Abstract
Thyroid carcinoma is the most common endocrine malignancy, and papillary thyroid carcinoma represents the most common thyroid cancer. Papillary thyroid carcinomas that invade locally or metastasize are associated with a poor prognosis. We found that, during epithelial–mesenchymal transition (EMT) induced by transforming growth factor-β1 (TGF-β1), papillary thyroid carcinoma cells acquired increased cancer stem cell-like features and the transcription factor paired-related homeobox protein 1 (PRRX1; alias PRX-1), a newly identified EMT inducer, was markedly up-regulated. miR-146b-5p was also transiently up-regulated during EMT, and in siRNA experiments miR-146b-5p had an inhibitory role on cell proliferation and invasion during TGF-β1–induced EMT. We conclude that papillary thyroid carcinoma tumor cells exhibit increased cancer stem cell-like features during TGF-β1–induced EMT, that miR-146b-5p has a role in cell proliferation and invasion, and that PRRX1 plays an important role in papillary thyroid carcinoma EMT and disease progression.
Thyroid carcinoma is the most common endocrine malignancy, and the most frequent type of thyroid cancer is papillary thyroid carcinoma (PTC), which makes up approximately 80% of thyroid cancers worldwide.1 Most patients with PTC have an excellent prognosis, but a small number of patients with aggressive PTC develop invasive tumors and/or distant metastases.1–3 To treat these patients more effectively, greater understanding of the mechanisms regulating tumor progression in aggressive PTC is needed.
Epithelial–mesenchymal transition (EMT) has been studied extensively during embryogenesis4 and in cancer progression.5–10 EMT inducers include cytokines, such as transforming growth factor beta-1 (TGF-β1),11,12 and transcription factors. TGF-β1 is a multifunctional cytokine that acts as a tumor suppressor in early tumor development and promotes tumor cell growth in later stages.13 Transcription factors linked to EMT include Snail, Slug, Twist, Zeb1, and Zeb2.14 Recent studies have linked EMT with cancer stem-like cells (CSCs) in mammary carcinomas.8,9 Some of the genes regulating EMT in thyroid cancer have been analyzed in recent years,10,15–17 but new inducers of EMT such as paired-related homeobox protein 1 (PRRX1; alias PRX-1) have also been reported.18,19 PRRX1 expression has been reported to play an important role as a key switch for neural cell lineage determination and in the maintenance of self-renewal of adult neural stem cells in the normal adult brain.18 Furthermore, in the anterior pituitary of rats, PRRX1 is produced in pituitary progenitor cells and may have a role in terminal differentiation during early pituitary organogenesis.19 PRRX1 has recently been observed to promote EMT in breast,20 pancreas,21 and colon22 cancers. In some tumors such as breast cancer, however, low PRRX1 levels were associated with metastasis and poor prognosis in clinical samples,20 whereas in colorectal adenocarcinomas abundant PRRX1 expression was associated with metastasis and poor prognosis.22 The role of PRRX1 in tumor progression of thyroid cancer is unknown.
miRNAs are a class of small noncoding RNAs of 18 to 25 nucleotides that are involved in post-transcriptional gene regulation by pairing with 3′ untranslated regions of target mRNAs.23 Studies have shown the importance of specific miRNAs in thyroid cancer development and progression.24–27 Several studies, especially in breast carcinomas, have shown a regulatory role of miR-146 on various proteins influencing tumor invasion and metastasis.28–31 Breast cancer metastasis-suppressor 1 (BRMS1) has been shown to influence miR-146 expression. Nuclear factor-κB (NF-κB), which is regulated by BRMS1 and miR-146, plays an important role in thyroid cancer progression, by controlling the proliferative and antiapoptotic signaling pathways of thyroid carcinomas.32 NF-κB is associated with regulation of anaplastic thyroid carcinoma (ATC) via up-regulation of miR-146a expression.33 Overexpression of miR-146b has been shown to reduce glioma growth in primary brain tumors.34,35 Some studies have shown that TGF-β1 regulates specific miRNAs such as miR-146b-5p in normal cells and in cancers such as PTC.24,27 Because TGF-β1 also regulates EMT in PTC,17 this growth factor may have important roles in both EMT and specific miRNA expression in thyroid cancer progression.
Our aim was to analyze the influence of TGF-β1 on PRRX1 and miR-146b-5p during EMT in PTC and to determine their influence on thyroid cancer progression. Here, we show that TGF-β1 up-regulates PRRX1 expression in PTC cell lines and that miR-146b-5p has a regulatory role on PTC proliferation and invasiveness during EMT.
Materials and Methods
Cell Culture and TGF Treatment
The papillary thyroid carcinoma cell line BCPAP was provided by Dr. Rebecca E. Schweppe (University of Colorado, Denver, CO) and the TPC-1 cell line was provided by Dr. Daniel T. Ruan (Brigham and Women’s Hospital, Boston, MA). Both cell lines were maintained in RPMI 1640 medium with 10% fetal bovine serum and 1% penicillin–streptomycin at 37°C in a 5% CO2–enriched atmosphere. The ATC cell lines THJ-16T and THJ-21T36 were kindly provided by Dr. John A. Copland III (Mayo Clinic, Jacksonville, FL) and were maintained in RPMI 1640 medium with 10% fetal bovine serum, 1% nonessential amino acids, 1% sodium pyruvate, and 1% penicillin–streptomycin at 37°C, 5% CO2.
To induce EMT, the cell lines were plated at a density of 1 × 105 per 75-mm flask, cultured with serum-free medium (modified from Fierabracci et al,37 with EGF and FGF omitted) with (treated) or without (control) 2 ng/mL human recombinant TGF-β1 (R&D Systems, Minneapolis, MN) for 30 days. Medium was replaced every 2 to 3 days. Control and treated cells were trypsinized, counted, and reinoculated at the original density every 7 days for 30 days; the remaining cells were collected for additional assays and analysis. Originally, the experimental design used a 10-day interval for analysis; however, for both cell lines the proliferation rate of the control exceeded the 10-day period, and the design was subsequently altered to a 7-day interval. To verify the authenticity of the TPC-1 and BCPAP cell lines, DNA short tandem repeat STR analysis was performed by DDC (DNA Diagnostics Center, Fairfield, OH). Both cell lines were tested using 19 markers and were found to be authentic.
Sphere Formation
TGF-β1–treated and control TPC-1 cells were collected at day 1 and day 14 of culture treatment, and 1 × 103 cells were plated into a six-well Ultra-Low attachment plate (Corning Life Sciences, Tewksbury, MA) with spheroid medium [modified from Fierabracci et al,37 with 1× B27 supplement added (Gibco; Life Technologies, Carlsbad, CA)] and were cultured for 5 days to allow sphere formation. Spheres were measured and counted to assess sphere-forming capacity. Only spheres ≥50 μm were counted.
Flow Cytometry
TGF-β1–treated and control TPC-1 and BCPAP cells were harvested and stained with a Molecular Probes dead cell apoptosis kit (Life Technologies) with annexin V, Alexa Fluor 488, and propidium iodide on days 7, 14, and 21 to analyze apoptosis. Tumor xenografts were harvested and enzymatically digested, and aldehyde dehydrogenase activity was analyzed with an ALDEFLUOR kit (STEMCELL Technologies, Vancouver, BC, Canada) according to the manufacturer’s instructions. All flow cytometry was performed on a BD FACSCalibur platform (BD Biosciences, San Jose, CA), and data were analyzed with Cyflogic software version 1.2.1 (CyFlo, Turku, Finland).
RNA Isolation and RT-qPCR
Total RNA was extracted from samples with TRIzol reagent (Life Technologies) according to the manufacturer’s instructions, and RNA quality and concentrations were assessed with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). To assess mRNA expression, total RNA of 1 μg was reverse-transcribed using an All-in-One first-strand cDNA synthesis kit (GeneCopoeia, Rockville, MD). RT-qPCR was performed on a CFX96 PCR detection system (Bio-Rad Laboratories, Hercules, CA) using Bullseye EvaGreen qPCR master mix (MIDSCI, St. Louis, MO), normalized to 18S rRNA; relative fold change was determined by the ΔΔ CT method. The PCR primers used are listed in Table 1 except E-cadherin, which was purchased from SABiosciences (Qiagen, Valencia, CA).
Table 1.
Primers Used for RT-qPCR Assays
| Primer | Forward | Reverse |
|---|---|---|
| 18S | 5′-GTAACCCGTTGAACCCCATT-3′ | 5′-CCATCCAATCGGTAGTAGCG-3′ |
| SOX2 | 5′-GCACATGAACGGCTGGAGCAACG-3′ | 5′-TGCTGCGAGTAGGACATGCTGTAGG-3′ |
| OCT4 | 5′-AGCCCTCATTTCACCAGGCC-3′ | 5′-CAAAACCCGGAGGAGTCCCA-3′ |
| Slug | 5′-ATGCATATTCGGACCCACAC-3′ | 5′-GCAGATGAGCCCTCAGATTT-3′ |
| Snail | 5′-CCCAATCGGAAGCCTAACTA-3′ | 5′-CAGGACAGAGTCCCAGATGAG-3′ |
| Vimentin | 5′-TCCAGCAGCTTCCTGTAGGT-3′ | 5′-CCCTCACCTGTGAAGTGGAT-3′ |
| Twist | 5′-CATCGACTTCCTCTACCAGGTC-3′ | 5′-TCCATTTTCTCCTTCTCTGGAA-3′ |
| Zeb1 | 5′-GCACAACCAAGTGCAGAAGA-3′ | 5′-GCCTGGTTCAGGAGAAGATG-3′ |
| Zeb2 | 5′-CAAGAGGCGCAAACAAGC-3′ | 5′-GGTTGGCAATACCGTCATCC-3′ |
| PRRX1 | 5′-CTGATGCTTTTGTGCGAGAA-3′ | 5′-ACTTGGCTCTTCGGTTCTGA-3′ |
| cMET | 5′-GATTTTAGTCATCCCAATGTCC-3′ | 5′-ATCCAGCATACAGTTTCTTGC-3′ |
| EGFR | 5′-ATGTCCGGGAACACAAAGAC-3′ | 5′-TTCCGTCATATGGCTTGGAT-3′ |
To assess miRNA expression, 1 μg of total RNA was reverse-transcribed using an All-in-One miRNA RT-qPCR detection kit (GeneCopoeia). RT-qPCR was performed on a CFX96 PCR detection system (Bio-Rad Laboratories), normalized to RN5; relative fold change was determined by the ΔΔ CT method. All primers used for miRNA detection were purchased from GeneCopoeia.
Western Blot Analysis
Treated and control TPC-1 and BCPAP cells at three time points (days 7, 14, and 21) were washed twice with phosphate-buffered saline (PBS), and then radioimmunoprecipitation assay lysis buffer was added. The protein lysates were prepared as described previously.38 In brief, protein concentration was quantified using a Pierce BCA protein assay kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Equal amounts of denatured protein were resolved by electrophoresis on 4% to 15% Criterion TGX precast gels (Bio-Rad Laboratories), transferred onto nitrocellulose membranes (Bio-Rad Laboratories), blocked in 5% nonfat milk solution, then incubated overnight at 4°C with the following primary antibodies: Twist (dilution 1:500; Abcam, Cambridge, MA); Snail (dilution 1:1000; Novus Biologicals, Littleton, CO); Slug (dilution 1:1000), E-cadherin (dilution 1:1000), and β-actin (dilution 1:2000) (Cell Signaling Technology, Danvers, MA); or PRRX1 (dilution 1:1000; Sigma-Aldrich, St. Louis, MO). Membranes were washed the next day and then incubated for 1 hour at room temperature with horseradish peroxidase–conjugated secondary antibodies (dilution 1:2000; Cell Signaling Technology). The immunoreactive protein bands were visualized by Immun-Star (Bio-Rad Laboratories) or by Pierce SuperSignal West Pico or SuperSignal West Femto (Thermo Fisher Scientific) chemiluminescent detection systems. β-Actin was used as the loading control.
siRNA Transfection, Proliferation, and Invasion Assays
Control and TGF-β1–treated cells were seeded into a 96-well plate at 1 × 104 cells per well (10 replicates each) in 100 mL serum-free/antibiotic-free medium with or without 2 ng/mL TGF-β1. Wells were transfected with 10 nmol/L of miRCURY LNA miRNA power inhibitor to hsa-mir-146b-5p (sequence: 5′-GCCTATGGAATTCAGTTCTC-3′) or miRCURY LNA microRNA Inhibitor Negative Control A (sequence: 5′-GTGTAACACGTCTATACGCCCA-3′) (Exiqon, Woburn, MA; Vedbaek, Denmark) or MISSION synthetic miRNA inhibitor to hsa-mir-146b-5p (sequence: 5′-UGAGAACUGAAUUCCAUAGGCU-3′) (Sigma-Aldrich) using X-tremeGENE siRNA transfection reagent (Roche Diagnostics, Indianapolis, IN) for 72 hours at 37°C in a 5% CO2–enriched atmosphere. After 72 hours of incubation, cell proliferation and invasion were assayed using a Vybrant MTT cell proliferation assay kit (Life Technologies) and a Cultrex BME cell invasion assay (R&D Systems) according to the manufacturer’s protocol. The 96-well plate for the invasion assay was coated with 0.1× BME basement membrane extract overnight before seeding cells. Read-out for the MTT assay was performed using a SpectraMax 190 absorbance microplate reader (Molecular Devices, Sunnyvale, CA); a Synergy 2 multimode microplate reader (BioTek Instruments, Winooski, VT) was used to analyze the cell invasion assay.
Tumor Xenografts
TGF-β1–treated and control cells were cultured for 30 days as described above. The cells were harvested and 1 × 105 cells were injected subcutaneously into 30 nude mice 10 mice per treatment group (Jackson Laboratory, Bar Harbor, ME) in 1:1 serum-free medium/growth factor–reduced Matrigel (BD Biosciences), with three injection sites per mouse for TPC-1 and BCPAP cells. Tumors were measured with calipers once a week, and they were harvested after 10 weeks and processed for further analysis.
Immunofluorescence Studies
In situ immunofluorescence staining for vimentin and cytokeratin 19 was performed on day 8 on TGF-β1–treated and control TPC-1 cells in a six-well plate. The cells were fixed with methanol for 5 minutes and washed three times with PBS. The cells were blocked with 2.5% horse serum, and primary antibody to vimentin (dilution 1:200) and cytokeratin 19 (dilution 1:20) was added overnight at 4°C. Alexa Fluor 488 goat anti-rabbit IgG (dilution 1:500; Life Technologies) was added for 30 minutes; after a wash with PBS, ProLong Gold antifade reagent with DAPI (Life Technologies) was added to the well to stain nuclei. Images were captured with an Eclipse TI fluorescence microscope (Nikon, Tokyo, Japan) and analyzed with NIS Elements software version 3.01 (Nikon).
Immunofluorescence staining was performed to detect E-cadherin in control and TGF-β1–treated cell lines. The TPC-1 cell line was prepared on cytospin slides after 14 days of treatment. Slides were fixed with 4% paraformaldehyde for 5 minutes and washed three times with PBS. Epitope retrieval was performed using 10 μmol/L of citrate buffer (pH 6) in a microwave oven for 5 minutes. The cells were then incubated with 2.5% horse serum for 1 hour before anti–E-cadherin rabbit monoclonal antibody (dilution 1:400; clone 24E10; Cell Signaling Technology) was added overnight at 4°C. Alexa Fluor 555 goat anti-rabbit IgG (dilution 1:500; Life Technologies) was added for 30 minutes, followed by a wash with PBS and mounting with ProLong Gold antifade reagent with DAPI (Life Technologies).
TMA
Three tissue microarrays (TMAs) were constructed as described previously19 from 193 case samples of formalin-fixed, paraffin-embedded tissues: 10 normal thyroid (NT), 10 nodular goiter (NG), 32 follicular adenoma (FA), 28 follicular carcinoma (FC), 57 PTC, 21 poorly differentiated carcinoma (PDC), and 35 ATC. The first TMA comprised 147 cases, including only 10 of the ATCs and omitting all PD carcinomas; the second TMA comprised the 21 PD carcinomas, and the third TMA comprised 25 ATCs. The TMA consisted of triplicate 0.6-mm cores made using a manual TMA (Beecher Instruments, Sun Prairie, WI). The NT consisted of tissues from the opposite (histologically normal) thyroid lobe in patients with follicular or papillary carcinomas.
The study was approved by the Institutional Review Board at the University of Wisconsin–Madison.
TMA and Whole Tissue Section IHC
Automated immunostaining of the TMAs for PRRX1 was performed as described previously.15 All immunolabeling was performed at room temperature using a Lab Vision 360 LV-1 Autostainer system (Thermo Fisher Scientific). Except as noted, reagents were from Biocare Medical (Concord, CA). PRRX1 rabbit polyclonal antibody (NBP2-13816; Novus Biologicals) was diluted 1:20 and incubated for 60 minutes, followed by incubation with biotinylated goat anti-rabbit IgG for 15 minutes and then 4+ streptavidin–horseradish peroxidase treatment for 15 minutes. Betazoid diaminobenzidene and Mayer’s hematoxylin were each incubated for 1 minute. Primary antibodies were omitted in negative controls, which resulted in no staining. For positive controls, ATCs previously shown to express the antigen of interest by immunohistochemistry (IHC) and by RT-qPCR were used.
PRRX1 staining on TMAs was evaluated based on the presence or absence of nuclear immunoreactivity in thyroid epithelial cells. Staining patterns were examined by three independent reviewers (R.V.L., C.M.G., and Z.G.). Nuclear staining for PRRX1 was interpreted based on intensity, which was scored as negative (0), weak (1+), moderate (2+), and strong (3+). Expression was considered focal if positive cells comprised 2% to 25% of all tumor cells in the TMA sample and diffuse if this proportion was greater than 25%. Focal staining of less than 1% of all tumor cells was considered to be negative.
Whole sections of formalin-fixed, paraffin-embedded lymph node tissues corresponding to seven PTCs and one ATC represented on the TMAs were also used for immunostaining for PRRX1. In addition, whole sections of formalin-fixed, paraffin-embedded tissue sections of five conventional PTCs, five tall-cell PTCs, and five PTCs with hobnail features were used for PRRX1 immunostaining.
Statistical Analysis
Continuous variables were assessed by Student’s t-test. Categorical variables were compared with the χ2 test. Multivariate prognostic analyses were performed with a Cox proportional hazards regression model. All statistical analyses were performed using IBM SPSS Statistics software version 21.0 (IBM, Armonk, NY) and GraphPad Prism software version 6.0 (GraphPad Software, La Jolla, CA). Two-tailed P values of <0.05 were considered to be statistically significant. Data are expressed as means ± SEM.
Results
TGF-β1 Treatment Induces EMT in PTC Cells
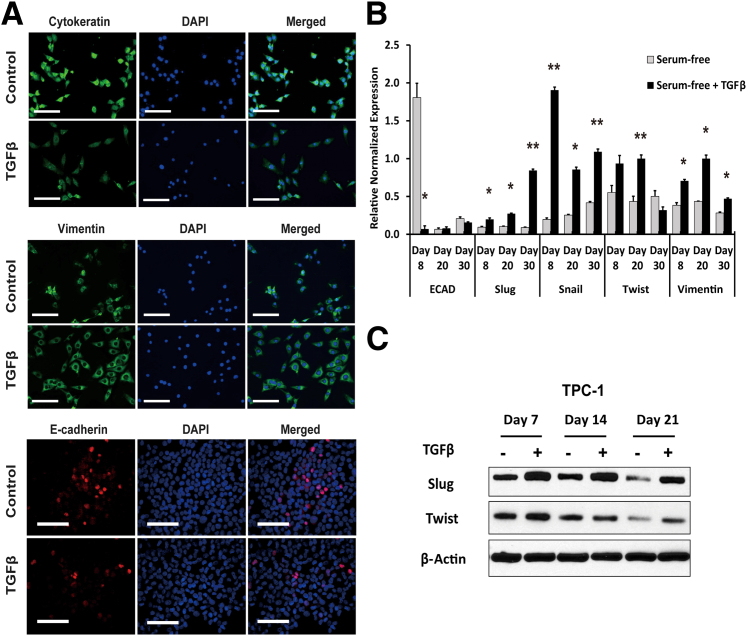
To investigate the role of TGF-β1 on the development of EMT in PTC cells, we used long-term in vitro culture with subsequent inoculation into nude mice. PTC cell lines TPC-1 and BCPAP were cultured in a modified serum-free medium for a period of 30 days and were analyzed at regular intervals to assess EMT marker expression. TGF-β1 treatment induced EMT in PTC cells, as evident from the production of spindle cells, down-regulation of E-cadherin and cytokeratin 19, and up-regulation of vimentin, Slug, Snail, and Twist in both TPC-1 cells (Figure 1) and BCPAP cells (Supplemental Figure S1). A significant decrease in the expression of E-cadherin was seen early in TGF-β1 treatment of both PTC cell lines; however, TPC-1 control cells also down-regulated E-cadherin, because of the serum-free environment of the culture medium (Figure 1B), whereas BCPAP control cells maintained significant E-cadherin expression (Supplemental Figure S1). The transcription factors Slug and Snail were significantly up-regulated in both cell lines at all time points analyzed, whereas Twist was expressed variably in both cell lines. Vimentin was increased by TGF-β1 treatment, but the increase was significant only in the TPC-1 cell line (Figure 1, B and C).
Figure 1.
TGF-β1 treatment up-regulates EMT marker expression in TPC-1 cells. A:In situ staining of attached cultures with cytokeratin 19 antibody on day 8 revealed decreased expression in spindle cells of TGF-β1–treated TPC-1 cells, as well as increased expression of the classic EMT marker vimentin. TGF-β1–treated and untreated TPC-1 cells were stained for the adhesion marker E-cadherin on day 14 cytospin-prepared slides; protein expression decreased in TGF-β1–treated cells. B: RT-qPCR results from treated and untreated TPC-1 cells at days 8, 20, and 30 indicated significant loss of E-cadherin expression and significant increase in the EMT markers Slug, Snail, Twist, and vimentin in TGF-β1–treated cells. C: Western blot analysis of the EMT markers Slug and Snail on TGF-β1–treated and untreated TPC-1 cells assessed on days 7, 14, and 21 of culture. Analysis shows increased slug and twist production in TGF-β1 treated cells consistent with RT-qPCR results. β-Actin was used as a control. Data are expressed as means ± SEM for five experiments. ∗P < 0.05; ∗∗P < 0.01. Scale bar = 50 μm. Original magnification: ×100 (A); ×200 (B). ECAD, E-cadherin; TGFβ, TGF-β1.
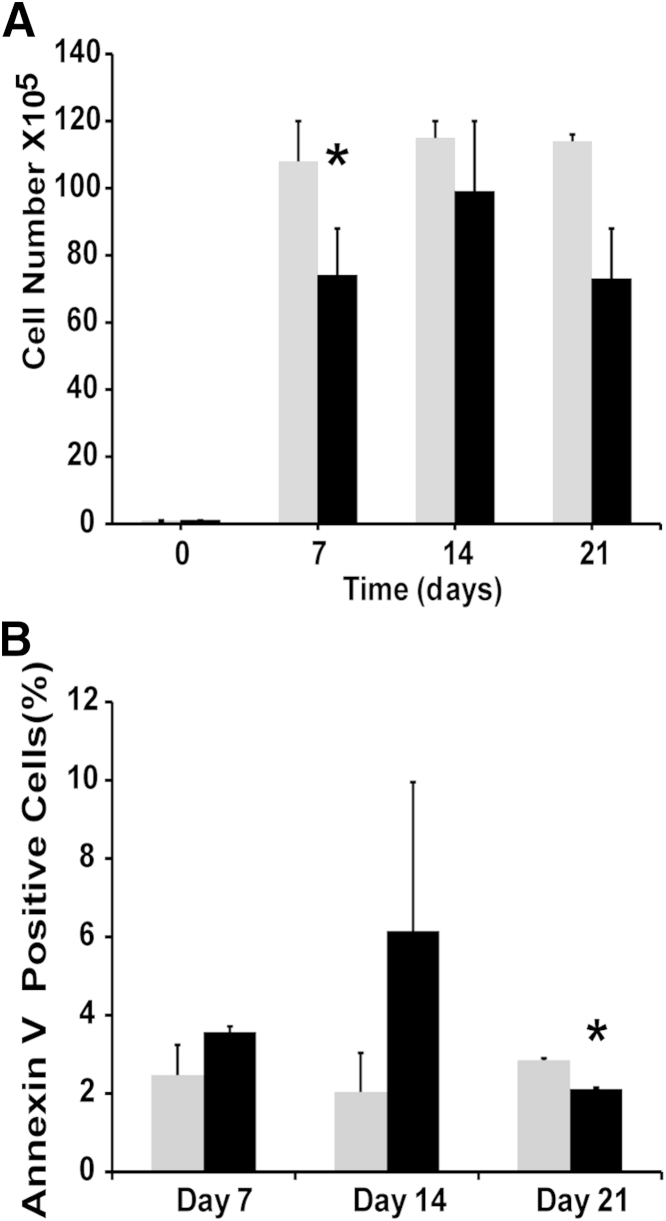
The effects of TGF-β1 on proliferation of TPC-1 and BCPAP cancer cell lines are not well known. Analysis of the proliferation rate of two cell lines of TGF-β1–treated and control cells revealed a reduction in growth in TPC-1 cells (Figure 2A) and early inhibition of BCPAP cell proliferation (data not shown) but with recovery by day 21. Flow cytometric analysis of annexin V staining revealed that this inhibition was due in part to TGF-β1–induced apoptosis in both TPC-1 cells (Figure 2B) and BCPAP cells (data not shown). RT-qPCR and IHC analyses showed that both cell lines had equal expression of TGF-β receptor type-1 (TGF-βR1), SMAD2, and SMAD4 (data not shown). Taking into account that the cell lines expressed pathway components, the exerted growth inhibition and induced apoptosis under TGF-β1 treatment indicate that the TGF-β1 pathway is intact and functional in both TPC-1 and BCPAP cells.
Figure 2.
In vitro TGF-β1 treatment inhibits growth of TPC-1 cells. A: TPC-1 cells were cultured in serum-free medium with (black bars) or without (gray bars) 2 ng/mL TGF-β1 for 21 days, with the culture medium renewed every 2 to 3 days. TGF-β1 exhibited in vitro growth inhibition on TPC-1 cells. B: Annexin V staining and flow cytometry indicated that growth inhibition was due in part to apoptosis. Data are expressed as means ± SEM for two experiments ∗P < 0.05.
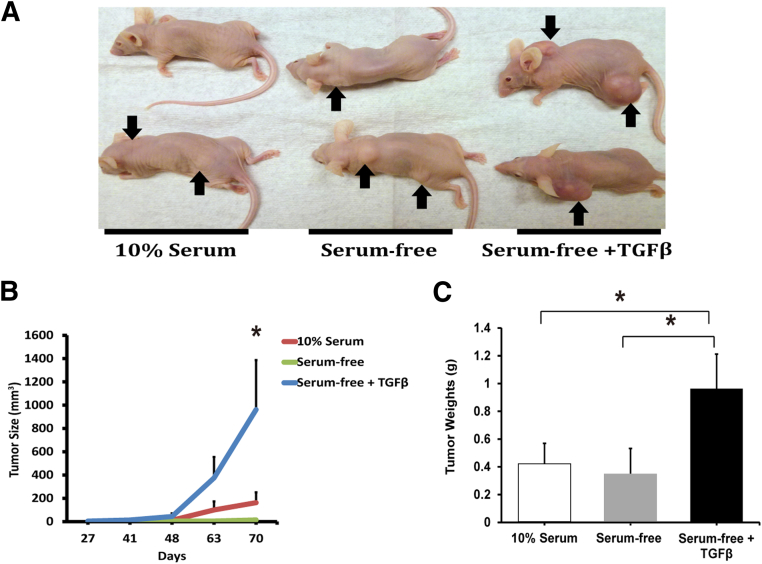
TGF-β1 Treatment Induces Tumorigenicity in PTC Cells
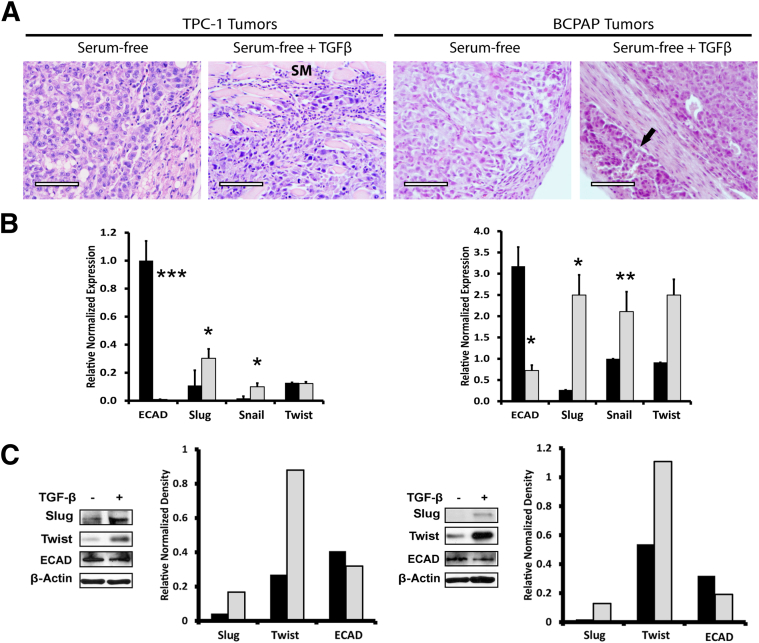
After the initial in vitro culture period of 30 days, 10% serum, serum-free, and TGF-β1–treated cells were injected subcutaneously into 30 nude mice (n = 10 mice per treatment group, with three injection sites per mouse) to assess tumor formation and growth (Figure 3A). TGF-β1–treated TPC-1 cells formed tumors at a significantly accelerated rate (Figure 3B) and tumor size increased significantly in 10 weeks, compared with control and cells grown in 10% serum (Figure 3C). TGF-β1 treatment also tended to increase tumor size of BCPAP cells, compared with control cells, but the difference was not significant because of marked variation in tumor size (Supplemental Figure S2). BCPAP cells cultured in 10% serum medium did not grow tumors. Tumors from the xenografts were analyzed for morphological features and for EMT marker expression (Figure 4). The morphology of serum-free treated TPC-1 and BCPAP tumors included thyroid carcinomas with epithelioid cells, whereas the TGF-β1–treated TPC-1 tumors exhibited a more spindle-cell morphology, with skeletal muscle invasion in some tumors (Figure 4A). Interestingly, the BCPAP tumors treated with TGF-β1 also exhibited an epithelioid morphology, but with vascular invasion in a few cases (Figure 4A). Tumors were also analyzed for EMT markers by RT-qPCR (Figure 4B) and Western blotting and densitometry (Figure 4C). Tumor EMT marker expression remained constant and correlated well with the in vitro analysis.
Figure 3.
TGF-β1 treatment on TPC-1 cells induces tumor formation in vivo. A: Six-week–old female nude mice were injected subcutaneously with 1 × 105 TPC-1 cells cultured for 30 days in medium containing 10% fetal bovine serum, serum-free medium, or serum-free medium with 2 ng/mL TGF-β1. There were 30 inoculations per treatment: 3 injection sites per mouse, and 10 mice per treatment group. Mice were monitored weekly, and tumor growth was assessed with calipers (height × length × width). After 10 weeks, mice were euthanized and the tumors were harvested, weighed, and processed for further analysis. Representative tumors are shown (arrows). B: Growth curves for TPC-1 tumors show that TGF-β1–treated tumors grew significantly faster than the serum-free control or 10% serum–treated tumor. C: TGF-β1–treated TPC-1 cells produced more tumors and significantly larger tumors (20/30, 67%) than cells treated with either 10% fetal bovine serum (15/30, 50%) or serum-free medium (17/30, 57%). Data are expressed as means ± SEM for three experiments. n = 10 mice per treatment group. ∗P < 0.05.
Figure 4.
TGF-β1 treatment induces EMT and aggressiveness in xenografts. A: To assess morphology, TPC-1 and BCPAP control and TGF-β1–treated tumors were stained with hematoxylin and eosin. In both cell lines, the control tumors were thyroid carcinoma with an epithelioid morphology. The TPC-1 TGF-β1–treated tumors were thyroid carcinoma with spindle-shaped cells, sometimes with skeletal muscle invasion (SM). The BCPAP TGF-β1–treated tumors were thyroid carcinoma with an epithelioid morphology, sometimes with evidence of vascular invasion (arrow). B: TGF-β1 (gray bars) and control (black bars) medium–treated PTC tumors were analyzed for the EMT markers E-cadherin, Slug, Snail, and Twist. RT-qPCR indicated significant down-regulation of E-cadherin and significant up-regulation of Slug and Snail in both TPC-1 (left) and BCPAP (right) TGF-β1–treated tumors. C: Analysis of EMT marker protein expression in PTC tumors by Western blotting and densitometry analysis revealed an increase in EMT proteins Slug and Twist and a decrease in E-cadherin in TGF-β1–treated tumors. Data are expressed as means ± SEM for two experiments. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bar = 75 μm. Original magnification, ×200.
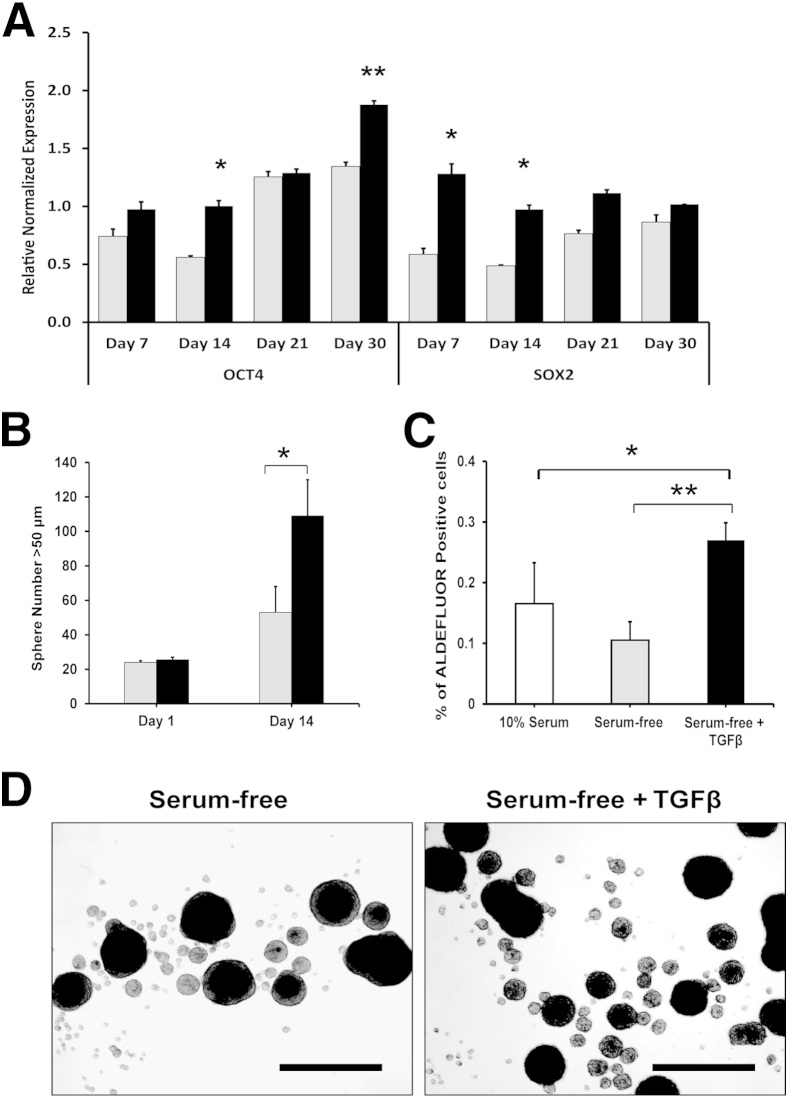
Because the significant growth of TGF-β1–treated TPC-1 cells was probably not explained solely by EMT induction, we analyzed stem cell markers. In vitro cultures of TPC-1 treated and control cells, as well as cells grown in 10% serum, were analyzed by RT-qPCR for the stem cell markers OCT4 and SOX2 (Figure 5A). The PCR results indicated a significant up-regulation of OCT4 by TGF-β1 treatment; however, control cells also up-regulated OCT4, because of serum-free culture stresses. Furthermore, TGF-β1 significantly up-regulated SOX2 very early in treatment, but the control cells also reached that threshold level by day 30. PCR results for the 10% serum condition showed very low levels of both transcription factors (data not shown).
Figure 5.
TGF-β1 up-regulates stem cell markers in TPC-1 cells. A: TPC-1 cells cultured for 30 days with (black bars) or without (gray bars) TGF-β1 were analyzed on days 7, 14, 21, and 30 for the stem cell markers OCT4 and SOX2. RT-qPCR analysis indicated up-regulation of the stem cell markers OCT4 and SOX2 after TGF-β1 treatment. B: Day 1 and day 14 TGF-β1–treated and untreated cells were incubated in ultra-low attachment six-well plates in spheroid medium for 5 days to assess thyrosphere formation. Spheres with diameter of ≥50 μm were counted. Day 14 TGF-β1–treated cells formed many more thyrospheres than did the untreated cells, indicating an increase in stem-like cells with self-renewal properties under TGF-β1 treatment. C: Xenograft tumors from 30-day cultured TPC-1 cells (Figure 2A) were harvested after 10 weeks without TGF-β1–treatment and analyzed for ALDEFLUOR activity by flow cytometry. TGF-β1–induced tumors exhibited a twofold increase in ALDEFLUOR activity, compared with controls, indicating a significant increase in stem-like cell populations within the tumors. D: Representative images of day 14 thyrospheres of TGF-β1–treated and control TPC-1 cells. Data are expressed as means ± SEM for two experiments. ∗P < 0.05; ∗∗P < 0.01. Scale bar = 250 μm.
Because in vitro markers did not fully elucidate the differences in tumor cell proliferation between the treated and control cells, we hypothesized that our experimental design selected for CSCs (ie, tumor-initiating cells). TGF-β1–treated and control cells from 24-hour to 14- day in vitro cultures were placed in ultra-low attachment plates with sphere formation medium for 5 days, to analyze self-renewal properties. There was no difference in the 24-hour culture assay between the treated and control cells; however, 14-day TGF-β1–treated cells produced a significantly greater number of spheres, compared with control cells (Figure 5, B and D), suggesting a greater self-renewal potential. Additionally, flow cytometric ALDEFLUOR analysis revealed that TGF-β1–treated tumor cells had a significantly greater amount of aldehyde dehydrogenase activity than either the control or 10% serum–treated tumor cells (Figure 5C), indicating a greater number of CSCs within the tumor population of TGF-β1–treated tumors.
TGF-β1 Induces PRRX1 Expression
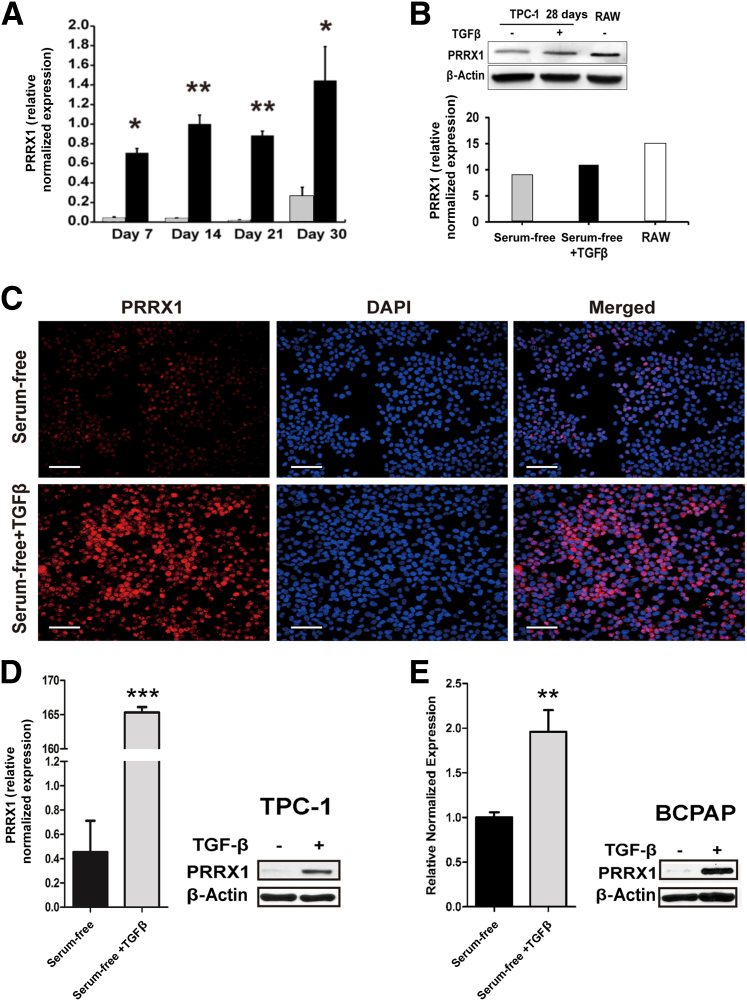
Recently, the novel homeobox transcription factor PRRX1 has been reported to be an EMT inducer and involved in tumorigenicity and metastasis20–22; however, the role of PRRX1 in thyroid carcinoma is unknown. To investigate whether PRRX1 plays a role in TGF-β1–induced EMT of PTC, TGF-β1–treated and control TPC-1 cells were tested for PRRX1 expression by RT-qPCR, Western blotting, and immunofluorescence (Figure 6). PRRX1 was significantly up-regulated in TGF-β1–treated cells (Figure 6A) from as early as day 7, and this effect remained significant through day 30. Control and TGF-β1–treated TPC-1 and BCPAP xenograft tumors were also tested for PRRX1 expression, and TGF-β1–treated tumors exhibited significant up-regulation of PRRX1, compared with the control medium–treated tumors (Figure 6, D and E). In two ATC cell lines tested for PRRX1 expression (THJ-16T and THJ-21T), the 21T cells had very high levels of PRRX1, whereas levels in 16T cells were similar those of TPC-1 cells (data not shown).
Figure 6.
TGF-β1 induces PRRX1 expression. A: RT-qPCR analysis of TGF-β1–treated (black bars) and untreated (gray bars) TPC-1 cells revealed significantly increased PRRX1 expression after TGF-β1 treatment at days 7, 14, 21, and 30. B: Immunoblotting and densitometry were performed for PRRX1 in 28-day TGF-β1–treated and control cells, as well as RAW cells used as a positive control, normalized to β-actin. C: PRRX1 immunostaining of control and TGF-β1–treated TPC-1 cells revealed a marked increase in PRRX1 protein expression on day 14. DAPI was used as a nuclear counterstain. D and E: RT-qPCR and Western blot analyses of TPC-1 and BCPAP xenografted tumors revealed significant up-regulation of PRRX1 in TGF-β1–treated cells, compared with the control medium–treated cells. Data are expressed as means ± SEM for four (A) or two (D and E) experiments. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bar = 50 μm.
Prognostic Implication of PRRX1 Expression in Thyroid Tissues
IHC analysis of the TMA is summarized in Table 2. IHC staining with PRRX1 antibody revealed nuclear localization of PRRX1 protein (Figure 7). The intensity of PRRX1 expression was moderate to strong. The ATC group had the highest percentage of cases staining for PRRX1, with nuclear expression in 20/35 (57.7%) ATCs in the TMAs, compared with all other groups, whereas PTCs, PDCs, and NGs were all negative for staining. PRRX1 staining was positive in 1/32 (3%) FAs, 1/28 (4%) FCs, and 2/10 (20%) NTs (Table 2). PRRX1 was also expressed in inflammatory cells, fibroblasts, and endothelial cells.
Table 2.
IHC Expression of PRRX1 in Thyroid Tissues
| Diagnosis | n | Positive | % Positive |
|---|---|---|---|
| Normal thyroid (NT) | 10 | 2 | 20∗ |
| Nodular goiter (NG) | 10 | 0 | 0 |
| Follicular adenoma (FA) | 32 | 1 | 3 |
| Follicular carcinoma (FC) | 28 | 1 | 4 |
| Papillary thyroid carcinoma (PTC) | 57 | 0 | 0 |
| Poorly differentiated carcinoma (PDC) | 21 | 0 | 0 |
| Anaplastic thyroid carcinoma (ATC) | 35 | 20 | 57.7† |
TMAs were constructed and used for IHC staining with PRRX1 antibody. Staining and quantitation of TMAs were performed as described under Materials and Methods.
P < 0.01 for NT versus ATC.
P < 0.001 for ATC versus NG, FA, FC, PTC, and PDC.
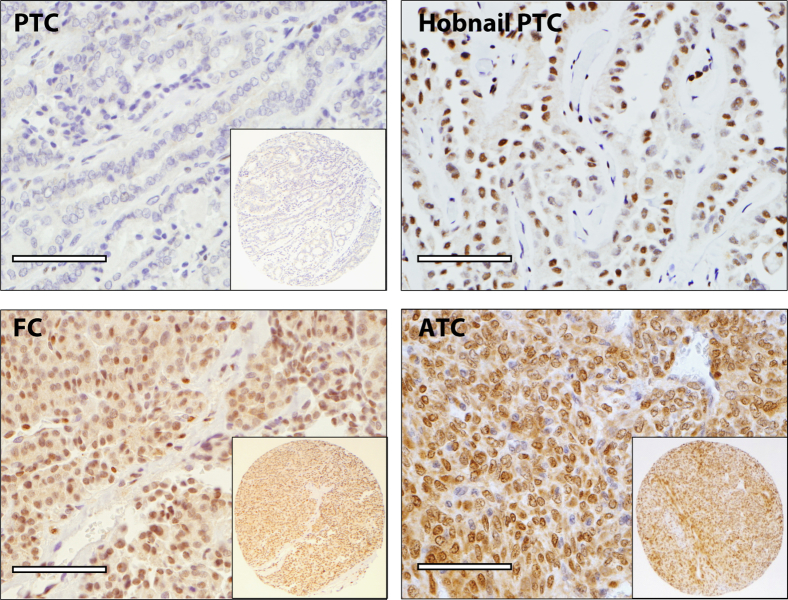
Figure 7.
PRRX1 is associated with higher grade thyroid cancers. In PRRX1 immunostaining of TMA with PTC, hobnail variant of PTC, FC, and ATC, nuclear staining (brown) indicates positive staining for PRRX1 expression. All PTCs (n = 57) on the TMA were negative, except 2 out of 5 hobnail variants of PTCs were positive. One out of 28 FC was positive, whereas 20 out of 36 ATCs were positive for PRRX1. Insets: low-power image of the corresponding tissue core. Scale bar = 75 μm.
The whole sections of lymph node tissues with metastatic carcinomas were analyzed for PRRX1 expression. The seven PTCs corresponding to cases on the TMA were all negative for PRRX1, but the one ATC case was positive in both the lymph node metastasis and in the primary tumor (data not shown). In another set of experiments, analysis of whole tissue sections of five conventional PTCs and five tall-cell variant PTCs were all negative for PRXX1; however, 2/5 (40%) PTCs with hobnail features were positive for PRRX1 (Figure 7).
miR-146b-5p Is Induced by TGF-β1 and Is Inhibitory to PTC Proliferation and Invasion
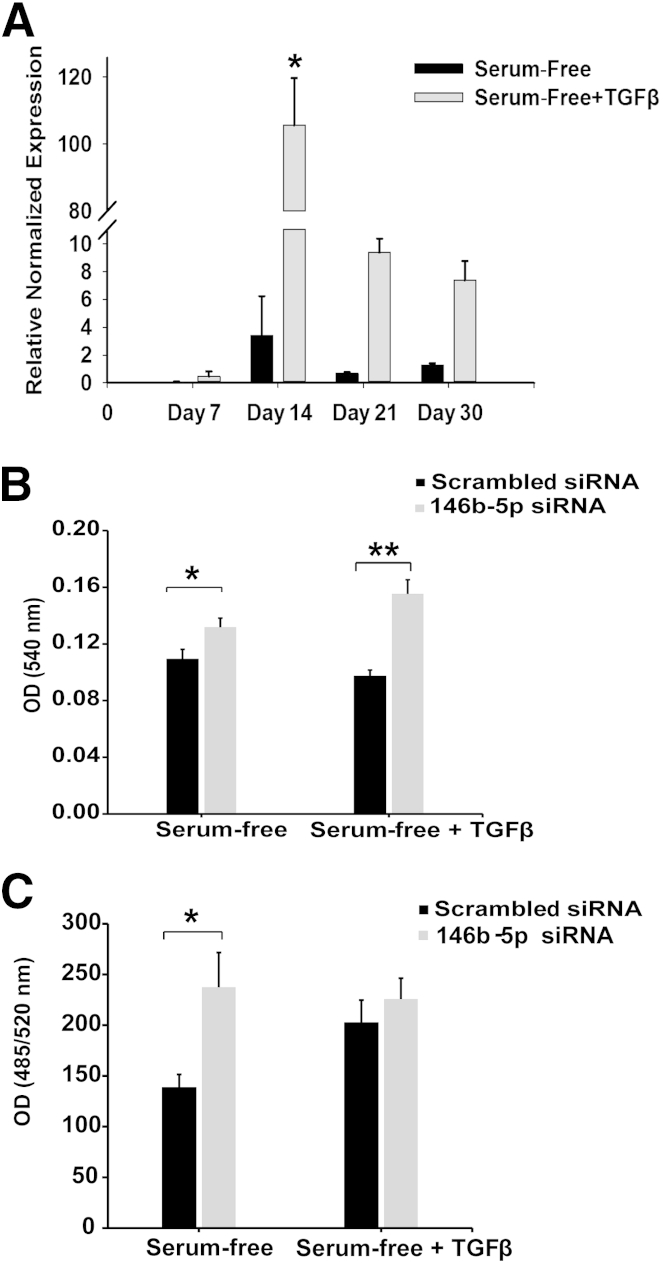
Several research groups have performed miRNA profiling of thyroid carcinomas and report that miR-146b-5p is one of the most up-regulated miRNA in PTC.24–26 Nonetheless, not many functional studies have been performed with this miRNA in thyroid carcinoma. We tested PTC cell lines by RT-qPCR for miR-146b-5p expression and found that miR-146b-5p was expressed at low levels in PTC-1 and BCPAP cells (data not shown). Interestingly, RT-qPCR showed a significant up-regulation of miR-146b-5p with TGF-β1 treatment in both cell lines; however, this up-regulation was transient (Figure 8A). BCPAP results were similar (data not shown). PCR analysis showed a significant up-regulation of miR-146b-5p by approximately days 10 to 14, with reduced expression by day 21 in five independent experiments. Given these findings, we conducted further investigations.
Figure 8.
TGF-β1 transiently up-regulates miR-146b-5p. A: RT-qPCR analysis of miR-146b-5p expression from TGF-β1–treated and untreated TPC-1 cells revealed a significant transient up-regulation on day 14 of TGF-β1 treatment, with subsequent return toward basal levels by day 21. B: Treated and untreated TPC-1 cells at day 14 were transfected with a scrambled negative control siRNA or a siRNA specific for miR-146b-5p and incubated for 3 days. At the end of the transfection incubation period, MTT assay indicated that down-regulation of miR-146b-5p significantly increased proliferation in both control and treated cells. C: Invasive properties of the transfected cells were assessed using a basement membrane extract assay. The invasiveness of TGF-β1–treated cells was unaffected by either the scrambled or the miR-146b-5p siRNA; however, a significant increase in the invasiveness of the control medium–treated cells occurred when miR-146b-5p was down-regulated. Data are expressed as means ± SEM for eight (A) or three (B and C) experiments. ∗P < 0.05; ∗∗P < 0.01. OD, optical density.
A miRNA silencing inhibitor (siRNA) comprising a pool of five separate sequences to miR-146b-5p was transfected into TGF-β1–treated and control cells on day 14, and proliferation and invasion studies were performed. RT-qPCR confirmed inhibition of miR-146b-5p by the chosen siRNA (Supplemental Figure S3). MTT assays indicated that the proliferation rate of both TGF-β1–treated and control TPC-1 cells was significantly increased by miR-146b-5p knockdown (Figure 8B). BCPAP cells exhibited similar changes, but the differences did not reach significance (data not shown). Invasion assays using a basement membrane extract were used to analyze invasive potential of TGF-β1–treated and control cells. Intriguingly, miR-146b-5p inhibition did not alter the invasive properties of TGF-β1–treated cells, but significantly increased the invasiveness of control cells to a level equal to or greater than that of TGF-β1–treated cells (Figure 8C). A second siRNA with a different pool of sequences to miR-146b-5p was then used, and this confirmed the results of the original siRNA (Supplemental Figure S4).
Discussion
The EMT program in which cancer cells acquire a mesenchymal phenotype has been proposed as a critical mechanism for the progression of epithelial cancers.8,9 Molecular changes in gene expression during EMT have been reported in thyroid carcinoma progression.10,39,40 In the present study, we found that the PTC cell lines TPC-1 and BCPAP undergo EMT under the influence of TGF-β1, with decreased expression of E-cadherin and increased expression of Snail, Slug, and Twist in a time-dependent manner. Although in a recent study Yasui et al41 did not detect E-cadherin in the TPC-1 cell line, we found low levels of E-cadherin in the cell membrane, with higher levels in the cytoplasm and in the nucleus of these cells, which is supported by our mRNA data. There was very little cell membrane–associated E-cadherin in the TPC-1 and BCPAP cell lines, compared with NT control tissues or control tissues of primary PTCs, suggesting that these two cell lines have aberrant expression of E-cadherin.
We observed an increase in CSCs after TGF-β1 treatment in the PTC cell lines, as evidenced by i) more rapid growth of tumors by tumor-initiating cells, ii) expression of higher levels of ALDEFLUOR-positive cells in the xenografted tumors, iii) up-regulation of the stem cell markers SOX2 and OCT4, and iv) formation of more thyrospheres after induction of EMT by TGF-β1. In their studies of transformed human mammary epithelial cells, Mani et al8 found that the mammary cells undergoing EMT had properties of stem cells, and stem-like cells from mammary carcinomas expressed EMT markers. Todaro et al5 found that, in primary thyroid cancers, ALDEFLUOR-high cells were the cells with CSC properties. However, in a recent study with thyroid carcinoma cell lines Yasui et al41 demonstrated that Snail can induce EMT and cancer stem cell-like properties in aldehyde dehydrogenase–negative ACT-1 thyroid cancer cells. Most studies of EMT in thyroid cancers have analyzed ATCs.41,42 Our present analysis of two PTC cell lines indicates a role of EMT and CSCs in PTC progression.
PRRX1 is a newly identified EMT inducer; like many EMT molecules, it has variable functionality, from development to adult stem cell maintenance to EMT and metastasis. In the present study, PRRX1 increased during TGF-β1 induction of EMT in the PTC cell lines in vitro, as well as in xenografted tumors, indicating that the increase in PRRX1 is relatively stable. In addition, we observed a parallel up-regulation of PRRX1 and stem cell-like properties. Ocaña et al20 reported a crucial difference between PRRX1 and other EMT transcription factors: PRRX1-induced EMT did not correspond with the induction of stem cell-like properties during Snail-, Twist-, or Zeb-mediated EMT in mammary cancer cells, and it was PRRX1 loss rather than gain that was associated with acquisition of stemness-related capabilities. Our findings are in general accord with the observation of Ocaña et al20 of TGF-β1–regulated expression of PRRX1; however, in our studies of PRRX1 expression in thyroid cancers we found that it is the higher grade cancers, such as ATCs, that have the highest percentage of cases expressing PRRX1. Our findings in thyroid cancer are similar to those of recent studies in colorectal carcinoma22 and in pancreatic adenocarcinoma21 showing that EMT and a cancer stem cell-like phenotype are associated with increased expression of PRRX1. The distinct differences across these studies may be related to cell type, stage of tumor development, or metastatic stage, such as dormant cells versus colonization of the new tumor cells.43 Further studies are needed to elucidate the various pathways regulating PRRX1 expression during tumor development and metastasis in thyroid and other tumors.
The IHC analysis of PRRX1 expression in the TMAs showed that mainly ATCs expressed this EMT marker. Interestingly, a few NTs (as well as one FA and one follicular carcinoma) also expressed PRRX1 in the TMA analysis. Studies in the anterior pituitary have shown PRRX1 expression in normal pituitary cells.19 In the present study, we also observed PRRX1 expression in fibroblast and endothelial cells in the thyroid in the present study. However, high-grade thyroid carcinomas expressed the highest level of this EMT marker. All of the conventional PTCs on the TMAs were negative for PRRX1, as were the corresponding metastases in seven cases. However, two of the PTCs with hobnail features were positive for PRRX1 (Figure 7). Our previous studies have shown that the hobnail variant of PTC is very aggressive and is associated with metastatic disease and a high rate of mortality.44,45 One of the two patients with hobnail variants of PTC positive for PRRX1 died of metastatic PTC. In the present study, in 6/35 cases of ATC there was PTC associated with ATC, which supports earlier observations that ATC may sometimes arise by dedifferentiation from well-differentiated thyroid carcinomas.46–49 The higher rate of positivity of the ATCs for PRRX1 may be associated with this dedifferentiation and progression from PTCs and FCs to ATCs. An alternative hypothesis is that some ATCs arise from cancer stem cells that give rise directly to ATCs, as suggested by Takano.50 However, based on the available evidence from morphological studies and from analysis of the CSC population in ATCs,5 both of these possible mechanisms may be important in the development of ATCs.
Conventional PTCs and their corresponding metastases to lymph nodes were negative for PRRX1; however, the one ATC and the matching metastases to lymph nodes were positive for PRRX1. Thus, interestingly, there was no change in PRRX1 expression in lymph node metastases in this small set of carcinomas. By contrast, Ocaña et al20 suggested a decrease in PRRX1 from the highest levels, observed in primary tumors during EMT, to lower levels in metastatic colonization in their metastatic models.
The differences between our observations in primary PTCs and the two PTC cell lines may be related to the long period of time that the cell lines have been in culture, with changes in the regulation of gene expression during this period. However, our observation that TGF-β1 stimulates up-regulation of PRRX1 (as well as other EMT markers, such as Snail and Slug) indicates that this is an excellent system for study of the mechanism of PRRX1 regulation in thyroid carcinomas. Further studies will be needed with primary and early and late metastatic thyroid carcinomas to determine the fate of PRRX1 during metastasis and to determine whether PRRX1 expression changes during EMT and during mesenchymal-to-epithelial transition in the process of colonization in thyroid carcinomas.
In studies using primary PTCs, Swierniak et al51 recently reported that almost all miRNAs exhibited isoforms of variable length and potentially distinct functions in thyroid tumorigenesis and that miR-146b-5p was the most highly deregulated miRNA. Analysis of miR-146b in primary PTCs has shown that this miRNA is highly expressed in more aggressive carcinomas.52 In the present study, miR-146b-5p expression increased 10- to 20-fold transiently during TGF-β1–induced EMT in the PTC cell lines at approximately day 14 of TGF-β1 treatment. The transient increase in miR-146b-5p observed in our PTC cell lines suggests that there might be an initial increase in this miRNA as an attempt to inhibit the EMT program. Both our present findings, that cell proliferation and invasion increased when the expression of miR-146b-5p was inhibited in the siRNA experiments, and the findings of Geraldo et al,24 that TGF-β1 had an inhibitory effect of miR-146b-5p translocation of SMAD4 to the cell nucleus, support this hypothesis. Furthermore, we observed a significant down-regulation of miR-146b-5p expression in the more aggressive, highly proliferative TGF-β1–treated xenograft tumors (Supplemental Figure S5A). Nonetheless, other factors regulating changes in miR-146b-5p will have to be elucidated in future experiments. Additionally, it has been shown that miR-146 inhibits EGFR and CXCR4 expression, which have a positive effect on tumor metastasis in breast carcinoma.28 EGFR and CXCR4 expression are also associated with thyroid cancer progression, with up-regulation of EGFR in undifferentiated thyroid cancers.53 In our studies of EGFR expression in the xenografted thyroid carcinomas derived from TPC-1, there was a significant increase in the expression of EGFR and c-MET in the more rapidly growing TGF-β1–treated tumors (Supplemental Figure S5B), which supports their role in thyroid cancer progression.
In summary, the PTC cell lines TPC-1 and BCPAP undergo EMT under the influence of TGF-β1 in vitro, which leads to increased tumor growth in xenografts and an increase in CSC features in vitro and in vivo. After 10 weeks of growth in vivo, the TGF-β1–treated tumors expressed higher levels of EMT biomarkers, compared with control tumors. Expression of the paired-related homeobox 1 transcription factor PRRX1 was increased in thyroid PTC cell lines by TGF-β1 treatment and was also associated with more aggressive thyroid cancers. Finally, miR-146-5p has an important role in regulating PTC cell proliferation and invasion, but the regulation of these changes remains to be elucidated.
Acknowledgments
We thank Dr. Rebecca E. Schweppe (University of Colorado, Denver, CO) for the BCPAP cell line, Dr. Daniel T. Ruan (Brigham and Women’s Hospital, Boston, MA) for the TPC-1 cell line, Dr. John A. Copland, III (Mayo Clinic, Jacksonville, FL) for the THJ-16T and THJ-21T cell lines, staff of the Translational Research in Pathology (TRIP) Laboratory (University of Wisconsin School of Medicine and Public Health) for TMA construction, and staff of the Flow Cytometry, 3P, and Experimental Pathology laboratories (University of Wisconsin School of Medicine and Public Health) for their services.
Footnotes
Supported by NIH grant R01-CA121115 (H.C.) and American Cancer Society MEN2 Thyroid Cancer Professorship (H.C.), by start-up funding from the Department of Pathology (R.V.L.), and by NIH Cancer Center Support Grant P30-CA014520-39 to the University of Wisconsin Carbone Cancer Center.
Disclosures: None declared.
Supplemental Data
TGF-β1 up-regulates EMT markers in BCPAP cells. BCPAP cells were cultured in serum-free medium with or without 2 ng/mL TGF-β1 and were assayed by RT-qPCR for EMT markers on days 8 and 20. TGF-β1 treatment significantly reduced the expression of E-cadherin, along with a significant up-regulation of Slug and Snail. TGF-β1 treatment significantly increased Twist expression on day 8, but levels were closer to the untreated condition by day 20. Vimentin expression increased slightly, but this did not reach statistical significance. Data are expressed as means ± SEM for two experiments. ∗P < 0.05, ∗∗P < 0.01.
In vitro TGF-β1 treatment of BCPAP cells increases tumorigenicity. TGF-β1 treatment produced larger tumors than control medium–treated cells. BCPAP cells cultured in 10% fetal bovine serum did not form tumors in this time period. Data are expressed as means ± SEM. n = 3 per group.
miR-146b-5p siRNA is effective in knocking down miRNA expression. At day 14, TGF-β1–treated and control cells were transfected with siRNA to miR-146b-5p (Figure 7). RT-qPCR results indicated a marked knockdown of miR-146b-5p expression by the siRNA at a concentration of 10 nmol/L. Data are expressed as means ± SEM.
miR-146b-5p inhibits growth of PTC cell lines. Day 14 TGF-β1–treated and control TPC-1 cells were transfected with siRNA to miR-146b-5p from Sigma-Aldrich comprising a different sequence pool than the Exiqon siRNA. Similar results were seen for both siRNA pools in both TPC-1 and BCPAP cell lines. Data are expressed as means ± SEM.
TGF-β1 treatment on TPC-1 cells reduces miR-146b-5p expression and induces c-MET and EGFR in xenograft tumors. A: RT-qPCR indicated a significant down-regulation of miR-146b-5p expression in TGF-β1–treated xenograft tumors. B: TGF-β1–treated TPC-1 cells significantly up-regulated c-MET and EGFR in tumors formed in nude mice. Data are expressed as means ± SEM for two experiments performed in duplicate. ∗P < 0.05; ∗∗P < 0.01.
References
- 1.Sipos J.A., Mazzaferri E.L. Thyroid cancer epidemiology and prognostic variables. Clin Oncol (R Coll Radiol) 2010;22:395–404. doi: 10.1016/j.clon.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Sherman S.I., Brierley J.D., Sperling M., Ain K.B., Bigos S.T., Cooper D.S., Haugen B.R., Ho M., Klein I., Ladenson P.W., Robbins J., Ross D.S., Specker B., Taylor T., Maxon H.R., 3rd Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–1021. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Kondo T., Ezzat S., Asa S.L. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 4.Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Todaro M., Iovino F., Eterno V., Cammareri P., Gambara G., Espina V., Gulotta G., Dieli F., Giordano S., De Maria R., Stassi G. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 2010;70:8874–8885. doi: 10.1158/0008-5472.CAN-10-1994. [DOI] [PubMed] [Google Scholar]
- 6.Davies T.F., Latif R., Minsky N.C., Ma R. Clinical review: the emerging cell biology of thyroid stem cells. J Clin Endocrinol Metab. 2011;96:2692–2702. doi: 10.1210/jc.2011-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin R.Y. Thyroid cancer stem cells. Nat Rev Endocrinol. 2011;26:609–616. doi: 10.1038/nrendo.2011.127. [DOI] [PubMed] [Google Scholar]
- 8.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin N., Campbell L.L., Polyak K., Brisken C., Yang J., Weinberg R.A. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J., Weinberg R.A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Vasko V., Espinosa A.V., Scouten W., He H., Auer H., Liyanarachchi S., Larin A., Savchenko V., Francis G.L., de la Chapelle A., Saji M., Ringel M.D. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci USA. 2007;104:2803–2808. doi: 10.1073/pnas.0610733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janda E., Lehmann K., Killisch I., Jechlinger M., Herzig M., Downward J., Beug H., Grünert S. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheel C., Eaton E.N., Li S.H., Chaffer C.L., Reinhardt F., Kah K.J., Bell G., Guo W., Rubin J., Richardson A.L. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaki-Yumoto M., Katsuno Y., Derynck R. TGF-[beta] family signaling in stem cells. Biochim Biophys Acta. 2013;1830:2280–2296. doi: 10.1016/j.bbagen.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peinado H., Olmeda D., Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 15.Buehler D., Hardin H., Shan W., Montemayor-Garcia C., Rush P.S., Asioli S., Chen H., Lloyd R.C. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol. 2013;26:54–61. doi: 10.1038/modpathol.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salerno P., Garcia-Rostan G., Piccinin S., Bencivernga T.C., Di Maro G., Doglioni C., Basolo F., Maestro R., Fusco A., Santoro M., Salvatore G. TWIST1 plays a pleiotropic role in determining the anaplastic thyroid cancer phenotype. J Clin Endocrinol Metab. 2011;96:E772–E781. doi: 10.1210/jc.2010-1182. [DOI] [PubMed] [Google Scholar]
- 17.Kim D.W., Walker R.L., Meltzer P.S., Cheng S.Y. Complex temporal changes in TGFbeta oncogenic signaling drive thyroid carcinogenesis in a mouse model. Carcinogenesis. 2013;34:2389–2400. doi: 10.1093/carcin/bgt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimozaki K., Clemenson G.D., Jr., Gage F.H. Paired related homeobox protein 1 is a regulator of stemness in adult neural stem/progenitor cells. J Neurosci. 2013;33:4066–4075. doi: 10.1523/JNEUROSCI.4586-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Susa T., Kato T., Yoshida S., Yako H., Higuchi M., Kato Y. Paired-related homeodomain proteins Prx1 and Prx2 are expressed in embryonic pituitary stem/progenitor cells and may be involved in the early stage of pituitary differentiation. J Neuroendocrinol. 2012;24:1201–1212. doi: 10.1111/j.1365-2826.2012.02336.x. [DOI] [PubMed] [Google Scholar]
- 20.Ocaña O.H., Córcoles R., Fabra A., Moreno-Bueno G., Acloque H., Vega S., Barrallo-Gimeno A., Cano A., Nieto M.A. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Reichert M., Takano S., von Burstin J., Kim S.B., Lee J.S., Ihida-Stansbury K., Hahn C., Heeg S., Schneider G., Rhim A.D., Stanger B.Z., Rustigi A.K. The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis. Genes Dev. 2013;27:288–300. doi: 10.1101/gad.204453.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi Y., Sawada G., Kurashige J., Uchi R., Matsumura T., Ueo H., Takano Y., Akiyoshi S., Eguchi H., Sudo T., Sugimachi K., Doki Y., Mori M., Mimori K. Paired related homoeobox 1, a new EMT inducer, is involved in metastasis and poor prognosis in colorectal cancer. Br J Cancer. 2013;109:307–311. doi: 10.1038/bjc.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 24.Geraldo M.V., Yamashita A.S., Kimura E.T. MicroRNA miR-146b-5p regulates signal transduction of TGF-β by repressing SMAD4 in thyroid cancer. Oncogene. 2012;31:1910–1922. doi: 10.1038/onc.2011.381. [DOI] [PubMed] [Google Scholar]
- 25.Nikiforova M.N., Tseng G.C., Steward D., Diorio D., Nikiforov Y.E. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93:1600–1608. doi: 10.1210/jc.2007-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pallante P., Visome R., Croce C.M., Fusco A. Deregulation of microRNA expression in follicular-cell-derived human thyroid carcinomas. Endocr Relat Cancer. 2010;17:F91–F104. doi: 10.1677/ERC-09-0217. [DOI] [PubMed] [Google Scholar]
- 27.Liao Y., Zhang M., Lönnerdal B. Growth factor TGF-beta induces intestinal epithelial cell (IEC-6) differentiation: miR-146b as a regulatory component in the negative feedback loop. Genes Nutr. 2013;8:69–78. doi: 10.1007/s12263-012-0297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurst D.R., Edmonds M.D., Scott G.K., Benz C.C., Vaidya K.S., Welch D.R. Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res. 2009;69:1279–1283. doi: 10.1158/0008-5472.CAN-08-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaidya K.S., Harihar S., Phadke P.A., Stafford L.J., Hurst D.R., Hicks D.G., Casey G., DeWald D.B., Welch D.R. Breast cancer metastasis suppressor-1 differentially modulates growth factor signaling. J Biol Chem. 2008;283:28354–28360. doi: 10.1074/jbc.M710068200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elsarraj H.S., Stecklein S.R., Valdez K., Behbod F. Emerging functions of microRNA-146a/b in development and breast cancer: microRNA-146a/b in development and breast cancer. J Mammary Gland Biol Neoplasia. 2012;17:79–87. doi: 10.1007/s10911-012-9240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cicek M., Fukuyama R., Welch D.R., Sizemore N., Casey G. Breast cancer metastasis suppressor 1 inhibits gene expression by targeting nuclear factor-kappaB activity. Cancer Res. 2005;65:3586–3595. doi: 10.1158/0008-5472.CAN-04-3139. [DOI] [PubMed] [Google Scholar]
- 32.Pacifico F., Leonardi A. Role of NF-kappaB in thyroid cancer. Mol Cell Endocrinol. 2010;321:29–35. doi: 10.1016/j.mce.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Pacifico F., Crescenzi E., Mellone S., Iannetti A., Porrine N., Liguoro D., Moscato F., Grieco M., Formisano S., Leonardi A. Nuclear factor-{kappa}B contributes to anaplastic thyroid carcinoma through up-regulation of miR-146a. J Clin Endocrinol Metab. 2010;95:1421–1430. doi: 10.1210/jc.2009-1128. [DOI] [PubMed] [Google Scholar]
- 34.Katakowski M., Buller B., Zheng X., Lu Y., Rogers T., Osobamiro O., Sju W., Jiang F., Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katakowski M., Zheng X., Jiang F., Rogers T., Szalad A., Chopp M. miR-146b-5p suppresses EGFR expression and reduces in vitro migration and invasion of glioma. Cancer Invest. 2010;28:1024–1030. doi: 10.3109/07357907.2010.512596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marlow L.A., D’Innocenzi J., Zhang Y., Rohl S.D., Cooper S.J., Sebo T., Grant C., McIver B., Kasperbauer J.L., Wadswart J.T., Casier J.D., Kennedy P.W., Highsmith W.G., Clark O., Milosevic D., Netzel B., Cradic K., Arora S., Beaudy C., Grebe S.K., Silverberg M.L., Azorsa D.O., Smallridge R.C., Copland J.A. Detailed molecular fingerprinting of four new anaplastic thyroid carcinoma cell lines and their use for verification of RhoB as a molecular therapeutic target. J Clin Endocrinol Metab. 2010;95:5338–5347. doi: 10.1210/jc.2010-1421. [Errata appeared in J Clin Endocrinol Metab 2013, 98:4213 and J Clin Endocrinol Metab 2013, 98:4546] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fierabracci A., Puglisi M.A., Giuliani L., Mattarocci S., Gallinella-Muzi M. Identification of an adult stem/progenitor cell-like population in the human thyroid. J Endocrinol. 2008;198:471–487. doi: 10.1677/JOE-07-0552. [DOI] [PubMed] [Google Scholar]
- 38.Sippel R.S., Carpenter J.E., Kunnimalaiyaan M., Lagerholm S., Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastointest Liver Physiol. 2003;285:G245–G254. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 39.Hardy R.G., Vincente-Dueñas C., González-Herrero I., Anderson C., Flores T., Hughes S., Tselepis C., Ross J.A., Sánchez-García I. Snail family transcription factors are implicated in thyroid carcinogenesis. Am J Pathol. 2007;171:1037–1046. doi: 10.2353/ajpath.2007.061211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knauf J.A., Ma X., Smith E.P., Zhang L., Mitsutake N., Lian X.H., Refetoff S., Nikiforov Y.E., Fagin J.A. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;65:4238–4245. doi: 10.1158/0008-5472.CAN-05-0047. [DOI] [PubMed] [Google Scholar]
- 41.Yasui K., Shimamura M., Mitsutake N., Nagayama Y. SNAIL induces epithelial-to-mesenchymal transition and cancer stem cell-like properties in aldehyde dehydroghenase-negative thyroid cancer cells. Thyroid. 2013;23:989–996. doi: 10.1089/thy.2012.0319. [DOI] [PubMed] [Google Scholar]
- 42.Braun J., Hoang-Vu C., Dralle H., Hüttelmaier S. Downregulation of microRNAs direct the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 43.Scheel C., Weinberg R.A. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asioli S., Erickson L.A., Sebo T.J., Zhang J., Jin L., Thompson G.B., Lloyd R.V. Papillary thyroid carcinoma with prominent hobnail features: a new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical and molecular study of eight cases. Am J Surg Pathol. 2010;34:44–52. doi: 10.1097/PAS.0b013e3181c46677. [DOI] [PubMed] [Google Scholar]
- 45.Lubitz C.C., Economopoulos K.P., Pawlak A.C., Dias-Santagata D., Faquin W.C., Sadow P.M. Hobnail variant of papillary thyroid carcinoma: an institutional case series and molecular profile. Thyroid. 2014;24:958–965. doi: 10.1089/thy.2013.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quiros R.M., Ding H.G., Gattuso P., Prinz R.A., Xiulong X. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103:2261–2268. doi: 10.1002/cncr.21073. [DOI] [PubMed] [Google Scholar]
- 47.Nikiforova M.N., Kimura E.T., Gandhi M., Biddinger P.W., Knauf J.A., Basolo F., Zhu Z., Giannini R., Salvatore G., Fusco A., Santoro M., Fagin J.A., Nikiforov Y.E. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 48.Albores-Saavedra J., Hernandez M., Sanchez-Sosa S., Simpson K., Angeles A., Hanson D.E. Histologic variants of papillary and follicular carcinomas associated with anaplastic spindle and giant cell carcinomas of the thyroid: an analysis of rhabdoid and thyroglobulin inclusions. Am J Surg Pathol. 2007;31:729–736. doi: 10.1097/01.pas.0000213417.00386.74. [DOI] [PubMed] [Google Scholar]
- 49.Begum S., Rosenbaum E., Henrique R., Cohen Y., Sidransky D., Westra W.H. BRAF mutations in anaplastic thyroid carcinoma: implications for tumor origin, diagnosis and treatment. Mod Pathol. 2004;17:1359–1363. doi: 10.1038/modpathol.3800198. [DOI] [PubMed] [Google Scholar]
- 50.Takano T. Fetal cell carcinogenesis of the thyroid: a modified theory based on recent evidence. Endocr J. 2014;61:311–320. doi: 10.1507/endocrj.ej13-0517. [My Opinion] [DOI] [PubMed] [Google Scholar]
- 51.Swierniak M., Wojcicka A., Czetwertynska M., Stachlewska E., Maciag M., Wiechno W., Gornicka B., Bogdanska M., Koperski L., de la Chapelle A., Jazdzewski K. In-depth characterization of the microRNA transcriptome in normal thyroid and papillary thyroid carcinoma. J Clin Endocrinol Metab. 2013;98:E1401–E1409. doi: 10.1210/jc.2013-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chou C.K., Yang K.D., Chou F.F., Huang C.C., Lan Y.W., Lee Y.F., Kang H.Y., Liu R.T. Prognostic implications of miR-146b expression and its functional role in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2013;98:E196–E205. doi: 10.1210/jc.2012-2666. [DOI] [PubMed] [Google Scholar]
- 53.Landriscina M., Pannone G., Piscazzi A., Toti P., Fabiano A., Tortorella S., Occhini R., Ambrosi A., Bufo P., Cignarelli M. Epidermal growth factor receptor 1 expression is upregulated in undifferentiated thyroid carcinomas in humans. Thyroid. 2011;21:1227–1234. doi: 10.1089/thy.2011.0172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TGF-β1 up-regulates EMT markers in BCPAP cells. BCPAP cells were cultured in serum-free medium with or without 2 ng/mL TGF-β1 and were assayed by RT-qPCR for EMT markers on days 8 and 20. TGF-β1 treatment significantly reduced the expression of E-cadherin, along with a significant up-regulation of Slug and Snail. TGF-β1 treatment significantly increased Twist expression on day 8, but levels were closer to the untreated condition by day 20. Vimentin expression increased slightly, but this did not reach statistical significance. Data are expressed as means ± SEM for two experiments. ∗P < 0.05, ∗∗P < 0.01.
In vitro TGF-β1 treatment of BCPAP cells increases tumorigenicity. TGF-β1 treatment produced larger tumors than control medium–treated cells. BCPAP cells cultured in 10% fetal bovine serum did not form tumors in this time period. Data are expressed as means ± SEM. n = 3 per group.
miR-146b-5p siRNA is effective in knocking down miRNA expression. At day 14, TGF-β1–treated and control cells were transfected with siRNA to miR-146b-5p (Figure 7). RT-qPCR results indicated a marked knockdown of miR-146b-5p expression by the siRNA at a concentration of 10 nmol/L. Data are expressed as means ± SEM.
miR-146b-5p inhibits growth of PTC cell lines. Day 14 TGF-β1–treated and control TPC-1 cells were transfected with siRNA to miR-146b-5p from Sigma-Aldrich comprising a different sequence pool than the Exiqon siRNA. Similar results were seen for both siRNA pools in both TPC-1 and BCPAP cell lines. Data are expressed as means ± SEM.
TGF-β1 treatment on TPC-1 cells reduces miR-146b-5p expression and induces c-MET and EGFR in xenograft tumors. A: RT-qPCR indicated a significant down-regulation of miR-146b-5p expression in TGF-β1–treated xenograft tumors. B: TGF-β1–treated TPC-1 cells significantly up-regulated c-MET and EGFR in tumors formed in nude mice. Data are expressed as means ± SEM for two experiments performed in duplicate. ∗P < 0.05; ∗∗P < 0.01.