Abstract
Aim
Large interindividual variability in morphine disposition could contribute to unpredictable variability in morphine analgesia and adverse events. Caucasian children have more adverse effects and slower morphine clearance than African–American children. To study variations in intravenous morphine pharmacokinetics in children, we examined the influence of genetic polymorphisms in OCT1
Methods
In 146 children undergoing adenotonsillectomy, 146 concentration–time profiles (2–4 measurements per patient) were available. Population pharmacokinetic ana lysis characterized the profiles in NONMEM® and tested OCT1 variants as covariates.
Results
Allometrically scaled post hoc Bayesian morphine clearance in homozygotes of loss-of-function OCT1 variants (n = 9, OCT1*2–*5/*2–*5 was significantly lower (20%) than in wild-type (n = 85, OCT1*1/*1) and heterozygotes (n = 52, OCT1*1/*2–*5; p < 0.05).
Conclusion
Besides bodyweight, OCT1 genotypes play a significant role in intravenous morphine pharmacokinetics. Relatively high allelic frequencies of defective OCT1 variants among Caucasians may explain their lower morphine clearance and possibly higher frequencies of adverse events compared with African–American children.
Keywords: children, morphine, OCT1, pediatrics, pharmacogenetics, pharmacokinetics, population pharmacokinetics, surgical pain
Morphine is one of the most commonly used opioids to manage perioperative pain in children. Inadequately treated pain is a major public health issue [1]. Clinically unpredictable variability in analgesic response and adverse events, along with narrow therapeutic indices of opioids, continue to pose a clinical problem in the perioperative setting, especially in children. Extreme clinical responses to opioids (inadequate analgesia and severe opioid-related adverse effects) often result in additional clinical interventions and a longer stay in hospital. Anesthesiologists are quite aware of interindividual variation in responses to drugs such as opioids [2]. There is a large body of literature focused on the determining factors including age, weight and race in the experience of clinical pain in adults [3–5]. Bodyweight/size and age are often used as primary covariates to predict morphine pharmacokinetics in pharmacokinetic models [2]. On the other hand, considerable evidence exists for racial influence on the response to treatment. It was reported that African–American and Hispanic patients have a lower tolerance for experimentally induced pain compared with Caucasian adults [3–5]. Earlier, we demonstrated that African–American children have inadequate surgical pain relief while Caucasian children have more adverse events with similar perioperative doses of morphine [6]. In addition, we also reported that African–American children had 23% higher morphine clearance than children of Caucasian descent [7]. In the study, we examined polymorphisms in UGT2B7, which is widely accepted as the predominant isozyme responsible for morphine metabolism [8], since common UGT2B7 polymorphisms (−161C>T and 802C>T [His268Tyr], linked with −900A>G) [101] have racial differences in their frequencies as the wild-type genotype was more frequent in African–American children. However, the racial difference in morphine clearance observed in our earlier study could not be explained by common UGT2B7 polymorphisms [7]. This points to the fact that the impact of factors important in personalizing care, such as race and genetic polymorphisms, has not been well characterized, although these factors should be equally important and be considered in predictive models [9].
A recent study showed that morphine is a substrate of OCT1, a member of the organic cation transporters (OCTs) family [10], also known as SLC22A1. OCTs are critical for the absorption, distribution and elimination of many small molecular weight basic compounds, including endogenous amines [11,12]. OCT1 is predominantly expressed in the sinusoidal membrane of the human liver [13–15]. It is hypothesized to mediate cellular uptake of morphine into hepatocytes (Figure 1). OCT1 is highly genetically polymorphic, as approximately 10% of Caucasians are compound homozygous carriers of one of the four common coding polymorphisms (Arg61Cys, Gly401Ser, Gly465Arg and deletion of Met420) that result in reduced or lost OCT1 transporter activity [16]. Homozygous carriers have a reduced ability to uptake drugs such as metformin, tropisetron or O-desmethyltramadol in the liver [17–20]. The recent report also indicated that carriers of the loss-of-function OCT1 genetic variants listed above resulted in increased plasma concentrations of morphine in healthy adult volunteers [10]. This alludes to the contribution of OCT1 polymorphisms to interindividual variability in morphine pharmacokinetics.
Figure 1. Hypothetical morphine transportation and biotransformation.
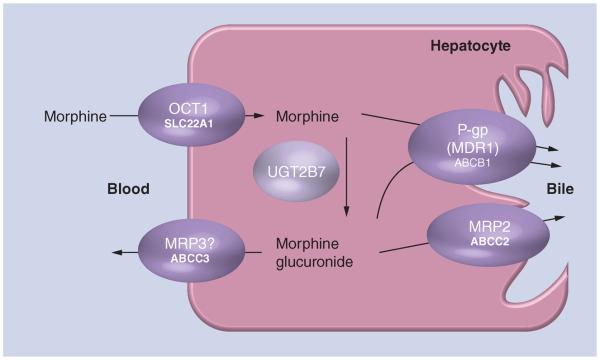
OCT1 is localized in the sinusoidal membrane of the human hepatocyte and mediates cellular uptake of substrates into the liver. ABCC3 (mrp3 in mice) is proposed to mediate the export of morphine back to blood [28]. The main metabolic enzyme of morphine is UGT2B7. P-gp and ABCC2 may mediate export of morphine and its metabolites to bile.
In the current study, using our homogeneous pediatric tonsillectomy cohort, we performed a follow-up pharmacogenetic study to evaluate the potential impact of OCT1 genetic variants on morphine pharmacokinetics in pediatric patients.
Methods
Study design & setting
This study is part of an ongoing clinical study, entitled Personalizing Perioperative Morphine Analgesia in Children, which is registered with clinicaltrials.gov (NCT01140724). The clinical study is designed as a prospective, genotype-blinded, clinical observational study with standard perioperative care in a large cohort of children undergoing outpatient adenotonsillectomy to evaluate factors contributing to inter individual variations in analgesic and adverse effect responses to perioperative opioids in children. In this study, the clinical care team and drug concentration measurement team are blinded to patients' genotypes. The study was approved by the institutional review board. Written informed consent was obtained from parents and assent obtained when appropriate from children >7 years of age before enrollment.
Participants
This pharmacogenetic study was performed using the same cohort of children previously reported [7]. Inclusion and exclusion criteria have been described in our previous report. In brief, children aged 6–15 years, classified as American Society of Anesthesiologists (ASA) physiological status 1 or 2 [102], scheduled for elective out patient adenotonsillectomy secondary to obstructive sleep apnea or recurrent adenotonsillitis were enrolled. Exclusion criteria for recruitment were non-English speaking patients/parents, allergy to morphine, developmental delay, liver or renal diseases, and presence of preoperative pain requiring narcotics. All participants were categorized as African–American, Caucasian or other according to race self-identified by their parents. All participants received standard perioperative care with a standard anesthetic consisting of an intra operative dose of 0.2 mg/kg of morphine (children with obstructive sleep apnea received 0.1 mg/kg of morphine).
Genotyping & genetic classification
Blood was drawn in order to collect genomic DNA from all participants in the operating room upon intravenous line placement under anesthesia for genotyping. The participants were genotyped for four nonsynonymous SNPs in the OCT1 gene, which cause reduction or loss of OCT1 transporter activity, including Arg61Cys (rs12208357), Gly401Ser (rs34130495), Gly465Arg (rs34059508) and the deletion of Met420 (rs72552763). In addition, OCT1 intron SNP (rs622342) was also determined since it has been proposed as a marker SNP for metformin response [21]. Furthermore, UGT2B7 −900A>G (rs7438135) was also determined to identify the variant allele possessing −900A>G, −161C>T and 802C>T. The genotyping was performed using commercially available TaqMan assays (Applied Biosystems, CA, USA) after cross validation by direct sequencing (see Supplementary table 1 for details; www.futuremedicine.com/doi/suppl/10.2217/pgs.13.94). In order to screen for an influence of the OCT1 genotype on morphine pharmacokinetics, all participants were classified into three groups based on the presence of the nonsynonymous OCT1 variants (table 1). The definition of alleles, OCT1*2, *3, *4 and *5 was based on a previous publication [22]. Combinations of the nonsynonymous OCT variants for determining the alleles are shown in table 2. Subjects who did not have any of four OCT1 exon variants were defined as being OCT1*1/*1.
Table 1.
OCT1 allele definition used in the current study†.
| Allele | Nucleotide | Amino acids | ||||||
|---|---|---|---|---|---|---|---|---|
| 286C>T | 1306G>A | 1365GAT>del | 1498G>C | Arg61Cys | Gly401Ser | Met420del | Gly465Arg | |
| OCT1*1 | C | G | GAT | G | Arg | Gly | Met | Gly |
| OCT1*2 | C | G | Del | G | Arg | Gly | Del | Gly |
| OCT1*3 | T | G | GAT | G | Cys | Gly | Met | Gly |
| OCT1*4 | C | A | GAT | G | Arg | Ser | Met | Gly |
| OCT1*5 | c | G | Del | C | Arg | Gly | Del | Arg |
Definition of allele is based on a previous publication [22].
Del: Deletion.
Table 2.
OCT1 genotype classification, observed genotypes and their frequencies.
| Diplotype (genotype) |
OCT1 SNPs |
In the current study |
Group definition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 286C>T | 1306G>A | 1365GAT>del | 1498G>C |
Total
|
Caucasians
|
African
–
Americans
|
|||||
| n † | Frequency | n | Frequency | n | Frequency | ||||||
| OCT1*1/*1 | C | G | GAT | G | 85 | 0.582 | 62 | 0.55 | 21 | 0.75 | Wild-types |
|
| |||||||||||
| OCT1*1/*2 | C | G | GAT/del | G | 27 | 0.185 | 21 | 4 | |||
| OCT1*1/*3 | C/T | G | GAT | G | 12 | 0.082 | 10 | 0.37 | 2 | 0.25 | Heterozygotes |
| OCT1*1/*4 | C | G/A | GAT | G | 6 | 0.041 | 6 | 0 | |||
| OCT1*1/*5 | C | G | GAT/del | G/C | 7 | 0.048 | 5 | 1 | |||
|
| |||||||||||
| OCT1*2/*2 | C | G | del | G | 2 | 0.014 | 2 | 0 | |||
| OCT1*2/*3 | C/T | G | GAT/del | G | 3 | 0.021 | 3 | 0 | |||
| OCT1*3/*3 | T | G | GAT | G | 0 | 0 | 0 | 0 | |||
| OCT1*2/*4 | C | G/A | GAT/del | G | 2 | 0.014 | 2 | 0 | |||
| OCT1*3/*4 | C/T | G/A | GAT | G | 0 | 0 | 0 | 0.08 | 0 | 0 | Homozygotes |
| OCT1*4/*4 | C | A | GAT | G | 1 | 0.007 | 1 | 0 | |||
| OCT1*2/*5 | C | G | Del | G/C | 1 | 0.007 | 1 | 0 | |||
| OCT1*3/*5 | C/T | G | GAT/del | G/C | 0 | 0 | 0 | 0 | |||
| OCT1*4/*5 | C | G/A | GAT/del | G/C | 0 | 0 | 0 | 0 | |||
| OCT1*5/*5 | C | G | Del | C | 0 | 0 | 0 | 0 | |||
|
| |||||||||||
| Total | 146 | 1 | 113 | 28 | |||||||
Number of subjects; 113, 28 and 5 were classified as Caucasians, African-Americans and others, respectively.
Del: Deletion.
Pharmacokinetic sampling
Blood samples (2–4) were obtained from an individual patient for morphine pharmaco-kinetic ana lysis before and 5 min, 10–15 min and 30–45 min after a bolus intravenous dose of morphine using individual venous needle sticks. The last blood sample was drawn in the recovery room typically between 30 and 45 min after morphine intravenous bolus and, for ethical reasons, a few minutes before the child fully recovered from anesthesia in the recovery room.
Drug measurement
Morphine and its active metabolites, morphine 3β-glucuronide (M3G) and morphine 6β-glucuronide (M6G), were quantified in EDTA plasma using an established and validated, semiautomated liquid chromatography–mass spectrometry/mass spectrometry assay [23]. The reliable limits of quantification were 0.25–1000 ng/ml (r2 > 0.99) for morphine, and 1–1000 ng/ml (r2 > 0.99) for both M3G and M6G. Total imprecision was less than 15%. The interday accuracy was within 85–115%. There was no carry over, matrix interferences or ion suppression/enhancement interfering with the quantification of the analytes.
Pharmacokinetic parameter estimate
To obtain individual pharmacokinetic parameter estimates from a limited number of samples per individual, population pharmacokinetic ana lysis and post hoc Bayesian estimation were used. First, a nonlinear, mixed effects, population pharmacokinetic model was developed for the current morphine data using NONMEM® (version 7.2, ICON Dev. Soln., MD, USA) with PsN-Toolkit (version 3.5.3) as the interface. A two compartment structure model (subroutine ADVAN3 TRANS4) described the morphine concentration–time profile well using first-order conditional estimation with interaction in NONMEM. A proportional error model was used to account for unexplained residual variability. The error model was developed by adding inter-individual variability on each pharmaco kinetic parameter (clearance [CL], central volume [V1], intercompartmental clearance [Q] and peripheral volume [V2]) one at a time in a stepwise manner as below:
| (Equation 1) |
The error model was finalized by evaluating the goodness-of-fit plots, objective function value and empirical Bayesian estimates-based diagnostics (η/ε-shrinkage). A drop in objective function value of 3.84 points (p < 0.05 assuming a χ2 distribution) was considered to be significant while evaluating the impact of one additional parameter among nested models. Models were also evaluated based on standard diagnostic plots (dependent variable [DV] vs population prediction [PRED], DV vs individual PRED [IPRED], conditional weighted residual [CWRES] vs PRED, CWRES vs time after first dosing [TIME]) to identify bias corresponding to model misspecification. Models with high η-shrinkage (>30%) were rejected as standard empirical Bayesian estimates-based diagnostics were rendered uninformative [24].
Standard diagnostic plots (DV vs PRED, DV vs IPRED, CWRES vs PRED, CWRES vs TIME) stratified by covariates were used to identify potential covariate effects. The effect of using bodyweight as a covariate was tested first and included in the pharmacokinetic model with an allometric model as depicted in
| (Equation 2) |
where Pi (BWi) is the parameter (weight) in the i th individual and θexp is the exponential scaling factor. Covariate ana lysis was also performed to test the significance of OCT genotype on CL as a categorical covariate.
In addition to the model diagnostic methods mentioned above, the final model was evaluated using normalized prediction distribution errors (npde) and bootstrap. In total, 1000 simulations of the current trial were performed using NONMEM and the observed data from the current trial was compared with the simulated data set to generate npde. The distribution of these npde's was plotted with respect to PRED, TIME and other covariates to identify potential model bias. Furthermore, the model stability and precision of the parameter estimates were determined by a bootstrap procedure within PsN. The mean parameter estimates with their coefficient of variation were determined by refitting the original model to 2000 new data sets that were generated by resampling with replacement from the original data set.
Statistical analysis
Numerical variables are summarized as mean ± standard deviation. Binary and categorical variables are summarized as ratio or frequency (%). Parametric and nonparametric analyses were undertaken using JMP8, a SAS-based statistics software package. Determination of the statistical differences in parameters between various groups was performed using either Student's t-test, Fisher's one-way ana lysis of variance (using the Tukey–Kramer multiple comparison test), or the Kruskal–Wallis test and the Mann–Whitney U test, as applicable. A p-value of <0.05 was considered statistically significant. When a multiple comparison analysis was performed, p < 0.05 was corrected for the number of groups (e.g., p < 0.05/3 when three groups were compared).
Results
In our cohort of 146 children, 329 morphine concentrations out of 475 measurements were available for the pharmacokinetic–pharmacogenomic ana lysis. We excluded 146 measurements before the initial morphine bolus dose, which had no detectable levels of morphine and metabolites. All participants were classified according to the presence of nonsynonymous OCT variants into three groups: 85 wild-types (OCT1*1/*1); 52 heterozygotes (OCT1*1/*2–*5); and nine homozygotes (OCT1*2–*5/*2–*5) (Tables 1, 2 & 3). Of the 146 patients, 113 (77%) were of Caucasian descent and 28 (19%) were of African–American descent. There were no homozygotes for the defective alleles in African–Americans, and the defective allele frequency (0.125) was much lower than that in Caucasians (0.265) (Table 3). Participant demographics are summarized by OCT genotype in Table 4.
Table 3.
OCT1 alleles and their frequencies.
| Allele | Allele frequency |
|||||
|---|---|---|---|---|---|---|
|
Caucasian
|
African
–
American
|
|||||
| n † | Diplotype frequency | Genotype group frequency | n † | Diplotype frequency | Genotype group frequency | |
| OCT1*1 | 166 | 0.735 | 0.735 | 49 | 0.875 | 0.875 |
|
| ||||||
| OCT1*2 | 31 | 0.137 | 4 | 0.071 | ||
| OCT1*3 | 13 | 0.058 | 0.265 | 2 | 0.036 | 0.125 |
| OCT1*4 | 10 | 0.044 | 0 | 0.000 | ||
| OCT1*5 | 6 | 0.027 | 1 | 0.018 | ||
|
| ||||||
| Total | 226 | 1 | 56 | 1 | ||
The number of alleles was calculated according to the genotypes.
Table 4.
Patient demographics and pharmacokinetic parameter estimates.
| Parameter | Wild-type (*1/*1) | Heterozygotes | Homozygotes | p-value (ANOVA) |
|---|---|---|---|---|
| Subjects (n) | 85 | 52 | 9 | |
| Age (years) | 9.2 ± 2.6 | 9.4 ± 2.6 | 8.7 ± 2.2 | 0.72 |
| Weight (kg) | 40.0 ± 15.1 | 39.9 ± 14.5 | 33.1 ± 8.4 | 0.39 |
| Sex(M/F) | 35/50 | 21/31 | 4/5 | |
| Race (Cau/AA/others) | 62/21/2 | 42/7/3 | 9/0/0 | |
| OSA (yes/no) | 42/43 | 29/23 | 5/4 | |
| Bayesian estimates with the weight-based model † | ||||
| CL (l/min) for all | 1.22 ± 0.18 | 1.25 ± 0.20 | 1.10 ± 0.11‡‡,* | 0.013 |
| CL (l/min) for Cau (n) | 1.21 ± 0.17 (62) | 1.24 ± 0.15 (42) | 1.10 ± 0.11 (9)‡‡,* | 0.022 |
| Bayesian estimates with the final genotype model † | ||||
| CL (l/min) for all | 1.22 ± 0.16 | 1.26 ± 0.13 | 1.02 ± 0.09‡‡‡,*** | <0.001 |
| CL (l/min) for Cau (n) | 1.21 ± 0.16 (62) | 1.24 ± 0.14 (42) | 1.02 ± 0.09 (9)‡‡‡,*** | <0.001 |
Data are shown as mean ± standard deviation.
Allometric scaling of bodyweight with exponents of 0.75 and 1.0 for clearance (CL and Q) and volume of distribution (V1 and V2), respectively (see Table 3). Individual Bayesian clearance estimates were compared among the three OCT1 genotypes after they were standardized to an individual weighing 70 kg.
p < 0.05,
p < 0.01,
p < 0.001 versus wild-type,
p < 0.05,
p < 0.01,
p < 0.001 versus heterozygotes (nonparametric comparisons for each pair using the Wilcoxon test).
AA: African–American; Cau: Caucasian; CL: Clearance; F: Female; M: Male; OSA: Obstructive sleep apnea.
For the two-step pharmacokinetic and pharmaco genetic association ana lysis, population pharmacokinetic ana lysis was performed to obtain individual pharmacokinetic parameter estimates by post hoc Bayesian analysis (Table 4, Supplementary Table 2). Data were best fit by a two-compartment pharmacokinetic structure model. Interindividual variability on CL was included as a random effect in the model since it was found to substantially improve the model fit (Supplementary Table 2). Model fits significantly improved when allometric scaling was incorporated to account for variation of body size. Allometric exponents when estimated were found to be similar to those proposed based on theoretical considerations. Therefore, allometric scaling on clearance and volume with fixed exponents of 0.75 and 1, respectively, was used in the final model (weight-based model).
Individual clearance estimates generated with the weight-based model were used to explore the influence of OCT1 genotype. Mean individual morphine clearance allometrically standardized to 70-kg weight in subjects homozygous for defective OCT1 alleles was significantly lower than those in subjects carrying less than one defective allele (Table 4). Age and bodyweight were not significantly different among the three OCT1 genotype groups. This significant difference in CL among three genotypes was observed when only Caucasians were included (Table 4). Of note, all homozygotes who showed low morphine clearance were Caucasians, and no homozygous subject was found among African–Americans in our study cohort (Table 1).
Consistent with the observation in the two-step approach, covariate analysis in the population pharmacokinetics analysis revealed that inclusion of the OCT1 genotype as a covariate on clearance in addition to weight significantly improved the model. Morphine clearance was approximately 17% lower in homozygotes for the defective alleles compared with the heterozygotes and OCT1*1/*1 carriers (p < 0.05) (Tables 4 & 5). Inclusion of race, in addition to the OCT genotype, did not significantly improve the model, although it was significant when applied to clearance in the weight-based model. Final model parameter estimates with the bootstrap results are summarized in Table 5 (evaluation plots, see Supplementary Figure 1). Individual clearance estimates by post hoc Bayesian analysis with the final model are displayed in Figure 2.
Table 5.
Population pharmacokinetics analysis.
| Parameters | Weight-based model, estimate (%CV) | Final model with OCT-dependent CL, estimate (%CV) | Bootstrap value, median (%CV) |
|---|---|---|---|
| Fixed effects | |||
| CL (l/min) | 1.2 (9.7) | 1.2 (9.9) | 1.2 (10.5) |
| Factor for OCT1 homozygotes | – | 0.83 (6.2) | 0.83 (6.4) |
| V1 (l) | 3.9 (38.9) | 3.9 (38.5) | 4.0 (36) |
| Q (l/min) | 1.6 (32.3) | 1.6 (32.1) | 1.6 (30.2) |
| V2 (l) | 21.3 (22.3) | 21.2 (22.3) | 20.9 (21.9) |
| γ CL | 0.75 | 0.75 | 0.75 |
| γ V1 | 1 | 1 | 1 |
| γ Q | 0.75 | 0.75 | 0.75 |
| γ V2 | 1 | 1 | 1 |
| Interindividual variability | |||
| Omega on CL | 0.1826 (13.2) | 0.173 (14.3) | 0.172 (29.9) |
| Residual error | |||
| Proportional | 0.2464 (9.7) | 0.2478 (9.7) | 0.245 (19.3) |
All reported pharmacokinetic parameters are for a standard adult weighing 70 kg.
γCL: Allometric coefficient for clearance; γQ; Allometric coefficient for intercompartmental clearance; γv1: Allometric coefficient for central volume; γV2: Allometric coefficient for peripheral volume; CL: Clearance; CV: Coefficient of variation; Q: Intercompartmental clearance; V1: Central volume; V2: Peripheral volume.
Figure 2. Morphine clearance standardized to a 70-kg person versus OCT1 genotype.
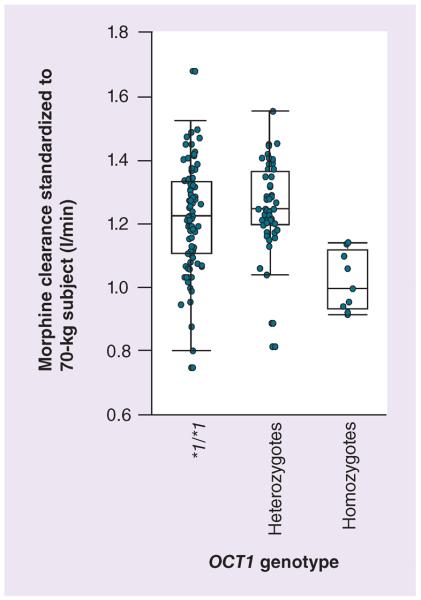
Variation of empirical Bayesian estimates for individual morphine clearance standardized to a 70-kg person with respect to the OCT1 genotype. Homozygotes for defective OCT1 alleles were found to have a significantly lower clearance as compared with subjects with at least one active allele.
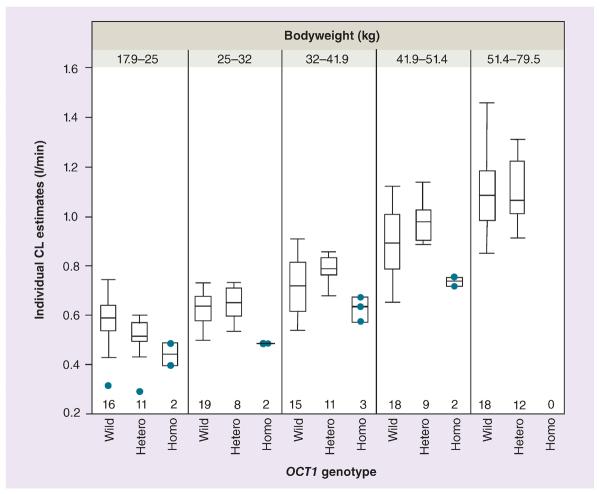
Owing to the wide age range (6–15 years), to explore developmental change on top of body size, study children were divided into five equal groups according bodyweight to minimize weight effect. As a result, the heterozygotes in the lightest weight group had a lower clearance than the wild-types (p = 0.031; the Kruskal–Wallis test; Figure 3). In addition, to explain the large variability in clearance in subjects with OCT1*1/*1, the OCT1 intron SNP (rs622342) and UGT2B7 -900A>G were considered for future study if one of these could provide further insight. The OCT1 intron SNP showed a negligible effect on clearance in the OCT1*1/*1 genotype groups (Supplementary Figure 2). Regarding the UGT2B7 genotype, there was a trend on clearance according to presence of the UGT2B7 -900A>G allele in relatively high-weight subjects with a OCT1*1/*1 genotype, although this trend did not reach significance (Supplementary Figure 3). Genetic contribution of the UGT2B7 variant (-900A>G) to morphine clearance was relatively minor compared with that of the OCT1 genotypes.
Figure 3. Individual morphine clearances versus OCT1 genotype among five different weight groups.
Variation of empirical Bayesian estimates for individual morphine clearance with respect to the OCT1 genotype stratified by weight. Homozygotes for defective OCT1 alleles consistently had significantly lower clearance as compared with subjects with at least one active allele while heterozygotes had lower clearance than the wild-type in the lowest weight groups. Circles represent individual clearance estimates from patients who were recognized as outliers or who were homozygous for the defective OCT1 variants. Numbers represent number of subjects (in each bodyweight category) with different OCT1 genotypes.
CL: Clearance; Hetero: Heterozygote; Homo: Homozygote; Wild: Wild-type.
Discussion
To our knowledge, this is the first study that demonstrates the contribution of the OCT1 genotype to interindividual variability in morphine pharmacokinetics in pediatric patients. Although large interindividual variability in the analgesic and adverse effect response to opioids such as morphine has been recognized, the underlying mechanisms have not been fully characterized [25]. Since this analgesic response is driven by morphine concentrations, we, as a first step, have focused on the interindividual variability in pharmacokinetic parameters as a part of our ongoing cohort study.
A recent in vitro study suggested that morphine can be a substrate of OCT1, and functional variants in the OCT1 gene cause a lower transport activity of morphine than wild-type OCT1 [10]. In addition, the subsequent clinical trial in healthy volunteers demonstrated that the OCT1 genotype might have an influence on the pharmacokinetics of morphine [10]. According to the Henderson–Hasselbalch equation [26] morphine has two pKa values, one for the phenol group (9.9) and one for the amine group (8.2). This means that the conformation of morphine could be changed to the ionic or nonionic form depending on the pH, and that the cationic conformation would be a major form of morphine at a pH of approximately 7. Thus, a basic physico-chemical property of morphine also supports the recent finding.
To date, functional OCT1 genetic variants have been studied intensively for metformin, a cationic compound [21,27], and also for 5-HT3 antagonists, such as tropisetron and ondansetron [19], as well as tramadol, an opioid agonist [20]. In particular, several clinical studies on metformin have demonstrated that OCT1 genetic variants significantly influence not only the pharmacokinetics [27] but also its pharmacodynamics [21]. Based on our literature search, four major defective exon variants and one intron SNP were selected for functional assessment in the current study. Of course, possible contributing minor variants cannot be ruled out, as the OCT1 gene is very polymorphic and has many minor variants that could have further explained the variability. More subjects enrolled in our ongoing study will allow us to evaluate the effect of these minor alleles of OCT1 on morphine pharmacokinetics. Our pediatric subjects represent a relatively homogeneous cohort in terms of disease background and study conditions. Therefore, with an increased number of subjects enrolled, we believe that we would be able to update our pharmacogenetic and pharmacokinetic inputs on an ongoing basis to help guide short-term postsurgical morphine treatment.
A higher morphine requirement to treat inadequate pain relief in African–American children compared with Caucasian children has been observed in the postoperative recovery room [6]. Relatively low clearance of morphine has also been observed in Caucasian children compared with African–American children. In addition, Caucasian children had significantly more related adverse effects than African–American children for similar doses of morphine [6,7] In our previous study, the observed variability in morphine clearance could not be explained by the most common UGT2B7 genetic polymorphisms (802C>T [His268Tyr]) although their allelic frequencies were different between the races [7]. From our current observation, we assume that lower frequency of defective OCT1 alleles in African–Americans compared with Caucasians might cause lower average morphine clearance in Caucasian children than African–American children. After inclusion of OCT1 genotype in the covariate analysis, race effect had decreased down to a 3% higher population clearance estimate in African–Americans compared with Caucasians (Supplementary Table 2).
There are a few limitations to note in this study. This study was carried out in children undergoing the most common and relatively short outpatient surgery where morphine is frequently used as the main perioperative opioid. Due to the relatively short surgical duration and recovery (most children recovered within 45 min of the morphine dose) and ethical issue of discomfort due to injecting a child while awake, we did not obtain additional late samples after 45 min. Late samples after 45 min would have been more relevant for elimination clearance in the terminal phase. For this reason, morphine clearance values estimated from the established model might not completely indicate clearance in the terminal phase. However, as low clearance values were driven by high morphine concentrations in the two-compartment population pharmacokinetic analysis, it would be reasonable to conclude that the observed difference in the apparent clearance was caused by OCT1 genotype status. In addition, we did not have quantifiable M3G and M6G metabolite concentrations over longer periods of time for some children. The relative short monitoring period might be a reason why the well-studied UGT2B7 genetic variation was not found to be associated with early variability in morphine clearance. Further studies with a longer sampling period are warranted to confirm our current observations.
In summary, subjects homozygous for defective OCT1 alleles had significantly lower morphine clearance than carriers of the active OCT1 alleles. Higher frequency of defective OCT1 alleles may cause lower average clearance of morphine in Caucasian children than that in African–American children. This finding provides a new mechanistic insight into interindividual differences in the pharmacokinetics of morphine and is an additional asset for guidance towards personalized care.
Future perspective
The primary focus of this study was to study the influence of different genetic variants of OCT1 on variability in early morphine pharmacokinetics in children to explain underlying racial differences in morphine clearance between Caucasian and African–American children. This study successfully demonstrated that besides bodyweight, genetic variants coding for loss-of-function of OCT1 play significant roles in early morphine clearances in children. Differences in allele frequency of OCT1 variants between Caucasian and African–American might contribute to differences in pharmacodynamic outcomes. In a larger study with adequate sample size, we will simultaneously study OCT1 and other genes important in morphine's pharmacokinetics and pharmacodynamics, and gene–gene interactions to address current knowledge gaps in personalizing pain management with opioids. Our two other ongoing prospective trials in younger individuals (<6 years) and adolescents (up to 18 years of age) receiving opioids for postoperative pain management in the hospital setting with serial opioid and metabolite assays beyond immediate perioperative periods will help study the effect of age and maturation along with other important factors such as weight, race and genetic factors on optimizing opioid doses and selection.
Supplementary Material
Executive summary.
Interindividual difference in morphine pharmacokinetics is caused in part by OCT1 genotype
-
■
Genetic variants of OCT1 alter morphine pharmacokinetics in children undergoing adenotonsillectomy.
-
■
Children homozygous for defective OCT1 alleles showed significantly lower apparent allometrically scaled morphine clearance than those heterozygous and homozygous for active OCT1 alleles.
-
■
Children heterozygous for defective OCT1 alleles did not show a significant difference in allometrically scaled morphine clearance than those homozygous for active OCT1 alleles except in relatively young/low weighted children.
Inter-racial difference in morphine pharmacokinetics may be caused by OCT1 genotype
-
■
Defective OCT1 alleles are more common in Caucasians than in African–Americans.
-
■
Relatively low morphine clearance in Caucasian children may be caused by a relatively high frequency of defective alleles compared with African–Americans.
Interindividual difference in morphine pharmacokinetics in children is caused by multiple factors
-
■
Future studies aimed at understanding the pharmacokinetic/pharmacodynamic heterogeneity of morphine should include at least, weight, age, race and genotypes of OCT1 and UGT2B7.
Acknowledgements
The authors would like to thank U Christians and C Clavijo at University of Colorado (CO, USA) for their help with measuring morphine and morphine metabolite concentrations.
This research study was supported by Children's Hospital Research Foundation's, Translational Research Award and the Outcomes Research Award, Children's Hospital Medical Center (OH, USA). AA Vinks was in part supported by NIH grant, 1K24HD050387. T Mizuno is supported by the Japanese Research Foundation for Clinical Pharmacology. This work was supported in part by USPHS Grant #UL1 RR026314 from the National Center for Research Resources, NIH and with the Place Outcomes Research Award and Translational Research Award, Cincinnati Children's Hospital Medical Center (OH, USA). Additional research funding support was provided by the Department of Anesthesia, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, USA.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
■ of interest
- 1.Quality improvement guidelines for the treatment of acute pain and cancer pain. American Pain Society Quality of Care Committee. JAMA. 1995;274(23):1874–1880. doi: 10.1001/jama.1995.03530230060032. [DOI] [PubMed] [Google Scholar]
- 2.Anderson BJ, Holford NH. Tips and traps analyzing pediatric PK data. Paediatr. Anaesth. 2011;21(3):222–237. doi: 10.1111/j.1460-9592.2011.03536.x. [DOI] [PubMed] [Google Scholar]
- 3.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom. Med. 2001;63(2):316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Edwards RR, Moric M, Husfeldt B, Buvanendran A, Ivankovich O. Ethnic similarities and differences in the chronic pain experience: a comparison of African–American, Hispanic, and white patients. Pain Med. 2005;6(1):88–98. doi: 10.1111/j.1526-4637.2005.05007.x. [DOI] [PubMed] [Google Scholar]
- 5.Rahim-Williams FB, Riley JL, Herrera D, Campbell CM, Hastie BA, Fillingim RB. Ethnic identity predicts experimental pain sensitivity in African–Americans and Hispanics. Pain. 2007;129(1–2):177–184. doi: 10.1016/j.pain.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadhasivam S, Chidambaran V, Ngamprasertwong P, et al. Race and unequal burden of perioperative pain and opioid related adverse effects in children. Pediatrics. 2012;129(5):832–838. doi: 10.1542/peds.2011-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Previous study showing racial differences in morphine pharmacodynamics.
- 7.Sadhasivam S, Krekels EH, Chidambaran V, et al. Morphine clearance in children: does race or genetics matter? J. Opioid. Manag. 2012;8(4):217–226. doi: 10.5055/jom.2012.0119. [DOI] [PubMed] [Google Scholar]
- 8.Coffman BL, Rios GR, King CD, Tephly TR. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab. Dispos. 1997;25(1):1–4. [PubMed] [Google Scholar]
- 9.Knibbe CA, Krekels EH, Danhof M. Advances in paediatric pharmacokinetics. Expert Opin. Drug Metab. Toxicol. 2011;7(1):1–8. doi: 10.1517/17425255.2011.539201. [DOI] [PubMed] [Google Scholar]
- 10.Tzvetkov MV, Stingl JC, Brockmoller J. Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in the OCT1 gene affect morphine pharmacokinetics. Clin Pharmacol. Ther. 2012;91(Suppl. 1):S105. doi: 10.1016/j.bcp.2013.06.019. [DOI] [PubMed] [Google Scholar]; ■ First study showing the contribution of OCT1 to morphine clearance.
- 11.Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1–3) J. Pharmacol. Exp. Ther. 2004;308(1):2–9. doi: 10.1124/jpet.103.053298. [DOI] [PubMed] [Google Scholar]
- 12.Koepsell H, Endou H. The SLC22 drug transporter family. Pflugers Arch. 2004;447(5):666–676. doi: 10.1007/s00424-003-1089-9. [DOI] [PubMed] [Google Scholar]
- 13.Nies AT, Koepsell H, Winter S, et al. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology. 2009;50(4):1227–1240. doi: 10.1002/hep.23103. [DOI] [PubMed] [Google Scholar]
- 14.Gorboulev V, Ulzheimer JC, Akhoundova A, et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16(7):871–881. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM. Cloning and functional expression of a human liver organic cation transporter. Mol. Pharmacol. 1997;51(6):913–921. doi: 10.1124/mol.51.6.913. [DOI] [PubMed] [Google Scholar]
- 16.Saadatmand AR, Tadjerpisheh S, Brockmoller J, Tzvetkov MV. The prototypic pharmacogenetic drug debrisoquine is a substrate of the genetically polymorphic organic cation transporter OCT1. Biochem. Pharmacol. 2012;83(10):1427–1434. doi: 10.1016/j.bcp.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Kerb R, Brinkmann U, Chatskaia N, et al. Identification of genetic variations of the human organic cation transporter hOCT1 and their functional consequences. Pharmacogenetics. 2002;12(8):591–595. doi: 10.1097/00008571-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Shu Y, Sheardown SA, Brown C, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Invest. 2007;117(5):1422–1431. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzvetkov MV, Saadatmand AR, Bokelmann K, Meineke I, Kaiser R, Brockmoller J. Effects of OCT1 polymorphisms on the cellular uptake, plasma concentrations and efficacy of the 5-HT(3) antagonists tropisetron and ondansetron. Pharmacogenomics J. 2012;12(1):22–29. doi: 10.1038/tpj.2010.75. [DOI] [PubMed] [Google Scholar]
- 20.Tzvetkov MV, Saadatmand AR, Lotsch J, Tegeder I, Stingl JC, Brockmoller J. Genetically polymorphic OCT1: another piece in the puzzle of the variable pharmacokinetics and pharmacodynamics of the opioidergic drug tramadol. Clin. Pharmacol. Ther. 2011;90(1):143–150. doi: 10.1038/clpt.2011.56. [DOI] [PubMed] [Google Scholar]
- 21.Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in the organic cation transporter 1 is associated with metformin response in patients with diabetes mellitus. Pharmacogenomics J. 2009;9(4):242–247. doi: 10.1038/tpj.2009.15. [DOI] [PubMed] [Google Scholar]
- 22.Tzvetkov MV, Vormfelde SV, Balen D, et al. The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin. Clin. Pharmacol. Ther. 2009;86(3):299–306. doi: 10.1038/clpt.2009.92. [DOI] [PubMed] [Google Scholar]; ■ OCT1 allele definition.
- 23.Clavijo CF, Hoffman KL, Thomas JJ, et al. A sensitive assay for the quantification of morphine and its active metabolites in human plasma and dried blood spots using high-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011;400(3):715–728. doi: 10.1007/s00216-011-4775-z. [DOI] [PubMed] [Google Scholar]
- 24.Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11(3):558–569. doi: 10.1208/s12248-009-9133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadhasivam S, Chidambaran V. Pharmacogenomics of opioids and perioperative pain management. Pharmacogenomics. 2012;13(15):1719–1740. doi: 10.2217/pgs.12.152. [DOI] [PubMed] [Google Scholar]
- 26.Po HN, Senozan NM. The Henderson–Hasselbalch equcation: its histroy and limitaions. J. Chem. Educ. 2001;78(11):1499–1503. [Google Scholar]
- 27.Shu Y, Brown C, Castro RA, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin. Pharmacol. Ther. 2008;83(2):273–280. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zelcer N, van de Wetering K, Hillebrand M, et al. Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine-6-glucuronide antinociception. Proc. Natl Acad. Sci. USA. 2005;102(20):7274–7279. doi: 10.1073/pnas.0502530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101. [(Accessed 18 December 2012)];UGT2B7 allele nomenclature. www.pharmacogenomics.pha.ulaval.ca/files/content/sites/pharmacogenomics/files/Nomenclature/UGT2B/UGT2B7.htm.
- 102. [(Accessed 18 December 2012)];ASA Physical Status Classification System. www.asahq.org/For-Members/Clinical-Information/ASA-Physical-Status-Classification-System.aspx.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.