Breast cancer is the most common malignancy affecting women and the second leading cause of cancer death in women in the United States [1]. Due to improvements in detection and adjuvant therapy, breast cancer specific mortality has decreased significantly in women with early stage disease, and the five-year relative survival rate for early stage disease has increased from 80% in 1950 to 89% today [1]. Increased breast cancer specific survival, however, is at risk of being offset by the potential late occurring cardiovascular toxic effects of oncologic therapy. Indeed, among women with early breast cancer, particularly those over age 65, cardiovascular disease (CVD) is now the predominant cause of mortality, and these women are also at increased risk of CVD compared with age-matched women without a history of breast cancer [2].
Long-term autonomic imbalance is associated with increased risk of CVD and mortality in non-cancer populations [3]. Importantly, there is evidence of a sustained increase in sympathetic activity and a reduction in parasympathetic input to the sinoatrial node in patients treated for early stage breast cancer. For example, the resting heart rate (RHR) of early stage breast cancer patients following the completion of primary adjuvant therapy is, on average, 9% to 16% [7–16 beats·min−1 (bpm)] higher compared to age-matched controls [4]. Several studies have also shown that heart variability (HRV) and baroreflex sensitivity is reduced among women with a history breast cancer [5]. Accordingly, based on a growing understanding of bi-directional interactions between the sympathetic and parasympathetic efferent pathways, autonomic imbalance is one potential pathway involved in both the etiology and the clinical course of breast cancer therapy-induced CVD (Fig. 1).
Fig. 1.
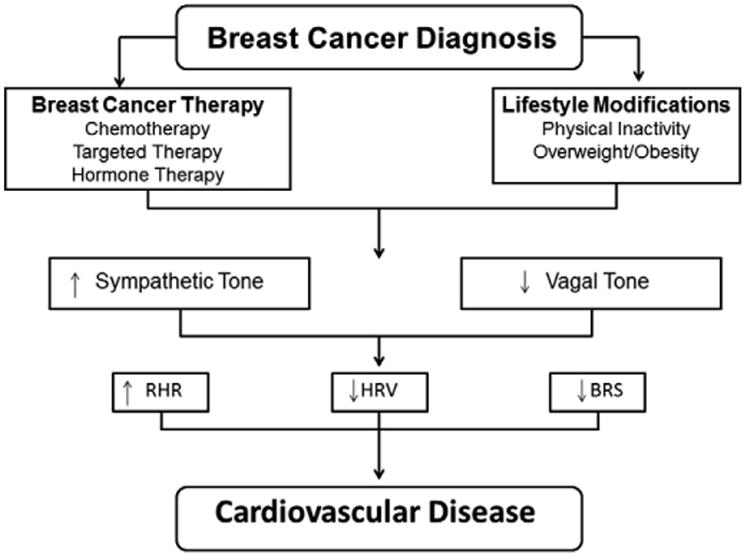
Relationship between breast cancer and autonomic dysfunction. Breast cancer diagnosis is associated with therapy-induced cardiovascular injury and lifestyle perturbations leading to increased sympathetic and decreased vagal tone in the heart. In turn, this autonomic imbalance increases RHR and decreases HRV and BRS leading to cardiovascular disease. RHR, resting heart rate; HRV, heart rate variability; BRS, baroreflex sensitivity.
Despite the potential long-term ramifications of autonomic dysfunction in early breast cancer, the development of safe and effective mitigation strategies remains elusive. Aerobic exercise training (AET) is one non-pharmacological therapy that may attenuate cardiovascular abnormalities in the early breast cancer setting; however the mechanisms by which AET mitigates autonomic dysfunction are not fully understood. The current evidence base indicates that the central pathways responsible for decreasing sympathetic outflow and increasing cardiac vagal tone after AET are, in part, dependent on changes in the renin–angiotensin–aldosterone system (RAAS), nitric oxide (NO), and reactive oxygen species (ROS) (Fig. 2). It is well established that the signaling pathway RAAS plays an important role in chemotherapy-induced cardiotoxicity [6]. Given that angiotensin II is known to exert powerful inhibitory effects upon the cardiac vagus nerve, suppression of this hormone or a precursor via AET could ultimately play an important role in the prevention of cardiac dysfunction. Breast cancer therapies also inhibit vascular NO release, consequently promoting vasoconstriction, increased peripheral resistance, and increased blood pressure [7]. Thus the upregulation of NO with AET could improve autonomic function. Interestingly, our group recently found that AET improved peripheral arterial endothelial function (a surrogate measure of NO bioavailability) in women with operable breast cancer receiving neoadjuvant doxorubicin [8]. Finally, chemotherapy-induced generation of reactive oxygen species (ROS) is the central mediator of numerous adverse acute and chronic biological effects in the cardiovascular system, including alterations in autonomic outflow [9]. Accordingly, attenuation of ROS generation and/or activity holds considerable therapeutic promise. Our group recently found that AET during chronic anthracycline exposure in mice lowered serum and cardiac levels of ROS and attenuated LV remodeling [10]. These results indicate that AET may protect cardiac cells against chemotherapy-induced toxicity through ROS inhibition.
Fig. 2.
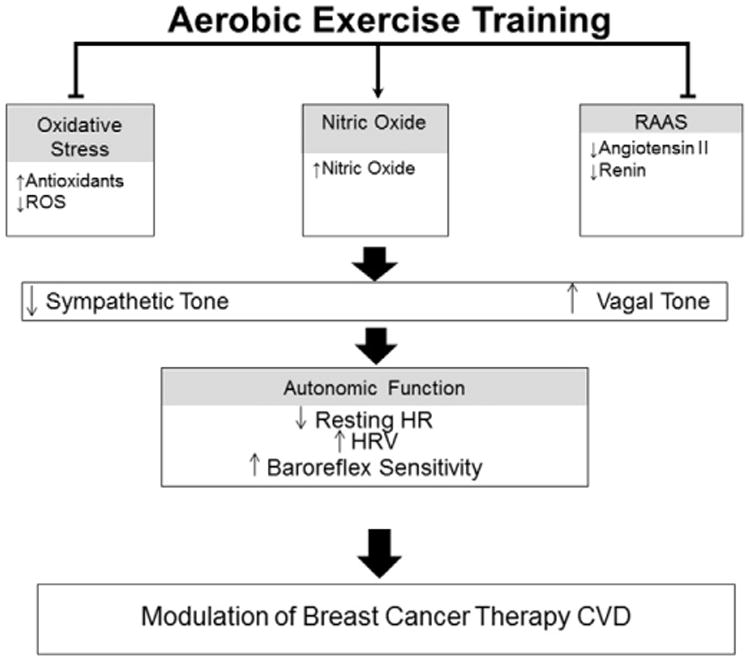
Modulation of autonomic dysfunction with aerobic exercise. Aerobic exercise training decreases oxidative stress and the RAAS while upregulating nitric oxide bioavailability. This results in decreased sympathetic tone and increased vagal tone, which in turn, decreases resting HR and increases HRV and baroreflex sensitivity. CVD, cardiovascular disease; RAAS, renin–angiotensin–aldosterone system; ROS, reactive oxygen species; HR, heart rate; HRV, heart rate variability.
In conclusion, CVD is a frequent and devastating adverse complication of breast cancer therapy leading to morbidity, poor quality of life, and premature mortality. As reviewed here, there is emerging evidence indicating that autonomic function is one component involved in the etiology and the clinical course of breast cancer therapy-induced CVD. Immediate work is now required to minimize, or optimally eliminate, breast cancer-associated CVD. As a first step in addressing knowledge gaps, hypothesis driven prospective studies evaluating the time course and clinical importance of RHR, HRV, and baroreflex sensitivity in early breast cancer are now warranted. Furthermore, elucidation of the potential molecular mechanisms by which AET reduces therapy-induced autonomic dysfunction is needed. For instance, the establishment of the existence of a relationship between AET, RAAS, NO and ROS would ascertain if AET can decrease sympathetic outflow and increase cardiac vagal tone in the setting of early breast cancer. Collectively, hypothesis-driven translational studies are required to define the nature and magnitude of autonomic dysfunction in early breast cancer, their relationship to cardiac dysfunction as well as to characterize the underlying mechanisms of AET in preventing and/or treating autonomic dysfunction and CVD. To this end, we propose that in combination with continual advancements in anticancer therapy, the testing and use of AET as adjuvant therapy may optimize health and longevity in cancer survivors by lowering therapy-associated alterations in autonomic function and CVD.
Acknowledgments
L.W.J. was supported by the National Institutes of Health grants nos. CA143254, CA142566, CA138634, and CA133895 and with funds from George and Susan Beischer.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.American Cancer Society. Cancer facts & figures. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Patnaik JL, Byers T, Diguiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piya MK, Shivu GN, Tahrani A, et al. Abnormal left ventricular torsion and cardiac autonomic dysfunction in subjects with type 1 diabetes mellitus. Metabolism. 2011;60(8):1115–21. doi: 10.1016/j.metabol.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30(20):2530–7. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meinardi MT, van Veldhuisen DJ, Gietema JA, et al. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol. 2001;19(10):2746–53. doi: 10.1200/JCO.2001.19.10.2746. [DOI] [PubMed] [Google Scholar]
- 6.Cadeddu C, Piras A, Mantovani G, et al. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am Heart J. 2010;160(3):487. doi: 10.1016/j.ahj.2010.05.037. e1-7. [DOI] [PubMed] [Google Scholar]
- 7.Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Am Heart J. 2010;160(3):487. doi: 10.1161/HYPERTENSIONAHA.109.129973. e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones LW, Fels DR, West M, et al. Modulation of Circulating Angiogenic Factors and Tumor Biology by Aerobic Training in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Cancer Prev Res. 2013;6(9):925–37. doi: 10.1158/1940-6207.CAPR-12-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 10.Dolinsky VW, Rogan KJ, Sung MM, et al. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. American Journal of Physiology - Endocrinol Metab. 2013;305(2):E243–53. doi: 10.1152/ajpendo.00044.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


