INTRODUCTION
Despite concerted research efforts, prevention and treatment of graft-versus-host disease (GVHD) have not changed significantly over the past 3 decades. The goal of this session is to provide an outline of the current state of the science with respect to the prevention, treatment, and evaluation of acute and chronic GVHD. In addition, we detail those strategies most likely to be at the forefront of clinical research in the upcoming years.
PREVENTION OF GVHD: RECENT DEVELOPMENTS AND FUTURE TRIALS
A calcineurin inhibitor (CNI) combined with a short course of methotrexate (MTX) is the most common GVHD prophylaxis regimen used in myeloablative or reduced-intensity conditioning (RIC) transplants, whereas a CNI combined with mycophenolate mofetil (MMF) is the most frequent regimen in nonmyeloablative conditioned or umbilical cord transplants. Recently, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) completed a Phase III trial comparing the mammalian target of rapamycin inhibitor sirolimus combined with tacrolimus and tacrolimus/MTX in matched related donor graft recipients receiving ablative conditioning. Accrual for patients receiving busulfan conditioning was closed early owing to a higher incidence of veno-occlusive disease of the liver in the sirolimus arm, thus limiting the applicability of the results from this trial [1].
Moving forward, there has been a wealth of single-center, early-phase trials examining novel GVHD prevention strategies that potentially warrant evaluation in larger, multi-center trials. The current challenge is to select the most promising of these approaches. Before we review these "newer" strategies, 2 "older" approaches—ex vivo and in vivo T cell depletion (TCD)—warrant brief discussion.
TCD
Ex Vivo TCD
Historically, techniques for ex vivo TCD have varied, resulting in inconsistent reductions in T cells. Modern methods for ex vivo TCD (CD34 selection) using magnetic bead columns result in consistent reductions of T cells by 4–5 logs [2], Recently, patients with acute myelogenous leukemia in first complete remission who received an ex vivo TCD transplant at Memorial Sloan Kettering Cancer Center (MSKCC) were compared with patients receiving T cell–replete transplants at M.D. Anderson Cancer Center [3]. The majority of patients at MSKCC received ablative conditioning, with ex vivo TCD (CD34 selection) used as sole GVHD prophylaxis. All the patients at M.D. Anderson received busulfan and fludarabine conditioning combined with tacrolimus/MTX. Patient characteristics were comparable in the 2 groups, except that the MSKCC cohort was slightly older (Table 1).
Table 1.
Characteristics of Recipients of Ex Vivo T Cell—Depleted Transplants at Memorial Sloan Kettering Cancer Center and T Cell—Replete Transplants at M.D. Anderson Cancer Center
| MSKCC; Ex Vivo T Cell Depletion | M.D. Anderson; T Cell Replete (Tacrolimus/MTX) |
P Value | |
|---|---|---|---|
| Number | 115 | 181 | |
| Follow-up, days, median (range) | 32 (1–108) | 29 (2–104) | |
| Diagnosis, n (%) | |||
| De novo acute myelogenous leukemia | 60 (52) | 144 (80) | <.001 |
| Acute myelogenous leukemia from myelodysplastic syndrome | 38 (33) | 24(13) | |
| Therapy-related acute myelogenous leukemia | 17(15) | 13(7) | |
| Time from diagnosis to transplantation, days, median (range) | 140 (59–1605) | 155 (57–532) | .08 |
| Age, years, median (range) | 52 (19–71) | 48 (18–63) | .0001 |
| Cytogenetic risk, n (%) | |||
| Favorable | 1(1) | 2(1) | .30 |
| Intermediate | 72 (63) | 103 (57) | |
| Poor | 42 (37) | 76 (42) | |
| Conditioning regimen, n (%) | |||
| Busulfan + fludarabine (rabbit ATC for matched unrelated donors) | 0(0) | 181 (100) | |
| Busulfan + fludarabine + melphalan | 61 (53) | ||
| Total body irradiation + thiotepa + Cy or fludarabine | 54 (47) | ||
| Donor type, n (%) | |||
| Matched related | 56 (49) | 103 (57) | .20 |
| Matched unrelated | 32 (28) | 64(35) | |
| 1 or 2 antigen MM related/unrelated | 27 (23) | 14(8) | |
| HLA matching, n (%) | |||
| 10/10 | 88 (77) | 167 (92) | <.001 |
| 9/10 | 23 (20) | 14(8) | |
| 8/10 | 4(3) | 0(0) |
The findings from this analysis challenge some previously held conceptions regarding the benefits and limitations of these 2 approaches. Not surprisingly, acute and chronic GVHD rates were lower in recipients of ex vivo TCD grafts; however, surprisingly, this did not translate into lower nonrelapse mortality (NRM). Equally surprising, relapse rates were not better in patients receiving T cell—replete transplants. Thus, overall survival (OS) rates were comparable in the 2 cohorts (Table 2). Similar results were seen in a BMT CTN analysis comparing outcomes for patients on a phase II trial of ex vivo TCD transplantation and patients who received a T cell—replete transplant on an earlier trial [2].
Table 2.
Comparison of Recipients of Ex Vivo T Cell Transplants at Memorial Sloan Kettering Cancer Center and T Cell—Replete Transplants at M.D. Anderson Cancer Center
| MSKCC; Ex Vivo T Cell Depletion (n = 115), %(95%CI) |
M.D. Anderson; T Cell Replete (Tacrolimus/MTX) (n = 181), %(95%CI) |
HR (95% CI) | P Value | |
|---|---|---|---|---|
| Acute GVHD II-IV at day 100 | 5(2–11) | 18 (13–24) | 3.9 (1.5–9.9) | .005 |
| Chronic GVHD at 3 years | 13 (8–22) | 53 (46–62) | 4.6(2.6–8.1) | <.001 |
| NRM | ||||
| Day 100 | 8 (4–15) | 3 (1–7) | 0.4(0.1–1.1) | .07 |
| 3 year | 24(17–34) | 16(11–23) | 0.6(0.3–1.1) | .10 |
| PFS at 3 years | 18 (12–27) | 25 (19–33) | 1.3 (0.8–2.3) | .30 |
| OS at 3 years | 57 (47–67) | 66 (58–74) | 0.7(0.5–1.1) | .20 |
In Vitro TCD
Incorporation of T cell—depleting antibodies in vivo is another commonly used method to lower GVHD rates. In an international study, Fresenius antithymocyte globulin (ATG) was found to lower rates of grade II–IV acute GVHD and chronic GVHD after ablative conditioning, with no impact on relapse [4]. Based on this finding, a licensing study is currently underway in the United States. Although these studies may define the role of anti—T cell antibodies in the ablative setting, the utility of ATG in nonmyeloablative or RIC transplantations remains unclear. A recent analysis suggests that in nonmyeloablative or RIC transplantions, the reduced rates of GVHD may be offset by higher rates of relapse [5].
Novel GVHD Prophylaxis Trials
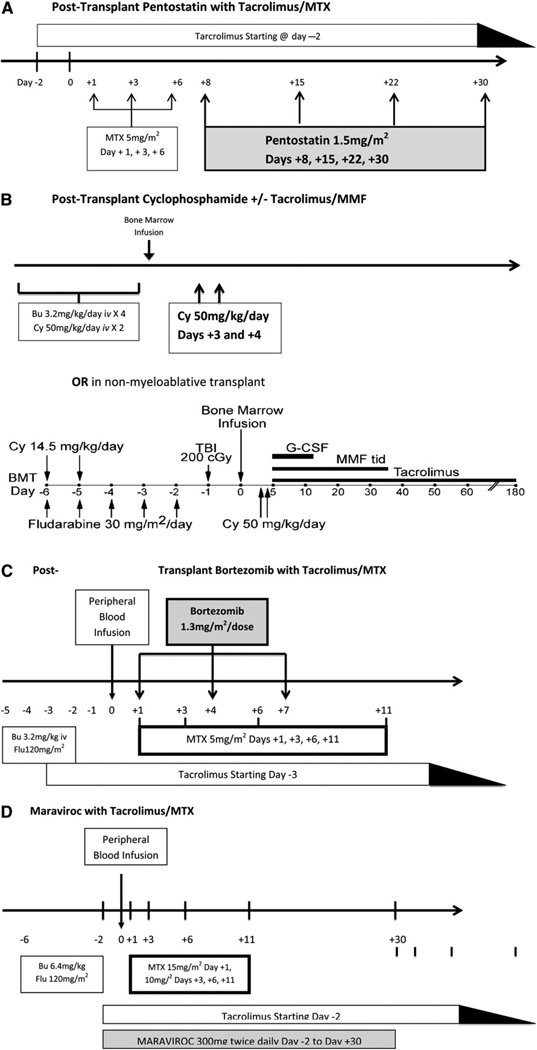
Four recently published primarily single-center GVHD prevention trials have yielded encouraging results and warrant consideration for future multicenter trials. These 4 trials entailed the study of pentostatin, cyclophosphamide, bortezomib, and maraviroc for GVHD prophylaxis (Figure 1).
Figure 1.
Schema of 4 novel GVHD prophylaxis regimens. (A) Pentostatin with tacrolimus/MTX. (B) Cyclophosphamide with or without tacrolimus/MMF. (C) Bortezomib with tacrolimus/MTX. (D) Maraviroc with tacrolimus/MTX.
Pentostatin
Researchers at M.D. Anderson Cancer Center have studied the addition of the nucleoside analog pentostatin to GVHD prophylaxis with CNI/MTX in matched unrelated or mismatched allogeneic transplant recipients [6]. A total of 147 patients with various malignancies and conditioning regimens were enrolled onto this Bayesian, adaptively randomized, controlled phase I/II trial. The majority of patients also received low-dose rabbit ATG. The results of that study show that pentostatin at a dose of 1.5 mg/m2/week for 4 weeks resulted in the highest fraction of patients alive and GVHD-free at day 100 posttransplantation with no previous grade III–IV acute GVHD compared with the control arm. The rate of grade III–IV acute GVHD for this arm was 10.7%, with no cases seen in HLA-mismatched transplant recipients.
Cyclophosphamide
Researchers at Johns Hopkins University have examined posttransplantation cyclophosphamide (Cy) as a sole GVHD prophylaxis strategy based on murine models demonstrating effective depletion of alloreactive T cells through the use of pulsed doses of Cy in the early posttransplantation period. Results of a Phase II trial of posttransplantation Cy in 117 recipients of matched related and unrelated bone marrow grafts after ablative conditioning with busulfan/Cy demonstrated a rate for acute GVHD grade II–IV (43%; 95% confidence interval [CI], 34%–52%) comparable to that for traditional prophylaxis regimens, whereas the rate for chronic GVHD was lower (10%; 95% CI, 5%–16%) [7]. Posttransplantation Cy as the sole GVHD prophylaxis seems to be beneficial after ablative conditioning, but not after RIC. A recent Phase II study at M.D. Anderson using this strategy with RIC demonstrated higher rates of acute GVHD compared with a matched cohort of recipients who received standard GVHD prophylaxis, suggesting that posttransplantation Cy may need to be combined with additional drugs when used with a less-intensive conditioning regimen (Table 3) [8]. In support of this, a study of the addition of posttransplantation Cy to tacrolimus/MMF GVHD prophylaxis in recipients of T cell—replete bone marrow grafts from haploidentical donors after non-myeloablative conditioning demonstrated a 34% incidence of grade II-IV acute GVHD and a 5% incidence of extensive chronic GVHD [9].
Table 3.
Matched Cohort Analysis of Tacrolimus/Methotrexate versus Posttransplantation Cyclophosphamide after RIC
| Posttransplantation CY (n = 49), % (95% a) | Tacrolimus and Methotrexate, % (95% CI) | HR (95% CI) | P Value | |
|---|---|---|---|---|
| Acute GVHD grade II-IV | 46(32–66) | 19 (10–37) | 2.8 (1.1–6.7) | .02 |
| Chronic GVHD at 1 year | 14 (6–32) | 21 (11–43) | 0.8 (0.2–2.6) | .70 |
| NRM at 2 years | 36 (23–55) | 16 (7–35) | 2.4 (0.8–6.7) | .10 |
| PFS at 2 years | 22 (10–37) | 33 (16–51) | 1.3 (0.7–23) | .40 |
| OS at 2 years | 26 (13–42) | 46 (26–64) | 1.8 (0.9–33) | .08 |
Bortezomib
The Dana-Farber Cancer Institute evaluated the addition of the proteasome inhibitor bortezomib to tacrolimus/MTX prophylaxis on days 1, 4, and 7 posttransplantation in 45 recipients of a 1 or 2 antigen-mismatched, T cell—replete, unrelated peripheral blood transplantations after RIC [10]. This Phase I/II study found a 22% (95% CI, 16%–34%) day 180 incidence of grade II–IV acute GVHD and a 29% (95% CI, 16%–43%) 1-year incidence of chronic GVHD, which are comparable to rates in a contemporaneous cohort of matched unrelated donor graft recipients who received sirolimus-based GVHD prophylaxis at their institution. The results of that trial suggest that immune modulation with bortezomib may reduce GVHD (and consequently NRM) in what is otherwise a high-risk, HLA-mismatched population.
Maraviroc
Another novel approach targeting T cell trafficking using the chemokine 5 receptor antagonist maraviroc was recently studied in a Phase I/II trial at the University of Pennsylvania [11]. Thirty-eight patients received a matched related or unrelated peripheral blood transplant after RIC, and then received maraviroc along with tacrolimus/MTX from day +2 to day +30 posttransplantation. Mean rates of day 100 and day 180 grade II–IV acute GVHD were 14.7% ± 6.2% and 23.6% ± 7.4%, respectively, with low rates of visceral GVHD (liver, 2.9% ± 2.9%; gut, 8.8% ± 5%). These clinical results appear to be consistent with murine models demonstrating the ability of CCR5 blocking antibodies to ameliorate visceral GVHD. However, the occurrence of late acute GVHD suggests that a longer duration of drug therapy may be needed.
Future Studies: BMT CTN GVHD Prophylaxis Trial
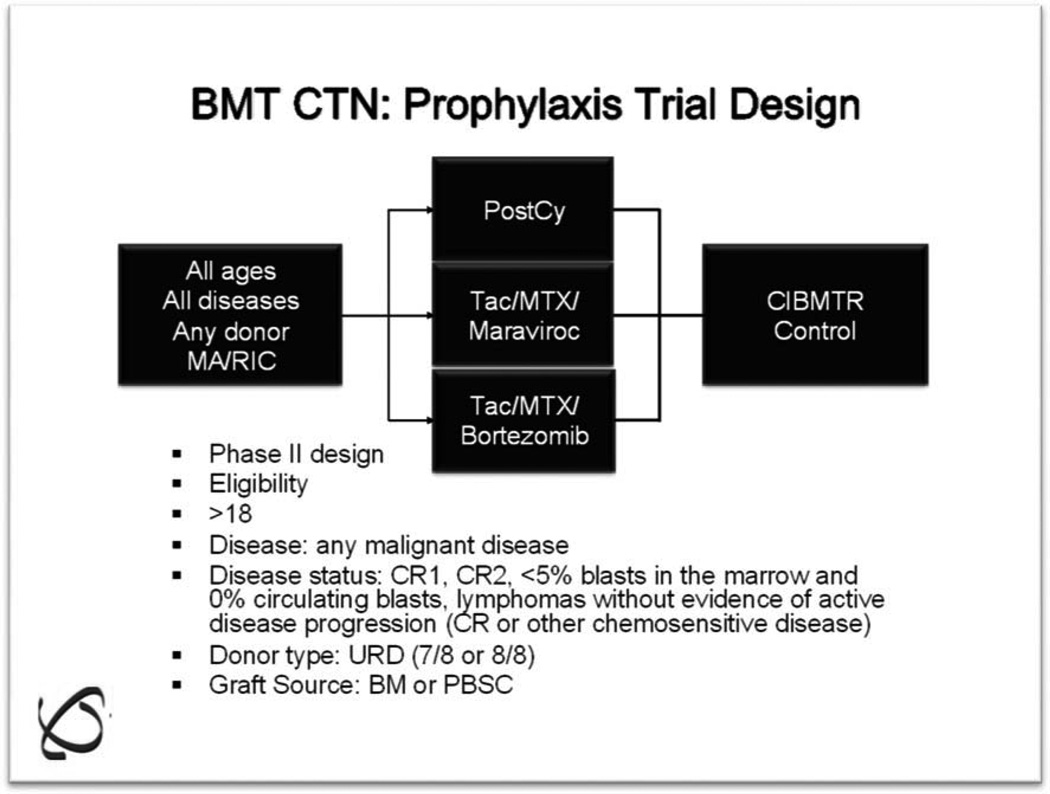
Differing study populations and trial endpoints make direct comparison of the foregoing studies problematic. In an effort to objectively evaluate each of these trials, the GVHD State-of-the-Science Committee of the BMT CTN recently conducted a benchmark analysis to determine the most promising prophylaxis regimens to move forward into a multicenter trial. Datasets from each of the 4 aforementioned trials, along with an ex vivo TCD dataset from MSKCC, were individually matched to controls within the Center for International Blood and Marrow Transplant Research (CIBMTR) who received CNI/MTX prophylaxis. The findings from this analysis are summarized in Table 4. Based on this information, along with an "assessment of interest" determined through a survey of core centers, a decision was made to proceed with a randomized Phase II trial comparing posttransplantation Cy/tacrolimus/MMF, bortezomib/tacrolimus/MTX, and maraviroc/tacrolimus/MTX in recipients of matched or 1 antigen-mismatched unrelated donor transplants after RIC (Figure 2). Each arm will be compared to a prospectively matched cohort of patients in the CIBMTR. GVHD and relapse-free survival at 1 year will be the primary endpoint.
Table 4.
Results from a Benchmark Analysis Comparing CIBMTR Matched Controls with GVHD Prophylaxis Strategies: Pentostatin, Cy, Maraviroc, Bortezomib, and Ex Vivo T Cell Depletion
| Measured Outcomes | Proposals Statistically Better Than Their CIBMTR Matched Controls |
|---|---|
| Acute GVHD grade II-IV | •Ex vivo T cell depletion |
| •Bortezomib | |
| Acute GVHD grade III/IV | •Ex vivo T cell depletion |
| •Pentostatin | |
| Chronic GVHD | •Ex vivo T cell depletion |
| •Pentostatin | |
| •Posttransplantation Cy | |
| DFS | •Ex vivo T cell depletion |
| •Pentostatin | |
| OS | •Ex vivo T cell depletion |
| •Bortezomib | |
| •Pentostatin |
Figure 2.
BMT CTN GVHD Prophylaxis Trial: randomized Phase II with CIBMTR matched controls.
TREATMENT OF ACUTE GVHD: LESSONS LEARNED FROM BMT CTN 0302 AND 0802
The current standard therapy for acute GVHD is steroids; however, durable responses are not the rule [12–14]. Although other agents have been added to steroids in the past, the combinations have failed to show an advantage, owing to excess toxicity or lack of superior control [15,16]. Here we review 2 recent multicenter trials of up-front therapy for acute GVHD.
BMT CTN 0302
BMT CTN 0302 was a 4-arm, randomized Phase II trial that evaluated the 4 drugs etanercept, MMF, denileukin diftitox, and pentostatin each in combination with steroids for patients with newly diagnosed acute GVHD [17]. A randomized Phase II study was chosen as an efficient method for selecting the most promising combination for a larger Phase III trial [18]. A total of 180 patients were randomized to the 4 study arms, with patients receiving MMF for GVHD prophylaxis randomized to a non-MMF arm. Day 56 complete response rates were 44% for etanercept, 73% for MMF, 55% for denileukin diftitox, and 62% for pentostatin. Corresponding 9-month OS rates were 47%, 64%, 49%, and 47%. The cumulative incidence of grade 3 or greater infections at day 270 was lowest in the MMF arm. Given that efficacy, survival, and toxicity all favored MMF, MMF was selected as the most promising therapy to be combined with steroids in a randomized, double-blinded. Phase III trial.
What Else Was Learned from 0302?
Pharmacokinetic analyses showed that approximately one-half of subjects did not achieve target mycophenolic acid (MPA) total and unbound trough concentrations. Therefore, administration of MMF 1 g twice daily as on BMT CTN 0302 produced low plasma concentrations in many patients [19]. MPA pharmacokinetic measurements from weeks 1 and 2 did not correlate with complete responses at either day 28 or day 56. In contrast, patients with mean total MPA troughs of >0.5 µg/mL or unbound troughs of >0.015 µig/mL at weeks 1 and 2 had significantly greater proportions of complete and partial responses at days 28 and 56, suggesting the need for a higher dose of MMF [19]. Levine et al. [20] examined time to maximum response as a predictor for long-term outcomes. Response at 14,28, and 56 days from initiation of acute GVHD treatment was used to categorize patients for NRM and survival. Day 28 response (complete or partial) best predicted NRM and survival at 9 months from the start of acute GVHD treatment. Levine et al. [21] also examined a panel of biomarkers initially identified at the University of Michigan by Paczesny et al. [22], including IL-2 receptor-α, tumor necrosis factor receptor 1, hepatocyte growth factor, IL-8, elafin, and regenerating islet-derived 3-α, to discriminate responders from nonresponders. Biomarkers measured on days 0, 14, and 28 were found to predict for day 28 posttherapy nonresponse and day 180 mortality [21].
BMT CTN 0802
This Phase III, double-blinded, randomized trial compared steroids/MMF and steroids/placebo. One notable difference between trial 0302 and trial 0802 was the use of an increased dose of MMF (1 g every 8 hours) based on the pharmacokinetic data from 0302 [19]. The primary study endpoint was GVHD-free survival at day 56. (The analysis of Levine et al. [20] demonstrating the significance of day 28 response was not yet available at the time the protocol was written.) Enrolled patients with any grade of newly diagnosed acute GVHD were started on prednisone at 2 mg/kg/day along with MMF or placebo. Tapering of steroids could commence after 3 days at the discretion of the treating physician, but all patients were required to receive a minimum of 0.25 mg/kg/day of prednisone on day 28. MMF/placebo was continued until day 56 or until steroid discontinuation, whichever came first.
The study was terminated early when a “futility rule” was met at a planned interim analysis following the enrollment of 236th (out of 372 planned) patient. A total of 117 patients were randomized to MMF and 119 patients were randomized to placebo at 36 participating centers. The 2 groups were well balanced in terms of patient characteristics. The distribution of acute GVHD grades at randomization was 65% I/II, 28% III, and 6% IV. GVHD-free survival at day 56 after randomization was 60.5% (95% CI, 51.6%–69.5%) for MMF (69 patients) and 52.2% (95% CI, 43%–61.3%) for placebo (60 patients) (P = .78). Chronic GVHD developed in 38 patients in the MMF arm (6-month estimate, 23.7%; 95% CI, 15.9%–31.6%) and in 39 patients in the placebo arm (6-month estimate, 26.5%; 95% CI, 18.3%–34.7%) (P = .69). OS at 6 months, rate of Epstein-Barr virus reactivation, and cumulative incidence of grade 3 infections were similar in the 2 arms, as were toxicity patterns. The cumulative incidence estimate for NRM at 6 months also was similar in the 2 arms: 15.5% for MMF and 20.1% for placebo (P = .83). The estimated 6-month OS was 71% for MMF and 73% for placebo (P = .25).
Four Hundred and Sixteen Patients Later, What Was Learned?
In the last decade, the BMT CTN conducted 2 randomized clinical trials evaluating acute GVHD therapies involving a total of 416 patients. At the end, steroid alone remains the first-line therapy. BMT CTN 0302 and 0802 have taught us many things: multicenter clinical trials for acute GVHD are possible (and encouraged); a large proportion of clinicians treat patients with grade I acute GVHD; MMF dosing is often subtherapeutic when given every 12 hours; day 28 response is predictive of NRM and OS; biomarkers may be able to discriminate patients who will respond from those who will not; and, most importantly, additional immuno-suppression with MMF did not improve outcomes for our patients [17,19–21]. Actually, given the diversity of transplantation regimens and the genetic uniqueness of each donor—recipient pair, the remarkable finding is that steroids continue to have such a high response rate. The development of a "magic bullet" able to control all of the diverse immune responses that we read clinically as acute GVHD is unlikely. The challenge for the future is to improve biomarkers to the point at which trials can be designed that individualize treatment assignments based on the likelihood for GVHD response (and, conversely, NRM and survival). In such a way, those most at risk could be rapidly identified and screened for future phase I/II trials.
CHRONIC GVHD: DO WE KNOW HOW TO MEASURE SEVERITY AND RESPONSE?
NIH Consensus Conference
Chronic GVHD affects 30%–50% of allogeneic hematopoietic cell transplantation (HCT) survivors and is a major cause of morbidity and mortality. In 2004, Pavletic and Vogelsang, with support from the National Cancer Institute, Office of Rare Diseases Research, Department of Defense, National Heart, Lung and Blood Institute, Health Resources and Services Administration, and the National Institute of Allergy and Infectious Diseases, convened a Consensus Conference with the goal of advancing research in chronic GVHD. Participants in this Consensus Conference wrote 6 papers to provide the recommendations for diagnosis and scoring [23], pathology [24], biomarkers [25], response criteria [26], supportive care [27], and design of clinical trials [28].
Before the Consensus Conference, criteria for the diagnosis of chronic GVHD had not been codified. Severity grading was dichotomous, with 2 broad categories of “limited” and “extensive” that were often erroneously interpreted to mean “not so bad” and “bad. ” Outcomes of clinical trials were based on clinician assessment of response: complete response (complete resolution of chronic GVHD), partial response (>50% improvement in at least one organ with no worsening in any other), stable disease, progression, or mixed response (improvement in one organ but worsening in another). Lack of objective data to support the clinician response assessments made it very difficult to have confidence in reported results or to compare agents tested in different trials. Phase II studies routinely reported very high response rates, whereas daily clinical experience did not seem to confirm the positive reports.
Major recommendations from the Consensus Conference included (1) a strict set of diagnostic criteria for chronic GVHD that no longer depends on time after HCT; (2) recognition of “overlap” syndrome when concurrent acute and chronic GVHD are active; (3) a 0–3 scoring system for severity in 8 organs; (4) an algorithm for calculating global severity based on individual organ scores of 0–3; and (5) a set of response criteria for skin, eye, mouth, upper gastrointestinal, esophagus, lower gastrointestinal, liver, and lung that includes scales and methods of calculating responses. Tools for capturing patient-reported outcomes and functional capacity were recommended as well. The conference was well attended, and clinicians and investigators have started to use these definitions and materials widely. However, most recommendations were based on consensus rather than data. In the 6 years since the publication of the Consensus criteria, do we have evidence of their validity?
What Have We Learned from the Longitudinal Study?
Data from a prospective multicenter observational study suggest that the severity scoring recommendations are valid and are able to segregate patients with chronic GVHD into 3 global severity groups (mild, moderate, and severe) with differing symptom burdens, quality of life, risks of NRM, and survival [29–31]. Additional studies that evaluated organs individually found that the severity scoring system was more valid and sensitive to change compared with the response measures [32,33]. These results suggest that the 0–3 scoring system and global severity score may be used with confidence to describe the current burden of chronic GVHD in a population, as well as an endpoint in prophylaxis trials.
Two recent studies examined the proposed response criteria, scoring the scales as recommended in a supplement to the original article [34,35]. Those studies suggested that the response categories do not correlate with clinician or patient perceptions of response to treatment and do not predict NRM or OS [34,35]. Thus, the response criteria as currently formulated are not adequate endpoints for clinical trials. How should we solve this problem? One way would be to start over from scratch, substituting instruments or designing new ones. Alternatively, members of the Chronic GVHD Consortium believe that there may be a way to work with the currently recommended scales by using different methods of aggregating the organ system measures or applying a new way of calculating responses [36]. However, much work remains to be done to design and validate response measures.
BMT CTN 0801
In 2010, the BMT CTN launched the largest intervention study for patients with chronic GVHD yet attempted (n = 300). This Phase II/III trial, designed to overcome previous limitations of single-center participation and small numbers, is collecting all of the recommended instruments of the NIH Chronic GVHD Consensus Conference. Initially launched as parallel, randomized Phase II studies comparing prednisone/sirolimus/CNI with prednisone/sirolimus or prenisone/sirolimus/extracorporeal photopheresis, the second Phase II study was dropped in 2011 owing to slow accrual. However, the remaining 2 arms have been achieving target accrual, and the Phase II portion of the study is scheduled to complete enrollment in early 2013. If the early results, based on clinician-reported complete plus partial responses at 6 months, look promising, the study will proceed to the larger Phase III. The primary endpoint of the Phase III study is resolution of all reversible manifestations of chronic GVHD at 2 years after study entry, a "hard" endpoint that is less susceptible to physician bias.
In summary, the lack of validated response measures is the greatest barrier to the design of future intervention studies, especially those that will include patients with heterogeneous chronic GVHD manifestations. We simply do not have the objective tools to be able to confirm a physician's or patient's impression of response or progression, nor do we have validated biomarkers or alternate ways of identifying patients responding to treatment. BMT CTN 0801 should be informative because it is protected from being uninterpretable because of the hard endpoint selected for the Phase III trial, but most chronic GVHD trials will not have the luxury of large numbers and 2-year endpoints. To make progress and prepare for the day when we do have better potential therapies for chronic GVHD, a focus on developing robust response measures is needed.
ACKNOWLEDGMENTS
We thank Drs Georgia B. Vogelsang and Joseph Antin for their thoughtful review of the manuscript.
Dr. Alousi received Research Funding from Therakos.
Footnotes
Financial disclosure: The other authors have nothing to disclose.
REFERENCES
- 1.Cutler C, Stevenson K, Kim HT, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112:4425–4431. doi: 10.1182/blood-2008-07-169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. / Gin Oncol. 2012;30:3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayraktar UD, De Lima M, Rima SM, et al. Ex vivo T celledepleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission: a comparison of outcomes in two institutions. Blood (ASH Annual Meeting Abstracts) Nov 2011. 2013;118 [Google Scholar]
- 4.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti—T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 5.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti–T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parmar S, Andersson BS, Couriel D, et al. Prophylaxis of graft-versus-host disease in unrelated donor transplantation with pentostatin, tacrolimus, and mini-methotrexate: a phase I/II controlled, adaptively randomized study. J. Clin Oncol. 2011;29:294–302. doi: 10.1200/JCO.2010.30.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alousi A, Saliba R, Chen J, et al. A matched controlled analysis of posttransplant cyclophosphamide (Cy) versus tacrolimus and mini-dose methotrexate in matched sibling and unrelated donor transplant recipients receiving reduced-intensity conditioning: post-transplant Cy is associated with higher rates of acute CVHD. 21. Vol. 120. Atlanta, Georgia: The University of Texas M D Anderson Cancer Center; 2012. Nov, p. 4200. Blood (ASH Annual Meeting Abstracts) [Google Scholar]
- 9.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using non-myeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Stood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30:3202–3208. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reshef R, Luger SM, Hexner EO, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367:135–145. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 13.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood. 1991;77:1821–1828. [PubMed] [Google Scholar]
- 14.Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–1030. [PubMed] [Google Scholar]
- 15.Cragg L, Blazar BR, Defor T, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:441–447. doi: 10.1016/s1083-8791(00)70036-x. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Zahrieh D, Agura E, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–1564. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- 17.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korn EL, Freidlin B, Abrams JS, et al. Design issues in randomized phase II/III trials. J Clin Oncol. 2012;30:667–671. doi: 10.1200/JCO.2011.38.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson PA, Huang J, Wu J, et al. Mycophenolate pharmacokinetics and association with response to acute graft-versus-host disease treatment from the Blood and Marrow Transplant Clinical Trials Network. Biol Blood Marrow Transplant. 2010;16:421–429. doi: 10.1016/j.bbmt.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine JE, Logan B, Wu J, et al. Graft-versus-host disease treatment: predictors of survival. Biol Blood Marrow Transplant. 2010;16:1693–1699. doi: 10.1016/j.bbmt.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine JE, Logan BR, Wu J, et al. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119:3854–3860. doi: 10.1182/blood-2012-01-403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease, I: Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease, II: Pathology Working Group report. Biol Blood Marrow Transplant. 2006;12:31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Schultz KR, Miklos DB, Fowler D, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease, III: Biomarker Working Group report. Biol Blood Marrow Transplant. 2006;12:126–137. doi: 10.1016/j.bbmt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease, IV: Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12:252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease, V: Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 200612:375–396. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Martin PJ, Weisdorf D, Przepiorka D, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease, VI: Design of Clinical Trials Working Group report. Biol Blood Marrow Transplant. 2006;12:491–505. doi: 10.1016/j.bbmt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Arai S, Jagasia M, Storer B, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118:4242–4249. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pidala J, Kurland B, Chai X, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117:4651–4657. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pidala J, Kurland BF, Chai X, et al. Sensitivity of changes in chronic graft-versus-host disease activity to changes in patient-reported quality of life: results from the Chronic Graft-versus-Host Disease Consortium. Haematologica. 2011;96:1528–1535. doi: 10.3324/haematol.2011.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inamoto Y, Chai X, Kurland BF, et al. Validation of measurement scales in ocular graft-versus-host disease. Ophthalmology. 2012;119:487–493. doi: 10.1016/j.ophtha.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobsohn D, Lee SJ, Kurland B, et al. Change in NIH skin score 0–3 correlates with provider- and patient-reported skin changes and overall survival: results from the Chronic GVHD Consortium. Blood. 2012;120:2545–2552. doi: 10.1182/blood-2012-04-424135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inamoto Y, Martin PJ, Chai X, et al. The clinical benefit of response in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18:1517–1524. doi: 10.1016/j.bbmt.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer JM, Lee SJ, Chai X, et al. Poor agreement between clinician response ratings and calculated response measures in patients with chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18(11):1649–1655. doi: 10.1016/j.bbmt.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chronic GVHD Consortium. Rationale and design of the Chronic GVHD Cohort Study: improving outcomes assessment in chronic GVHD. Bio! Blood. Marrow Transplant. 2011;17:1114–1120. doi: 10.1016/j.bbmt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]