacity (FVC) over a 60-week period.
Results
Over the 60-week treatment period, there was no difference between the NAC and placebo groups in the decline of FVC (60-week change of −0.18 liters for NAC vs. −0.19 liters for placebo, p=0.77). In addition, there were no significant differences between NAC and placebo for mortality (6 [4.9%] vs. 3 [2.5%] events, p=0.50) or acute exacerbation (3 [2.3%] vs. 3 [2.3%] events, p>0.99).
Conclusions
Compared to placebo NAC offered no benefit for the preservation of FVC in IPF patients with mild-to-moderate physiological abnormalities.
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive lung disease of unknown cause characterized by the histopathologic and/or radiological patterns of usual interstitial pneumonia (UIP) in a typical clinical setting.1,2 To date, no pharmacologic therapies have been shown to improve survival.3
The IFIGENIA study (Idiopathic Pulmonary Fibrosis International Group Exploring N-Acetylcysteine I Annual) with a three-drug regimen (combined prednisone, azathioprine, and NAC) found that this treatment preserved pulmonary function better than the two-drug regimen (azathioprine plus prednisone).4 The Prednisone, Azathioprine, and N-acetylcysteine: a study THat Evaluates Response in Idiopathic Pulmonary Fibrosis: A randomized, double-blind, placebocontrolled trial (PANTHER-IPF) examined the three-drug regimen of prednisone plus azathioprine plus NAC, or NAC alone (plus matched placebos for prednisone and azathioprine), compared to matched placebos for each of the active therapies in IPF patients with mild-to-moderate impairment in pulmonary function.5 Following safety concerns identified by the Data and Safety Monitoring Board (DSMB), the three-drug regimen was stopped by the NHLBI on October 14, 2011, and a clinical alert was issued. [http://www.nlm.nih.gov/databases/alerts/2011_nhlbi_ifp.html accessed on December 20, 2013] The NAC-alone and matched placebo arms of the study continued to recruit and were followed for the pre specified duration. This is a report of the results of NAC compared to the placebo arm.
METHODS
Study Oversight
The study was designed and conducted by the IPFnet Steering Committee and was carried out at 25 clinical centers (see supplementary appendix for a complete listing of IPFnet sites and for the PANTHER-IPF protocol). An independent protocol review committee, appointed by the National Heart, Lung, and Blood Institute (NHLBI), reviewed and approved the protocol for scientific merit. An NHLBI-appointed DSMB and all local institutional review boards approved the protocol and all amendments. The DSMB met multiple times per year to review data for safety and overall trial progress. All patients provided written informed consent. The Duke Clinical Research Institute served as the data-coordinating center and the IPFnet Steering Committee oversaw all aspects of the study’s conduct. The PANTHER-IPF Protocol Committee (a subcommittee of the IPFnet Steering Committee) developed the design and concept of the study, and approved the statistical plan; the IPFnet Steering Committee had full access to all of the data. The writing committee wrote the first draft of the manuscript, and the steering committee made subsequent revisions. The source and dose of the NAC and matching placebo was Zambon S.p.A. (Milan, Italy). Zambon reviewed and provided comments on a draft of the manuscript before submission for publication; as a result minor changes were made. All authors assume responsibility for the overall content and integrity of the article.
Study Patients
The inclusion criteria for this study have been previously published.4 IPF patients aged 35 to 85 with mild-to-moderate pulmonary function impairment (as defined by a forced vital capacity [FVC] of ≥ 50% and DLCO ≥ 30% predicted) were potentially eligible. All patients met the modified criteria of the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association for the diagnosis of IPF.1,6 Patients were diagnosed with IPF using high resolution computed tomography (HRCT) or biopsy and with a 48-month or less duration of illness before enrollment.
Patients were excluded if they met any of the following criteria: non-idiopathic fibrotic lung disease, qualitatively assessed extent of emphysema on HRCT greater than fibrotic change, physiological evidence of airflow obstruction (FEV1/FVC < 0.65 or residual volume > 120 %), any current signs or symptoms of severe, progressive or uncontrolled co-morbid illnesses as determined by the site investigator, on the active list for lung transplantation, or receiving combination azathioprine plus prednisone and NAC for more than 12 weeks in the previous four years. Patients who were originally randomized to the discontinued three-drug regimen of the three-arm study were not allowed to participate in the two-arm study. Detailed criteria are enumerated in the PANTHER-IPF protocol.
Study Design
The initial PANTHER-IPF study was a randomized, double-blind, placebo-controlled, three-arm trial comparing the three-drug regimen versus NAC alone (plus matching placebos for azathioprine and prednisone) versus matched placebos for each of the active therapies. Following the termination of the three-drug regimen arm, an additional 105 patients were randomized to either NAC or placebo. All patients randomized to the NAC or placebo arms were followed for the planned 60 weeks. This report details the comparison of the NAC vs. placebo-treated patients. The original research protocol with all subsequent amendments and statistical analysis plan are posted with the article at www.nejm.org.
Outcome Measures
The primary outcome measure was the change in FVC over 60 weeks. Secondary outcome measures included: mortality, time to death, frequency of acute exacerbations, frequency of maintained FVC, time-to-disease progression, change in DLco, composite physiological index (CPI),7 alveolar–arterial oxygen gradient [P(A-a)O2], 6-minute walk distance (6MWD) during a 6-minute walk test (6MWT), oxygen saturation area under the curve during 6MWT, 6MWD to desaturation < 80%, 6MWT 7 minutes walked, health status and wellbeing (measured by Medical Outcomes Study 36-Item Short-Form Health Survey [SF-36], the EuroQoL Group 5-Dimension Self-Report Questionnaire [EQ-5D], and St George’s respiratory questionnaire [SGRQ]), dyspnea as measured by the University of California at San Diego Shortness of Breath Questionnaire (UCSD-SOBQ), Investigating Choice Experiments for the Preferences of Older People CAPability measure for older people [ICE-CAP]), frequency and types of adverse events (AEs), infectious and noninfectious respiratory complications, and the frequency of all-cause and respiratory-related hospitalizations.
Adjudication
The IPFnet Adjudication Committee was tasked with reviewing all deaths and hospitalizations for cause, as well as, all cases of suspected acute exacerbation. The definition of acute exacerbations was pre-specified and was in accordance with published criteria.8
Statistical Design and Analysis
Randomization
A permuted, block-randomization scheme was created with varying block sizes stratified by clinical center. Once the screening process was completed, patients were randomized to receive the available treatment regimens with equal probability (1:1:1 prior to the clinical alert and 1:1 following the clinical alert) via telephone contact with a central interactive voice response system.
Sample Size Justification
After accounting for potential dropouts (assuming 80% of patients are followed for 60 weeks) and imperfect compliance (2% non-compliance for each arm),9 the target overall sample size of 130 patients per group provided 93% power to obtain a statistically significant difference between the treatments for the hypothesized difference between treatment groups of 0.15 L over 60 weeks.10
Data Analysis
All analyses are based on intent-to-treat principles using all randomized patients. Patients who prematurely discontinued study medication but did not withdraw consent were followed to the 60 week time point. For continuous baseline factors, summary measures are presented using mean (standard deviation) and median (25th and 75th percentiles). For categorical variables, counts and percentages are presented.
For the primary analysis, a repeated measures analysis (using PROC MIXED in SAS) was used to compare differences in the slope of FVC measurements across the treatment groups over the 60-week study period with planned measurements at baseline and weeks 15, 30, 45 and 60.11 This model assumes data were missing at random and no data were imputed. Variables in the regression model included treatment, time, time by treatment, age, sex, race, and height. The slope estimates capture the change in FVC over time. Contrast estimates of differences in slopes of treatment by time (along with confidence intervals) were used to estimate the treatment effect. A sensitivity analysis for the FVC endpoint was conducted using the worst-rank approach which assigns missing data the worst possible value.10 This analysis was conducted at each of the scheduled follow-up assessment points (15, 30, 45, and 60 weeks). For binary endpoints, statistical comparisons were based on two-sided Fisher’s exact tests or Chi-square tests. Kaplan-Meier curves and log-rank tests were used to display event rates and test statistical hypotheses, respectively. Statistical comparisons were two-sided and p-values<0.05 were considered statistically significant unless otherwise specified.
Subgroup Analyses
Pre-defined groups of interest included higher baseline FVC, typical versus atypical baseline HRCT, recent versus more remote IPF diagnosis, lower enrollment CPI, medical therapy for gastroesophageal reflux, ethnic background, gender, smoking history, and emphysema > 25% on HRCT. Continuous subgroup factors were split into two groups based on the median value. Given the major protocol modifications related to the termination of the three-drug regimen, we analyzed the cohorts of patients randomized prior to versus following the clinical alert (‘pre and post clinical alert’ subgroups) to explore the possibility of any differences between these subgroups. This comparison was not specified in the updated statistical analysis plan. For subgroup analyses (PANTHER-IPF protocol, section 2.4), a conservative level of 0.001 was used for statistical significance.
RESULTS
Baseline Characteristics
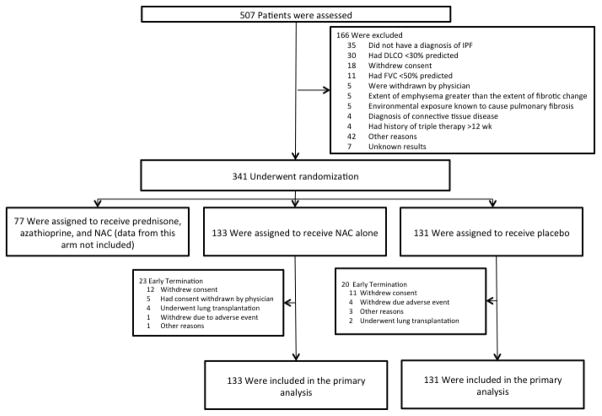
Between December 2009 and October 2011 (pre-alert) and between January 2012 and July 2012 (post-alert), 264 patients were enrolled into the study arms: 133 in the NAC and 131 in the placebo group (Figure 1). Between October 2011 and January 2012 enrollment was suspended while the protocol was amended and approved by the Steering Committee, DSMB, and local IRBs. The study groups were well matched—the mean age for the population was 67 years, 22% of the patients were females and 96% were white (Table 1). The mean percent predicted FVC and DLCO were 73% and 45%, respectively. The mean 6MWT distance was 373 meters. HRCT findings were sufficient to diagnose definite UIP in 77% of cases. A total of 139/264 (52.6%) of participating subjects underwent surgical lung biopsy.
Figure 1.
Enrollment and Outcomes
Table 1.
Patient Characteristics at Baseline
| Characteristic | Overall | Pre-Alert | Post-Alert | |||
|---|---|---|---|---|---|---|
| NAC (N=133) | Placebo (N=131) | NAC (N=81) | Placebo (N=78) | NAC (N=52) | Placebo (N=53) | |
| Age – years | 68.3+/−8.4 | 67.2+/−8.2 | 68.7+/−7.7 | 67.9+/−8.1 | 67.5+/−9.4 | 66.1+/−8.3 |
| Female sex | 26 (19.5) | 33 (25.2) | 17 (21.0) | 21 (26.9) | 9 (17.3) | 12 (22.6) |
| White race | 126 (94.7) | 126 (96.2) | 78 (96.3) | 75 (96.2) | 48 (92.3) | 51 (96.2) |
| History of smoking – never | 36 (27.3) | 33 (25.2) | 20 (25.0) | 20 (25.6) | 16 (30.8) | 13 (24.5) |
| Time since diagnosis - years | 1.0+/−1.0 | 1.1+/−1.0 | 1.0+/−1.1 | 1.1+/−1.0 | 1.0+/−0.9 | 1.3+/−1.1 |
| Concomitant illnesses | ||||||
| Coronary artery disease | 32 (24.1) | 28 (21.5) | 21 (25.9) | 18 (23.1) | 11 (21.2) | 10 (19.2) |
| Diabetes | 28 (21.2) | 23 (17.7) | 14 (17.3) | 14 (17.9) | 14 (27.5) | 9 (17.3) |
| Gastroesophageal reflux disease | 79 (59.8) | 82 (62.6) | 50 (61.7) | 46 (59.0) | 29 (56.9) | 36 (67.9) |
| Pulmonary physiology | ||||||
| Forced vital capacity (liters) | 2.9+/−0.8 | 2.9+/−0.8 | 2.9+/−0.9 | 2.8+/−0.7 | 3.0+/−0.8 | 3.1+/−0.8 |
| Forced vital capacity (% of predicted) | 72.2+/−15.9 | 73.4+/−14.3 | 71.9+/−15.4 | 72.1+/−14.5 | 72.7+/−16.6 | 75.2+/−13.8 |
| Carbon monoxide diffusion corrected for hemoglobin (mL/min/mmHg) | 13.2+/−3.7 | 13.5+/−3.8 | 12.8+/−3.8 | 13.1+/−3.6 | 13.8+/−3.5 | 14.1+/−4.0 |
| Carbon monoxide diffusion corrected for hemoglobin (% of predicted value) | 44.7+/−10.8 | 46.0+/−12.2 | 43.9+/−10.3 | 45.3+/−12.2 | 46.0+/−11.5 | 47.1+/−12.2 |
| PaO2 breathing ambient air (mm Hg) | 80.7+/−10.5 | 81.5+/−11.8 | 79.7+/−11.0 | 79.3+/−11.4 | 82.1+/−9.6 | 84.7+/−11.8 |
| 6-minute walk distance (meters) | 371+/−116 | 375+/−105 | 371+/−116 | 369+/−117 | 373+/−116 | 385+/−83 |
| Patient reported outcomes | ||||||
| UCSD Shortness of Breath Questionnaire (range 0–120)§ | 26.1+/−17.1 | 27.1+/−18.7 | 26.7+/−17.6 | 28.8+/−19.2 | 25.2+/−16.4 | 24.5+/−17.8 |
| SGRQ – total score (range 0–100)¶ | 40.2+/−16.5 | 38.0+/−17.2 | 40.1+/−15.8 | 39.2+/−17.2 | 40.4+/−17.9 | 36.3+/−17.2 |
| SF-36 aggregate physical score (range 0–100)** | 41.4+/−8.8 | 40.7+/−9.3 | 41.2+/−7.9 | 40.7+/−9.2 | 41.7+/−10.1 | 40.7+/−9.5 |
| SF-36 aggregate mental score (range 0–100)** | 53.2+/−9.1 | 55.3+/−7.5 | 53.0+/−9.9 | 55.9+/−7.4 | 53.7+/−7.8 | 54.3+/−7.6 |
| ICECAP Summary Score (range 0–1) | 0.87+/−0.09 | 0.88+/−0.09 | 0.88+/−0.09 | 0.89+/−0.08 | 0.86+/−0.09 | 0.87+/−0.09 |
| EuroQoL Visual Analog Score (range 0–100) | 78.3+/−15.0 | 77.7+/−14.3 | 78.5+/−13.6 | 78.4+/−15.1 | 78.1+/−17.0 | 76.7+/−13.3 |
Race was self-reported by the study subject.
Categorical variables presented as N (%).
Continuous variables presented as mean +/− SD
Scores on the Shortness of Breath Questionnaire range from 0 to 120, with higher scores indicating worse function. The minimally clinically important difference is 5 points.11
Scores on St. George’s Respiratory Questionnaire range from 0 to 100, with higher scores indicating more limitations. The suggested minimal important difference for patients with idiopathic pulmonary fibrosis has been estimated at 5 to 8 points.12
Scores on the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) range from 0 to 100, with higher scores indicating better function. The suggested minimal important difference for patients with idiopathic pulmonary fibrosis has been estimated at 2 to 4 points.
There are no published minimal important difference scores for the EuroQoL or ICECAP in idiopathic pulmonary fibrosis.
No statistically significant differences in baseline patient characteristics results were observed.
Abbreviations: SGRQ denotes St George’s respiratory questionnaire; EuroQoL denotes EuroQoL Group 5-Dimension; SF-36 denotes the Medical Outcomes Study 36-Item Short-Form Health Survey; and ICECAP denotes ICEpop CAPability measure for Older people.
Study Drug Adherence
A total of 34 of 133 patients in the NAC group and 29 of 131 in the placebo group discontinued study medications (p=0.53). At 30-weeks, 93.3% in the NAC arm and 91.7% in the placebo arm reported taking more than 80% of the recommended doses of study drug. Similarly, at 60-weeks, 90.4% in the NAC arm and 94.4% in the placebo arm reported taking more than 80% of the recommended doses of study drug.
Primary Outcome Measure
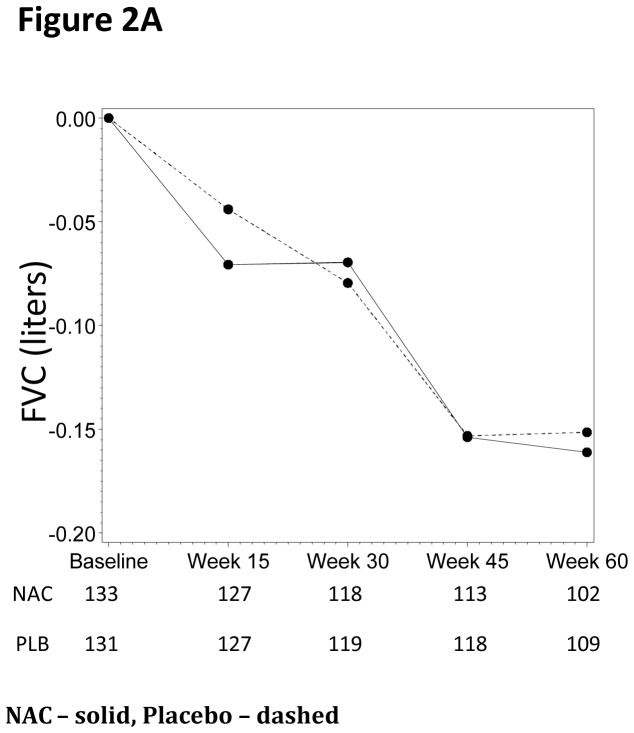
Using the worst-rank score analysis, there were no statistically significant differences in FVC % predicted between the treatment groups at any of the time points (p=0.77, Table 2, Figure 2A and Figure S1). There were no statistically significant differences in the primary endpoint in the predefined subgroups.
Table 2.
Outcome Measures – Estimated Changes over 60 Weeks
| Endpoint | NAC (N=133) | Placebo (N=131) | Treatment Difference (95% Confidence Interval) | P-value |
|---|---|---|---|---|
| Forced vital capacity (liters) | −0.18 | −0.19 | 0.01 (−0.06, 0.09) | 0.77 |
| Pre-alert | −0.17 | −0.22 | 0.06 (−0.04, 0.15) | 0.27 |
| Post-alert | −0.19 | −0.13 | −0.06 (−0.18, 0.06) | 0.32 |
| Carbon monoxide diffusion corrected for hemoglobin (mL/min/mmHg) | −1.3 | −1.4 | 0.2 (−0.4, 0.8) | 0.56 |
| Pre-alert | −1.1 | −1.9 | 0.8 (0.1, 1.5) | 0.028 |
| Post-alert | −1.4 | −0.8 | −0.7 (−1.6, 0.3) | 0.18 |
| 6-minute walk test distance (meters) | −23.4 | −47.5 | 24.2 (−2.6, 50.9) | 0.076 |
| Pre-alert | −27.2 | −67.7 | 40.5 (6.1, 74.8) | 0.021 |
| Post-alert | −14.1 | −15.8 | 1.7 (−38.7, 42.1) | 0.93 |
| UCSD shortness of breath questionnaire | 6.0 | 5.8 | 0.2 (−4.1, 4.4) | 0.94 |
| Pre-alert | 5.6 | 7.6 | −2.1 (−8.3, 4.2) | 0.52 |
| Post-alert | 7.4 | 2.8 | 4.6 (−0.7, 9.8) | 0.086 |
| SGRQ – total score | 4.3 | 5.5 | −1.2 (−4.9, 2.4) | 0.51 |
| Pre-alert | 4.5 | 7.7 | −3.3 (−8.3, 1.7) | 0.20 |
| Post-alert | 4.3 | 2.7 | 1.6 (−3.7, 6.9) | 0.55 |
| SF-36: aggregate mental score | 0.0 | −2.6 | 2.6 (0.3, 5.0) | 0.025 |
| Pre-alert | 0.8 | −3.5 | 4.3 (1.3, 7.3) | 0.005 |
| Post-alert | −1.3 | −1.2 | −0.1 (−3.8, 3.6) | 0.97 |
| ICECAP summary score | 0.01 | −0.02 | 0.03 (0.01, 0.05) | 0.013 |
| Pre-alert | −0.00 | −0.05 | 0.04 (0.01, 0.08) | 0.008 |
| Post-alert | 0.02 | 0.00 | 0.01 (−0.02, 0.05) | 0.40 |
| EuroQoL visual analog score | 0.9 | −3.3 | 4.2 (−0.3, 8.8) | 0.069 |
| Pre-alert | −1.7 | −7.6 | 5.9 (−0.1, 11.9) | 0.054 |
| Post-alert | 4.5 | 1.6 | 2.8 (−3.8, 9.4) | 0.40 |
For each endpoint the first row is the overall result, the second row is the pre-alert cohort, and the third row is the post-alert cohort.
Abbreviations: SGRQ denotes St George’s respiratory questionnaire; EuroQoL denotes EuroQoL Group 5-Dimension; SF-36 denotes the Medical Outcomes Study 36-Item Short-Form Health Survey; and ICECAP denotes ICEpop CAPability measure for Older people.
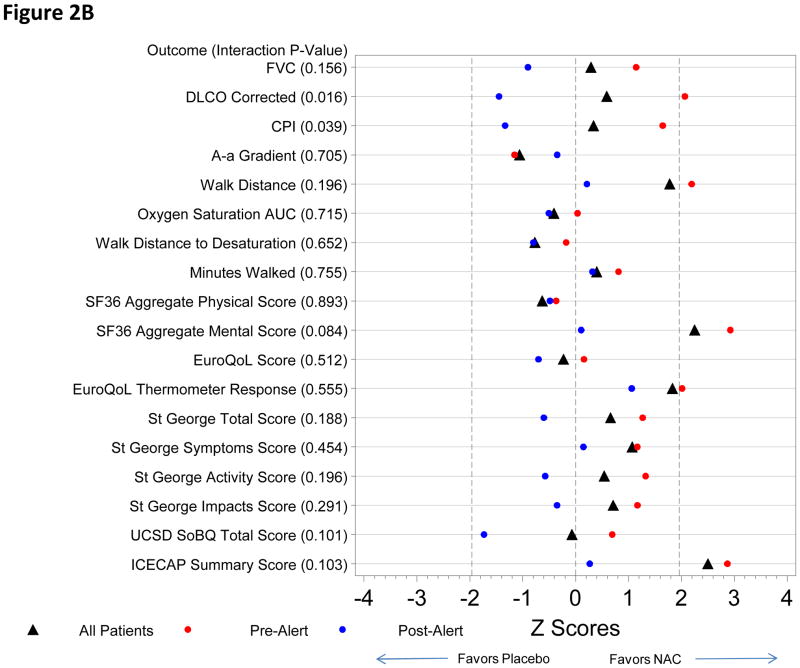
Figure 2.
Figure 2A. FVC Changes from Baseline (Liters)
Figure 2B. Model Results Pre- and Post Alert for NAC vs. Placebo
Secondary Outcome Measures
For the majority of pre-defined secondary endpoints there was no difference between NAC and placebo (Table 2), including DLco (Figure S3(a)). However, a trend favoring NAC in 6MWD (p=0.076; Figure S3(b)), EuroQoL Visual Analog Scale (p=0.069), improvement in SF-36 Mental Score (p=0.025) and ICECAP summary score (p=0.013) were noted (Table 2).
Over the 60-week treatment period there were no significant differences between NAC and placebo for mortality (6 [4.9%] vs. 3 [2.5%] events, p=0.50) or acute exacerbation (3 [2.3%] vs. 3 [2.3%] events, p>0.99). Among other measures, there were no statistically significant differences between study groups for respiratory mortality, all-cause hospitalizations, respiratory hospitalizations, or the proportion of patients experiencing disease progression (all-cause mortality or a 10% decline in FVC) (Table 3 and Figure S2(a–c)).
Table 3.
Clinical Events and Safety
| Outcome | NAC (N=133) | Matched Placebo (N=131) | P-value |
|---|---|---|---|
| Mortality – all-cause† | 6 (4.5) | 3 (2.3) | 0.50 |
| Pre-alert | 4 (4.9) | 3 (3.8) | >0.99 |
| Post-alert | 2 (3.8) | 0 (0.0) | 0.24 |
| Mortality – respiratory† | 5 (3.8) | 3 (2.3) | 0.72 |
| Pre-alert | 3 (3.7) | 3 (3.8) | >0.99 |
| Post-alert | 2 (3.8) | 0 (0.0) | 0.24 |
| Acute exacerbation† | 3 (2.3) | 3 (2.3) | >0.99 |
| Pre-alert | 2 (2.5) | 2 (2.6) | >0.99 |
| Post-alert | 1 (1.9) | 1 (1.9) | >0.99 |
| Hospitalization – all-cause† | 20 (15.0) | 18 (13.7) | 0.76 |
| Pre-alert | 14 (17.3) | 9 (11.5) | 0.30 |
| Post-alert | 6 (11.5) | 9 (17.0) | 0.43 |
| Hospitalization – respiratory† | 11 (8.3) | 10 (7.6) | 0.85 |
| Pre-alert | 9 (11.1) | 5 (6.4) | 0.30 |
| Post-alert | 2 (3.8) | 5 (9.4) | 0.44 |
| Serious Adverse Events | 25 (18.8) | 20 (15.3) | 0.45 |
| Respiratory | 9 (6.8) | 9 (6.9) | 0.97 |
| Infectious | 6 (4.5) | 6 (4.6) | 0.98 |
| Cardiac | 9 (6.8) | 2 (1.5) | 0.033 |
| Gastrointestinal | 0 (0.0) | 6 (4.6) | 0.014 |
| Death (%) | 4.9 (2.2, 10.6)* | 2.5 (0.8, 7.5) | 0.30 |
| Disease Progression** (%) | 27.1 (20.1, 36.0)* | 26.5 (19.5, 35.4) | 0.82 |
| Death or Hospitalization (%) | 17.5 (11.9, 25.4)* | 15.6 (10.2, 23.3) | 0.59 |
For each endpoint the first row is the overall result, the second row is the pre-alert cohort, and the third row is the post-alert cohort. Presented as # of individuals with an event (percentage)
60-week Kaplan-Meier Estimates (95% Confidence Intervals)
Death or >10% decline in FVC
Adverse Events
The overall incidence of serious adverse events is presented in Table 3. There were no significant differences in serious adverse events between the NAC and placebo groups except for cardiac disorders (which occurred in 6.8 percent of patients receiving acetylcysteine [9 of 133] and in 1.5 percent of those receiving placebo [2 of 133] [P=0.033]) and gastrointestinal disorders (which occurred in 0 percent of patients receiving acetylcysteine and in 4.6 percent of those receiving placebo [6 of 133] [P=0.014]).
Subgroup Analyses
None of the outcome measures reached a pre-specified conservative p-value (p<0.001). There were no differences between the NAC and placebo groups in the primary endpoint over the 60 weeks of follow-up either pre-alert or post-alert (p=0.27 and p=0.32 respectively) (Table 2). For a number of other comparisons a trend toward a favorable response in the NAC group (versus placebo) was noted in the pre-alert compared to the post-alert period (Tables 2–3, Figure 2B).
DISCUSSION
NAC 600mg tid has been suggested to benefit patients with IPF by favorably altering the oxidative state of the lung.12 The IFIGENIA study of the three-drug regimen (NAC, azathioprine plus prednisone) found that this treatment preserved FVC and DLco better than a two-drug regimen (azathioprine plus prednisone).4 The current study shows that NAC 600mg tid was not associated with preservation of FVC compared with a matched placebo in IPF patients with mild-to-moderate impairment in pulmonary function. The patients treated with NAC monotherapy reported better mental wellbeing (based on the SF-36 mental score and ICECAP summary score) over a 60 week period. NAC monotherapy was associated with more cardiac events and less GI events compared to placebo.
The responses for the NAC patients were similar in the pre- and post-alert periods. There were no differences between the NAC and placebo groups in the decline of FVC, all-cause mortality, respiratory mortality, all-cause hospitalizations, respiratory hospitalizations, acute exacerbations or the proportion of patients experiencing disease progression between these groups. A trend toward benefit in other outcome measures in subjects receiving placebo in the post-alert period compared to the pre-alert period was noted; however, an explanation for this finding is not evident. It must be emphasized that our results are applicable only to IPF patients who met the inclusion and exclusion criteria of this trial, and not to patients with more advanced disease or other forms of idiopathic interstitial pneumonia and interstitial lung disease.
Treatment with NAC did not help preserve FVC in IPF patients with baseline mild-to-moderate physiological abnormalities.
Supplementary Material
Acknowledgments
Prednisone, Azathioprine, and N-acetylcysteine: a study THat Evaluates Response in Idiopathic Pulmonary Fibrosis: A randomized, double-blind, placebo-controlled trial (PANTHER-IPF) and the IPFnet were funded by the National Heart, Lung, and Blood Institute (NHLBI) and The Cowlin Family Fund at the Chicago Community Trust; NAC and matching placebo were a gift from Zambon S.p.A.
Supported by grants from the NHLBI: U10HL080413 (data coordinating center), U10HL080274, U10HL080370, U10HL080371, U10HL080383, U10HL080411, U10HL080509, U10HL080510, U10HL080513, U10HL080543, U10HL080571, U10HL080685 (clinical centers). ClinicalTrials.gov number, NCT00650091
We are indebted to the PANTHER-IPF DSMB (Gerald S. Davis, M.D., chair; Robert Levine, M.D., Steven D. Nathan, M.D., Sharon Rounds, M.D., B. Taylor Thompson, M.D., Bruce Thompson, Ph.D., and Gilbert White, M.D.), its NHLBI representatives (Hannah Peavy, M.D., and Barry Schmetter, B.S.), and the PANTHER-IPF protocol review committee (Peter B. Bitterman, M.D., chair; Teri J. Franks, M.D., Steven Idell, M.D., Steven Piantadosi, M.D., Ph.D., William N. Rom, M.D., M.P.H., Moises Selman, M.D., and David S. Wilkes, M.D.) for their dedication and oversight. We are indebted to the patients who volunteered to participate in this study, to the study coordinators and to the generous provision of study medications (NAC and matched placebo effervescent tablets from Zambon).
Footnotes
ASSURANCES
The authors are solely responsible for the content of this article, which does not necessarily represent the official views of the NHLBI or the National Institutes of Health. The members of the writing committee assume responsibility for the overall content and integrity of the article. Kevin J. Anstrom, PhD and Eric Yow, MS analyzed the data.
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peikert T, Daniels CE, Beebe TJ, Meyer KC, Ryu JH. Assessment of current practice in the diagnosis and therapy of idiopathic pulmonary fibrosis. Respir Med. 2008;102:1342–8. doi: 10.1016/j.rmed.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–42. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Anstrom KJ, King TE, Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968–77. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 6.Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167:962–9. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 7.Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636–43. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics. 1986;42:507–19. [PubMed] [Google Scholar]
- 9.Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med. 1998;17:1643–58. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Lachin JM. Worst-rank score analysis with informatively missing observations in clinical trials. Control Clin Trials. 1999;20:408–22. doi: 10.1016/s0197-2456(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 11.Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD. 2005;2:105–10. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 12.Swigris JJ, Brown KK, Behr J, et al. The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med. 2010;104:296–304. doi: 10.1016/j.rmed.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.