Abstract
β-Arrestin-1 (βArr1), a scaffolding protein critical in G-protein coupled receptor desensitization has more recently been found to be important in the pathogenesis of various inflammatory diseases. We sought to understand the role of βArr1 in sepsis pathogenesis using a mouse model of polymicrobial sepsis. Although in previous studies we established that βArr1 deficiency protects mice from endotoxemia, here we demonstrate that the absence of βArr1 remarkably renders mice more susceptible to mortality in polymicrobial sepsis. In accordance with the mortality pattern, early production of inflammatory mediators was markedly enhanced in βArr1 knockout mice systemically and locally in various organs. In addition, enhanced inflammation in the heart was associated with increased NFκB activation. Compared to these effects, immune cell infiltration, thymic apoptosis, and immune suppression during polymicrobial sepsis were unaffected by a deficiency of βArr1. Additionally, enhanced inflammation and consequent higher mortality were not observed in heterozygous mice, suggesting that one allele of βArr1 was sufficient for this protective negative regulatory role. We further demonstrate that, unexpectedly, βArr1 in nonhematopoietic cells is critical and sufficient for inhibiting sepsis-induced inflammation, whereas hematopoietic βArr1 is likely redundant. Taken together, our results reveal a novel and previously unrecognized negative regulatory role of the nonhematopoietic βArr1 in sepsis-induced inflammation.
Sepsis is a serious medical condition that to date incurs high mortality (approximately 30% to 50%) despite high expenditure in terms of patient care. Early detection, antibiotics, and life support to maintain organ homeostasis remain the only line of defense with no specific treatments available. Therefore, understanding the mechanistic basis of sepsis progression is critical in identifying potential therapeutic targets for future drug development. Among the various pathophysiological events that occur through sepsis progression, inflammation remains a double-edged sword for the host, with dysregulated proinflammatory phase causing tissue destruction1 and a prolonged immunosuppressed phase causing excessive microbial burden. A balanced inflammatory response protects patients from sepsis-induced morbidity and mortality. Therefore, elucidating mechanisms regulating inflammation is critical to our understanding of sepsis pathogenesis.
β-Arrestins (1 and 2), initially identified as being involved in G-protein coupled receptor (GPCR) desensitization are now known to have diverse array of roles in GPCR-dependent and -independent signaling.2 This gives arrestins an opportune foothold in various physiological functions and places them as important regulators of homeostasis. Arrestins' role in mediating inflammation stems from their ability to modulate chemotaxis,3 cytokine production, and signaling via noncanonical regulators of inflammation, such as beta-adrenergic,4 angiotensin,5 lipid, and other receptors. Additionally, they can act as scaffolding proteins for major signaling complexes, including mitogen-activated protein kinase (MAPK)6,7 and NFκB pathways.8,9 In vitro studies have demonstrated a negative regulatory role for β-arrestin-1 (βArr1) in Toll-like receptor-4 (TLR-4) and tumor necrosis factor receptor (TNFR) signaling.8,10,11 However, we demonstrated in previous studies that βArr1 is a critical mediator of inflammation and mortality in the endotoxemia model of sepsis.12 Consistent with that, other studies have also shown that βArr1 mediates the pathogenesis of various inflammatory diseases, such as rheumatoid arthritis,13 colitis,14 cancer,15 and multiple sclerosis.16,17
Despite the limitations of mouse models in replicating human sepsis, cecal ligation and puncture (CLP)-induced polymicrobial sepsis has been shown to encompass several immunopathological features of human sepsis.18 Based on a previously reported role of βArr1 in endotoxemia and other inflammatory models, we hypothesized that βArr1-deficient mice will be protected from polymicrobial sepsis-induced inflammation and mortality. Our studies, however, reveal a previously unappreciated negative regulatory role of βArr1 in polymicrobial sepsis and consequent mortality. We further demonstrate in this study that βArr1 in the nonhematopoietic compartment is required and sufficient to regulate polymicrobial sepsis-induced inflammation.
Materials and Methods
Animals
βarr1 knockout (KO) mice on a C57BL/6 background (provided by Dr. Robert Lefkowitz, Duke University) have been described earlier.12 Wild-type (WT) C57BL/6 mice were purchased from the National Cancer Institute, and all mice were bred or housed at Michigan State University (East Lansing, MI) in rooms maintained at 22°C to 24°C with 50% humidity and a 12-hour light–dark cycle. Mouse chow and water were provided ad libitum to all animals. All experiments were performed with age- and sex-matched mice between 8 and 12 weeks of age. Animal procedures were approved by Michigan State University Institutional Animal Care and Use Committee and conformed to NIH guidelines.19
CLP Surgery
Animals were subjected to CLP as described earlier.20 Briefly, mice were anesthetized using i.p. injection of 5 mg/kg xylazine and 80 mg/kg ketamine. The cecum was exteriorized, ligated, and punctured either once [single puncture (SP)] with a 16-guage needle (16G-SP) or twice [double puncture (DP)] with a 20-guage needle (20G-DP). The cecum was then reinserted and the peritoneal cavity sutured with 5.0 silk (Syneture; Covidian, Mansfield, MA). Sham surgery wherein the cecum was exteriorized, but neither ligated nor punctured, was used as control. All animals were given a s.c. injection of 1 mL of saline (prewarmed to 37°C) after the surgery. For mortality studies, mice were observed for 7 days after surgery.
Generation of Chimeric Mice
Chimeric mice were generated using lethal irradiation and bone marrow reconstitution. Briefly, mice were irradiated with a total dose of 11 gy (5.5 gy × 2, 3 hours apart) and 12 hours later injected with 5 × 106 bone marrow cells from a donor. Immediately after irradiation and reconstitution, mice were put on water with antibiotics (sulfamethoxazole and trimethoprim; Hi-Tech Pharmacal, Amityville, NY) for 4 weeks, and mice were used for experiments 8 weeks after reconstitution.
Sample Processing
At a predetermined time of harvesting, mice were euthanized using carbon dioxide asphyxiation. Peritoneal fluid, plasma, and organs were harvested and processed as previously stated.21 Briefly, the peritoneal cavity was flushed with R10 medium (RPMI 1640 with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 55 μmol/L β-mercaptoethanol), the cells collected and then processed for fluorescence-activated cell-sorting analysis. The first peritoneal wash was done in 4 mL of medium, and the supernatant was saved for further analysis. The cavity was then lavaged twice with 10 mL of medium, and cells from all washes were collected and counted for further analysis. Blood was centrifuged at 300 × g for 5 minutes, and the supernatant was stored at −80°C for enzyme-linked immunosorbent assay (ELISA). The organs were harvested, flash frozen, and then stored at −80°C. Spleen was crushed, subjected to red blood cell lysis, and then filtered through 40-μm nylon mesh. For further stimulations, cells were counted, resuspended in R10 at a concentration of 10 × 106 cells/mL, and then incubated at 37°C for 18 hours with or without lipopolysaccharide (LPS; 100 ng/mL). For flow cytometric analysis, 2 × 106 cells were used and processed. Thymus was processed similar to the splenocytes, and samples were prepared for cytometric analysis. For bronchoalveolar lavage collection, the thoracic cavity was opened, and the trachea was cannulated and then secured with ligation. The bronchoalveolar space was lavaged three times using R10 medium, and the cells pooled from the three washes for cytometric analysis.
Flow Cytometry
Peritoneal, bronchoalveolar-lavage, spleen, and thymus cells collected from septic mice were processed as described above. They were then stained with an antibody cocktail made in 2.4G2 supernatant fcγ-receptor blocking antibody to block nonspecific binding, and then washed with staining buffer (phosphate-buffered saline with sodium azide and bovine calf serum). The antibodies against cell-surface markers CD11b, F4/80, granulocyte-differentiation antigen (Gr-1), CD3, CD19, CD4, and CD8 were obtained from eBiosciences (San Diego, CA) and used as per the manufacturer's instructions. Cells were run on a BD LSR II flow cytometer (BD Biosciences, San Jose, CA), and data were analyzed using FlowJo software version 10.0 (TreeStar, Ashland, OR). Neutrophils were gated as CD3−CD19−CD11b+Gr-1+ cells, macrophages as CD3−CD19−CD11b+F4/80+ cells, and T cells as CD19−CD3+ cells. T cells were further marked as CD4+ (T helper), CD8+ (cytotoxic), and CD4+CD8+ (double-positive) T cells based on CD4 and CD8 expression.
Cytokine/Chemokine Measurements
Cytokines were measured from plasma, splenic culture supernatant, and peritoneal fluid using ELISA kits from eBiosciences, as per the manufacturer's protocol. To pool the data from multiple bone-marrow transfer experiments, the raw values were converted to fold change over the average WT concentrations for each experiment.
Bacterial Counts
Bacterial load was determined in peritoneal fluid and blood. Briefly, the sample was serially diluted and plated on Difco Mueller Hinton agar plates (Becton, Dickinson and Company, Franklin Lakes, NJ). The plates were then incubated at 37°C for 24 hours, and the number of colony-forming units (CFU) were counted and recorded.
Preparation of Polymicrobial Culture
Polymicrobial culture was obtained as described previously.21 Briefly, cecal contents collected from WT mice were inoculated in sterile medium (BD Bacto Brain Heart Infusion; Becton, Dickinson and Company) and cultured at 37°C with 220 rpm shaking for 18 hours. The contents were then centrifuged at 432 × g for 10 minutes; the bacterial pellet was resuspended in 40% glycerol and then stored at −80°C. For CFU measurements, 100 μL of the polymicrobial culture was inoculated in 100 mL of medium and grown for 14 hours, washed with phosphate-buffered saline, and then plated on Mueller Hinton agar (Becton, Dickinson and Company) plates using serial dilution. Once the CFU/mL for the culture was determined, it was diluted to obtain the required CFU count for the experiments.
Bacterial Killing Assay
Intracellular and total killing assays were performed using thioglycollate-elicited neutrophils. To obtain neutrophils, mice were injected with 1 mL of thioglycollate i.p., and 4 hours later, cells were collected using peritoneal lavage. Escherichia coli (ATCC 25922; ATCC, Manassas, VA) was cultured in tryptic soy broth overnight, and a secondary culture was started from it for the assay. Four hours later, the culture was spun at 5000 rpm for 15 minutes, washed twice with sterile PBS, and the expected CFU count was calculated based on the optical density value and previously determined growth curve. It was then opsonized for 1 hour at 37°C with heat-inactivated serum (55°C for 1 hour) with mild shaking (100 rpm). Neutrophils and bacteria were mixed at a multiplicity of infection of 1:5 and incubated at 37°C with mild shaking (100 rpm). For the total killing assay, a bacteria-alone control was set up as well, and at indicated times, both control and experimental groups were serially diluted and plated to obtain CFU counts. For the intracellular killing assay, 10 μg/mL gentamicin was added to kill extracellular bacteria 20 minutes later. Following gentamycin treatment (20 minutes), the cells were washed in phosphate-buffered saline to remove gentamicin and then incubated for the indicated time periods at optimum conditions. At the end of each time period, the cells were lysed in 0.1% Triton X-100, serially diluted, and then plated to obtain CFU counts.
Quantitative RT-PCR
To determine the relative levels of a specific RNA transcript, RNA was isolated from snap-frozen tissue using an RNeasy mini kit (Qiagen, Valencia, CA) using the manufacturers' protocol. Reverse transcription was performed with 1 μg of RNA using a cDNA synthesis kit (Promega, Madison, WI). Quantitative RT-PCR was performed with an ABI 7500 Fast Real-Time PCR System (Applied Biosystems; Life Technologies, Foster City, CA), and all genes were normalized to HPRT as previously described.21 Primer sequences are provided in Table 1. Data were normalized to WT for all genes.
Table 1.
Primer Sequences Used in qPCR Analysis
| Primer/gene name | Forward primer | Reverse primer |
|---|---|---|
| TNFA | 5′-TCTCATCAGTTCTATGGCCC-3′ | 5′-GGGAGTAGACAAGCTACAAC-3′ |
| IL6 | 5′-ACAAGTCGGAGGCTTAATTACACAT-3′ | 5′-TTGCCATTGCACAACTCTTTTC-3′ |
| NFKBIA | 5′-TGGCCAGTGTAGCAGTCTTG-3′ | 5′-GACACGTGTGGCCATTGTAG-3′ |
| NOS2 | 5′-TCTTTGACGCTCGGAACTGTAGCA-3′ | 5′-ACCTGATGTTGCCATTGTTGGTGG-3′ |
| NOS3 | 5′-CTGCTGCCCGAGAATATCTTC-3′ | 5′-CTGGTACTGCAGTCCCTCCT-3′ |
| ICAM | 5′-GGCACCCAGCAGAAGTTGTT-3′ | 5′-GCCTCCCAGCTCCAGGTATAT-3′ |
| VCAM | 5′-GGAGAGACAAAGCAGAAGTGGAA-3′ | 5′-ACAACCGAATCCCCAACTTG-3′ |
| SERPINE1 | 5′-GGCACAGTGGCGTCTTCCT-3′ | 5′-TGCCGAACCACAAAGAGAAAG-3′ |
| F3 coagulation factor III | 5′-CATGGAGACGGAGACCAACT-3′ | 5′-CCATCTTGTTCAAACTGCTGA-3′ |
| PROCR | 5′-AGCGCAAGGAGAACGTGT-3′ | 5′-GGGTTCAGAGCCCTCCTC-3′ |
qPCR, quantitative PCR.
Western Blotting
Snap-frozen heart tissue was homogenized in lysis buffer [20 mmol/L Tris-HCl (pH 7.4), 1 mmol/L EDTA, 150 mmol/L NaCl] containing 1% Triton X-100 and protease and phosphatase inhibitors. Homogenized tissue was spun at 15000 × g for 10 minutes at 4°C. Protein concentration of the supernatant was determined using the Bradford method. Western blots were performed as previously described.21 Briefly, equivalent concentrations of protein samples were run on polyacrylamide gels and then transferred to nitrocellulose membranes. Primary antibodies used were phospho-extracellular-signal-regulated kinase (p-ERK) (9101L; Cell Signaling Technology, Danvers, MA), ERK2 (Sc-1647; Santa Cruz Biotechnology, Santa Cruz, CA), phosphorylated inhibitor of NF-κB-alpha (pIκBα) (9246S; Cell Signaling Technology), and IκBα (9242S; Cell Signaling Technology), and secondary (anti-rabbit or anti-mouse) antibodies were bought from Invitrogen (Life Technologies, Carlsbad, CA) or LI-COR (Lincoln, NE). Blots were probed with the primary antibody followed by IR-dye/horseradish peroxidase-conjugated secondary antibody and then scanned. Bands were quantified using a LI-COR Odyssey scanner or ImageJ software version 1.46r (NIH, Bethesda, MD). For data analysis, pERK1/2 was normalized to ERK2, and pIκBα to IκBα, as loading controls.
Caspase Activity
Caspase 3 activity was assessed in thymocytes using the fluorescence assay described earlier.22 Briefly, thymocytes were lysed in CHAPS buffer (50 mmol/L HEPES, 0.1% CHAPS, 1 mmol/L dithiothreitol, 0.1 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, and 10 μg/mL leupeptin). The cell lysate was quantified, and 10 μg protein was incubated with a fluorescent substrate (Ac-DEVD-AFC; Enzo Life Sciences, Farmingdale, NY) in assay buffer [100 mmol/L HEPES, 10% sucrose, 0.1% CHAPS, and 10 mmol/L EDTA (pH 7.4)]. Fluorescence of the cleaved product was measured (excitation at 400 nm and emission at 505 nm) using a Tecan SpectraFluor Plus fluorescence plate reader (M1000 infinite, Tecan, Switzerland). Data were analyzed and presented as picograms of cleaved AFC fluorophore per milligram protein per minute, calculated using standard curve of free AFC.
Phagocytosis and ROS Potential
Cells harvested from septic mice 12 hours after surgery were used as the source of neutrophils for phagocytic potential and reactive oxygen species (ROS) generation. Briefly, the peritoneal cavity was lavaged with R2 media (RPMI 1640 with 2% fetal bovine serum), and cells counted for the assay. For phagocytosis, pHrodo E. coli BioParticle conjugate (Invitrogen; Life Technologies) was used as described in the manufacturer's manual. Cells (1 × 105) were incubated with bioparticles for 30 minutes at either 37°C (experimental) or 4°C (control), and the reaction stopped by washing with cold fluorescence-activated cell-sorting wash buffer. For ROS detection, 1 × 105 cells were preloaded with 5 mmol/L DHR-123 dye (Invitrogen; Life Technologies) and stimulated with phorbol myristate acetate (1 ng/mL). The cells were stained with Gr-1 antibodies to detect neutrophils and the increase in mean fluorescence intensity over control, recorded as phagocytic potential and ROS generation, respectively.
Histopathology
Liver, kidney, spleen, and lung tissues were collected from mice subjected to CLP, and fixed in 10% formalin overnight. The tissues were then embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The hallmarks of injury and inflammation (infiltration) were assessed by a board-certified pathologist (P.C.L) in a blinded manner.
Statistical Analysis
All experimental data in the figures are expressed as means ± SEM and analyzed using GraphPad Prism Software version 5 (La Jolla, CA). Each n represents an individual mouse. The Student's t-test (for comparing groups with equal variances) or Mann-Whitney U-test (for comparing groups with unequal variances) was used to compare two experimental groups. Differences in the survival were determined using the log-rank test. P < 0.05 was considered statistically significant.
Results
βArr1 Inhibits Sepsis-Induced Mortality and Inflammation
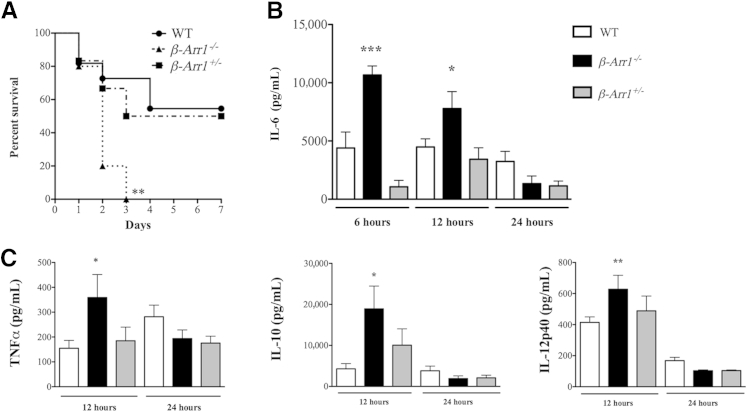
To assess the importance of βArr1 in modulating progression of sepsis, we subjected WT and βArr1 KO mice to CLP surgery (16G-SP) and followed their survival over the course of 7 days. We also included βArr1 heterozygous (HET) mice to assess the gene dosage effect of βArr1 in sepsis-induced mortality. In contrast to the role of βArr1 in endotoxemia-induced mortality, we observed that all of the KO mice succumbed to sepsis within 3 days of surgery, whereas only approximately 45% of the WT mice died (P = 0.0032) (Figure 1A). Interestingly, HET mice had a mortality rate similar to WT mice, suggesting that one allele of βArr1 is sufficient to inhibit sepsis-induced mortality (Figure 1A). Because all of the KO mice died within 3 days of surgery, we hypothesized that the deaths likely were consequent to an early dysregulated inflammatory response.23–25 Given that plasma cytokine levels, especially IL-6, at an early time point are predictive of mortality in this model, we assessed IL-6 production in response to the septic insult. Associated with accelerated mortality, plasma IL-6 levels were significantly elevated in septic KO mice at both 6 and 12 hours after sepsis compared to the WT (Figure 1B). At 24 hours after sepsis, IL-6 levels decreased significantly and were similar between the WT and KO mice. Similarly, tumor necrosis factor α (TNFα), IL-12p40, and IL-10 levels were also significantly elevated in septic KO mice as compared to the WT 12 hours after CLP and decreased to WT levels by 24 hours after sepsis (Figure 1C). This exaggerated early cytokine response was not observed in septic HET mice, consistent with their mortality being similar to that of WT.
Figure 1.
Role of β-arrestin-1 (βArr1) in sepsis-induced mortality and inflammation. A: Wild-type (WT), βarr1 knockout (KO) (β-Arr1−/−), and βArr1 heterozygous (β-Arr1+/−) mice were subjected to 16-guage needle single-puncture surgery and observed for mortality over 7 days. B and C: Mice from the different genotypes were subjected to cecal ligation and puncture as indicated in A, and plasma cytokine concentrations in septic mice determined at the indicated time points after surgery. n = 10 to 12 mice for each genotype (A); n = 8 to 14, with data pooled from at least two independent experiments (B and C). Error bars on the figure denote SEM. ∗∗P < 0.01 compared to WT by log-rank (Mantel Cox) test (A); ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 compared to WT using t-test (B and C).
Regulation of Bacterial Clearance and Cellular Infiltration by βArr1
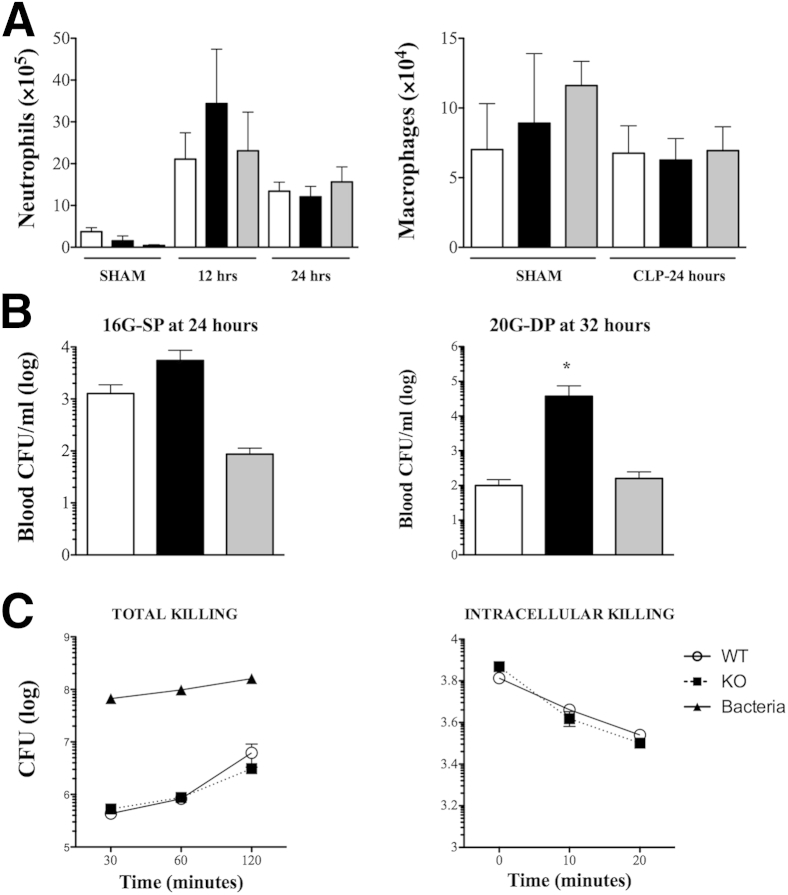
Cellular infiltration into the site of injury and bacterial clearance are critical factors impacting both inflammation and mortality26,27 and could potentially contribute to a hyperinflammatory phenotype in septic KO mice. Examination of cellular infiltrate into the peritoneal cavity revealed marked neutrophil infiltration in response to septic insult, but no significant difference between the three genotypes at both 12 and 24 hours after surgery (Figure 2A). In this grade of sepsis, peritonitis did not induce an increase in macrophage numbers at the site of infection even by 24 hours, which was nonetheless similar between the genotypes (Figure 2A). This suggests that cellular infiltration to the site of infection is likely not regulated by βArr1.
Figure 2.
Role of β-arrestin-1 (βArr1) in cellular infiltration and bacterial killing. Wild-type (WT; white bars), βArr1 knockout (KO; β-Arr1−/−; black bars), and βArr1 heterozygous (β-Arr1+/−; gray bars) mice underwent sham or cecal ligation and puncture surgeries and were euthanized at the defined time points. A: Neutrophil and macrophage infiltration in the peritoneal cavity of sham mice at 24 hours and septic mice at indicated time points after surgery were determined using flow cytometry. B: Bacterial load was represented as colony-forming units (CFU)/mL in the blood of septic mice at the indicated time points and grades of sepsis. C: Bacterial killing capacity of thioglycollate-elicited neutrophils from WT and KO mice was depicted as surviving bacteria (CFU) recovered from the extracellular medium at the indicated time points for total bacterial killing, and from cellular lysate at various time points after 20 minutes uptake for the intracellular killing assay. Data for septic mice were pooled from three independent experiments. n = 9 to 14 for septic mice and n = 3 for sham (B); n = 3 to 5 (C). Error bars denote SEM. ∗P < 0.05 using Student's t-test. 16G-SP, 16-guage needle single puncture; 20G-DP, 20-guage needle double puncture.
Interestingly, bacterial load in blood, too, was similar between WT and KO septic animals subjected to 16G-SP (Figure 2B). Bacterial load, however, was higher in the septic KO mice subjected to 20G-DP, suggesting that the role of βArr1 in bacterial clearance in vivo is perhaps dependent on the severity of sepsis induction (Figure 2B).28 HET mice, however, had bacterial loads similar to septic WT mice in both CLP models. To probe the potential role of βArr1 in bactericidal activity independent of the confounding effect of ensuing inflammation, we examined the ability of thioglycollate-elicited neutrophils from WT and KO mice to kill bacteria in vitro. As shown, we did not observe any role for βArr1 in the intracellular or total in vitro bacterial killing assays (Figure 2C). It is possible that even though βArr1 KO neutrophils do not seem to have any apparent defect in their ability to perform efficient bacterial killing, βArr1 might differentially regulate the effect of inflammation on bactericidal activity. It must be noted that in both models (16G-SP and 20G-DP), early levels of plasma IL-6 and IL-10 were significantly higher in septic KO mice as compared to the WT (Figure 1 and Supplemental Figure S1A), even though bacterial clearance was differentially affected (Figure 2B). Additionally, peritoneal infiltration of neutrophils and macrophages was similar between the septic WT and βArr1 KO animals in both grades of sepsis (Figure 2 and Supplemental Figure S1B). Thus, the exacerbated systemic cytokine levels in the septic KO mice are likely independent of systemic bacterial load or cellular infiltration to the site of infection.
βArr1 Inhibits Tissue Inflammation
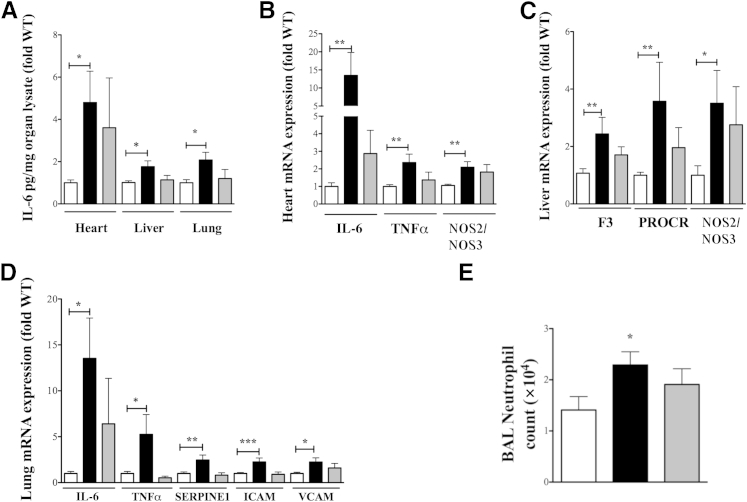
Inflammatory mediators induced by septic insult are capable of inflicting host tissue damage due to excessive/injudicious production.1,29 Even though histopathological lesions of tissue damage are not evident in early sepsis in major tissues including lung and liver,30,31 mortality is consequent to multiple organ dysfunction in sepsis. Given the mortality pattern of septic KO mice, we hypothesized that expression of inflammatory mediators (associated with organ dysfunction) would be higher in septic KO mice. Similar to plasma, IL-6 levels were significantly higher in heart, liver, and lung of septic KO as compared to WT mice 12 hours after CLP (Figure 3A). In addition to assessing IL-6 protein levels, we also examined the mRNA expression of several inflammatory mediators in various tissues as follows:
Figure 3.
Role of β-arrestin-1 (βArr1) in sepsis-induced organ inflammation. A: Wild-type (WT; white bars), βArr1 knockout (β-Arr1−/−; black bars), and βArr1 heterozygous (β-Arr1+/−; gray bars) mice were subjected to 16G-single puncture surgery, and the indicated organs were collected at defined time points for analysis. IL-6 levels (pg/mg) in organ lysates (determined by ELISA) from septic mice 12 hours after surgery. B–D: Quantitative real-time PCR analysis of inflammatory mediators in heart (B), liver (C), and lung tissue (D) from septic mice 12 hours after surgery. E: Total number of neutrophils as determined by flow cytometry isolated from bronchoalveolar lavage of septic mice 24 hours after surgery. mRNA expression was normalized to HPRT before converting to fold WT. Protein and RNA data are represented as fold WT and are pooled from at least two independent experiments. n = 8 to 17 for each genotype. Error bars denote SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 using Student's t-test.
Heart
Cardiac mRNA expression of IL-6 and TNFα was significantly higher in the septic KO, as compared to septic WT mice (Figure 3B). Associated with these two proinflammatory cytokines, expression of nitric oxide synthase 2/3 (NOS2/NOS3) was also markedly enhanced in KO hearts, suggesting increased nitric oxide (NO) production from an inducible NOS allele and possible loss of protective effects of endothelial NOS.32 Together in the presence of higher levels of cytokines such as IL-6, this could lead to exacerbated cardiac dysfunction in the KO mice.
Liver
In contrast to cardiac tissue, liver mRNA expression of IL-6 and TNFα was similar in septic mice of both genotypes (data not shown), even though the IL-6 protein level was higher in tissue from septic KO mice (Figure 3A). However, mRNA expression of coagulation factors, coagulation factor III [F3 (tissue factor)] and protein C receptor (PROCR), endothelial PROCR (EPCR), as well as NOS2/NOS3, were markedly elevated in septic KO livers compared to the WT mice (Figure 3C). Increased PROCR expression in the endotoxemia model has been shown to occur via proteinase-activated receptor 1 (PAR-1)–dependent thrombin signaling, linking it to increased thrombin production and impaired anti-inflammatory and anticoagulation pathways.33 This procoagulant and increased NO production is potentially detrimental for liver tissue and could lead to excessive liver dysfunction in the KO mice.34
Lungs
Similar to the cardiac tissue, lungs from septic KO mice also exhibited significantly higher mRNA expression of IL-6 and TNFα, as compared to the septic WT mice (Figure 3D). In addition, we also observed enhanced mRNA expression of procoagulant factor serpin peptidase inhibitor, clade E, member 1 (SERPINE1) (plasminogen activator inhibitor (PAI) as well as that of the adhesion molecules intercellular adhesion molecule (ICAM) and vascular cell-adhesion molecule 1 (VCAM) in the septic KO compared to the WT mice lungs (Figure 3D). Consistent with enhanced adhesion molecule expression, the number of bronchoalveolar lavage neutrophils was significantly higher in KO compared to the WT septic mice, at a later time point (24 hours) (Figure 3E). Because lung neutrophil sequestration has been shown to correlate with lung tissue damage in a sepsis model,35 enhanced inflammation in the septic KO lung could lead to higher pulmonary dysfunction in septic KO animals.
Overall, the tissues examined showed an exacerbated inflammatory gene expression profile in KO mice compared to the WT mice, which together with the higher mortality suggest a greater extent of organ dysfunction in response to polymicrobial sepsis. In contrast to the KO mice, and as expected, tissues for septic HET mice had similar levels of inflammatory mediators to that of septic WT mice (Figure 3, A–E), consistent with comparable mortality between WT and HET mice. Histological examination of major organs (including lung, liver, kidney, and heart) from septic animals, however, displayed minimal differences between WT and KO animals at both 24 (data not shown) and 48 hours after surgery (Supplemental Figure S2). Histopathological assessment of organ injury in the CLP model has been a bit controversial, and recent studies have demonstrated that there are minimal histopathological differences in major organs of septic mice predicted to live versus those predicted to die, even up to 48 hours after surgery.29,30
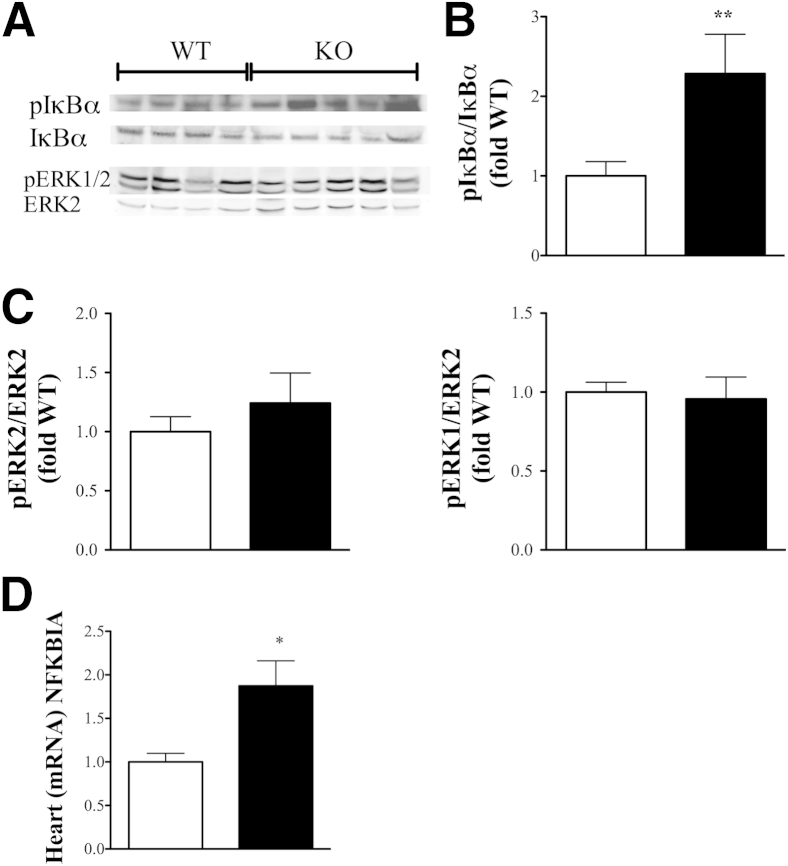
βArr1 Inhibits Cardiac IκBα Phosphorylation
The enhanced proinflammatory signature observed in tissues from βArr1 KO mice suggested enhanced activation of signaling pathways. βArr1 has been shown to be an important regulator of NFκB8 and MAPK pathways involved in production of inflammatory mediators.7,9,36,37 To ascertain whether βArr1 modulates signaling via any of these pathways and to correlate the gene expression to signaling, we determined activation status of these pathways in the heart lysates from septic mice. Consistent with the expected pattern, pIκBα levels (Figure 4, A and B) were enhanced in the heart lysate from septic KO mice as compared to WT mice. This was specific for IκBα because the phosphorylation status of other pathways, including ERK (Figure 4, A and C), was comparable in heart lysates from septic WT and KO mice. Note that phosphorylation of other NFκB and MAPK molecules, including P65, P105, c-Jun N-terminal kinase (JNK), and p38, in septic mice of either genotype was either undetectable or very low (data not shown). However, because this was a single time point analysis, differential activation of these signaling players during the course of the infection cannot be denied. Nonetheless, because there was enhanced activation of IκBα phosphorylation in the KO mice, we examined the mRNA levels of NFKBIA (IκBα), whose promoter is activated by NFκB.38 Consistent with enhanced IκBα phosphorylation in the KO hearts, mRNA expression of IκBα was significantly enhanced in the KO compared to the WT hearts (Figure 4D). Together, these results suggest a negative regulatory role for βArr1 in NFκB activation in cardiac tissue in response to a septic insult and potentially links increased inflammatory mediator production to higher NFκB activation in the βArr1 KO mice.
Figure 4.
β-arrestin-1 (βArr1) inhibits cardiac NFκB signaling. Wild-type (WT; white bars) and βArr1 knockout (KO) (β-Arr1−/−; black bars) mice were subjected to 16-guage needle single-puncture surgery, and heart tissue was collected 12 hours after surgery. A–C: Representative blots (A) and quantitative presentation of phosphorylation status of IκBα (B) and ERK1/2 (C) in heart lysates from septic mice. B and C: Note that pIκBα was normalized to IκBα, and pERK1/2 to ERK2, for loading control. D: Real-time quantitative RT-PCR for NFKBIA (IκBα) mRNA expression in heart tissue from septic mice. Data are pooled from two independent experiments. n = 8 to 10 for each genotype. Error bars denote SEM. ∗P < 0.05, ∗∗P < 0.01 using Student's t-test.
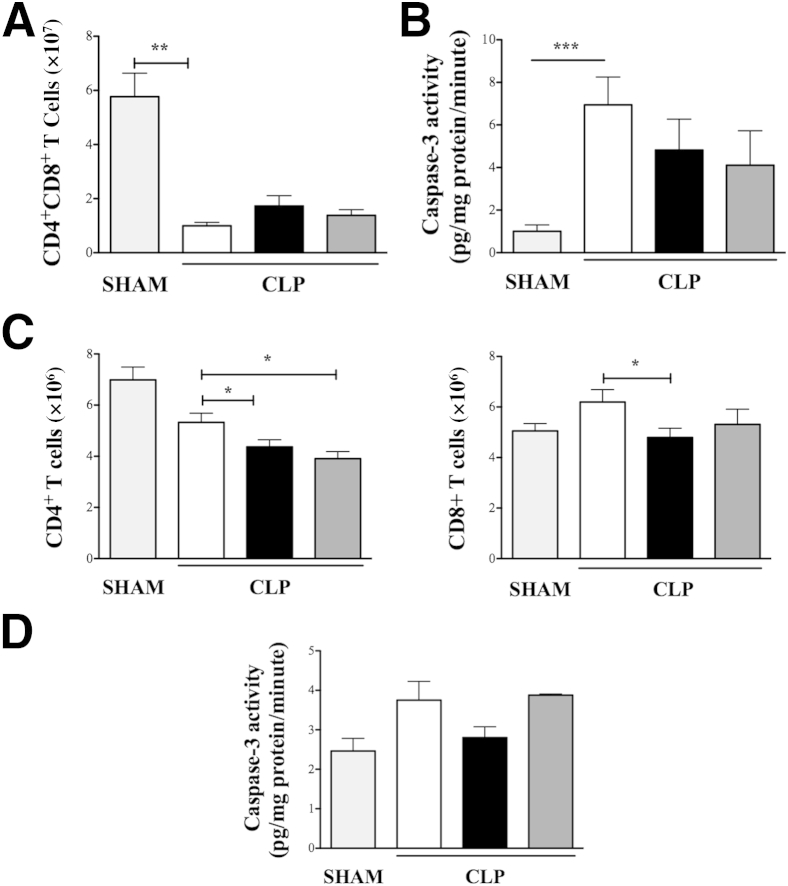
Thymus Apoptosis and Immune-Suppression Are Unaffected by Loss of βArr1
Lymphocyte apoptosis and innate immune suppression have drastic consequences in progression of sepsis39 and have been correlated with a dysregulated inflammatory cascade associated with mortality.40,41 Given that sepsis progression was worse in the KO mice, we wanted to examine whether the detrimental effects of lymphocyte apoptosis and immune suppression played any role in the final outcome. To evaluate the extent of apoptosis induced in response to sepsis, lymphocyte cellular profile was evaluated in thymus and spleens of sham and septic mice. Note that the cellular profile in thymus and spleen of sham mice was unaffected by the zygosity of βArr1 (Table 2). Sepsis induced a drastic reduction in number (and proportion) of thymic CD4+CD8+ (double positive) T cells, that was comparable between WT, KO, and HET septic mice (Figure 5A). Additionally, caspase-3 activity that was significantly induced in response to sepsis was similar between thymic tissues from septic mice of all genotypes (Figure 5B). Although septic WT spleen did not exhibit significant loss in CD4+ (P = 0.0563) or CD8+ T cells compared to sham mice, the numbers of both T cell types were significantly lower in septic KO mice as compared to septic WT (Figure 5C). Splenic CD4+ T cells were also lower in septic HET mice, similar to that of the KO. Additionally, caspase-3 activity in spleen, even though slightly induced at this time point, was similar between the three genotypes in response to sepsis (Figure 5D). The discrepancy in the spleen and thymus of KO septic mice with regard to the effect on T cells may be due to lower expression of βArr1 in thymus as compared to the spleen.16 Additionally, CD4+ T cells have a greater amount of nuclear βArr1, hence might be affected to a greater extent by its zygosity as compared to CD8+ T cells.
Table 2.
T-Cell Distribution in Lymphoid Organs of Sham Mice
| Organ | WT | βArr1−/− | βArr1+/− |
|---|---|---|---|
| Thymus | |||
| CD4+CD8+ | 5.0 ± 0.6 × 107 | 5.7 ± 1.6 × 107 | 3.8 ± 1.2 × 107 |
| Spleen | |||
| CD4+ | 6.6 ± 0.3 × 106 | 6.3 ± 0.6 × 106 | 7.3 ± 0.9 × 106 |
| CD8+ | 4.8 ± 0.2 × 106 | 4.5 ± 0.5 × 106 | 5.3 ± 0.8 × 106 |
n = 4 to 6 for each genotype.
WT, wild type.
Figure 5.
Role of β-arrestin-1 (βArr1) in sepsis-induced lymphocyte apoptosis. Wild-type (WT; white bars), βArr1 knockout (β-Arr1−/−; black bars), and βArr1 heterozygous (β-Arr1+/−; dark gray bars) mice were subjected to 16-guage needle single-puncture surgery, and thymus and spleen were collected 24 hours after surgery for the indicated parameters/analysis. A and B: CD4+CD8+ T cells in thymus as determined by flow cytometry (A) and caspase-3 activity in thymic lysates of septic mice as compared to WT/sham (light gray bars) (B). C and D: CD4+ and CD8+ T cells in spleen as determined by flow cytometry (C) and caspase-3 activity in splenic lysates from septic mice as compared to WT/sham (D). Data are pooled from at least three independent experiments for septic mice. n = 4 to 6 for sham and n = 10 to 19 for septic mice for each genotype (A–C); n = 4 to 5 (D). Error bars denote SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 using Student's t-test.
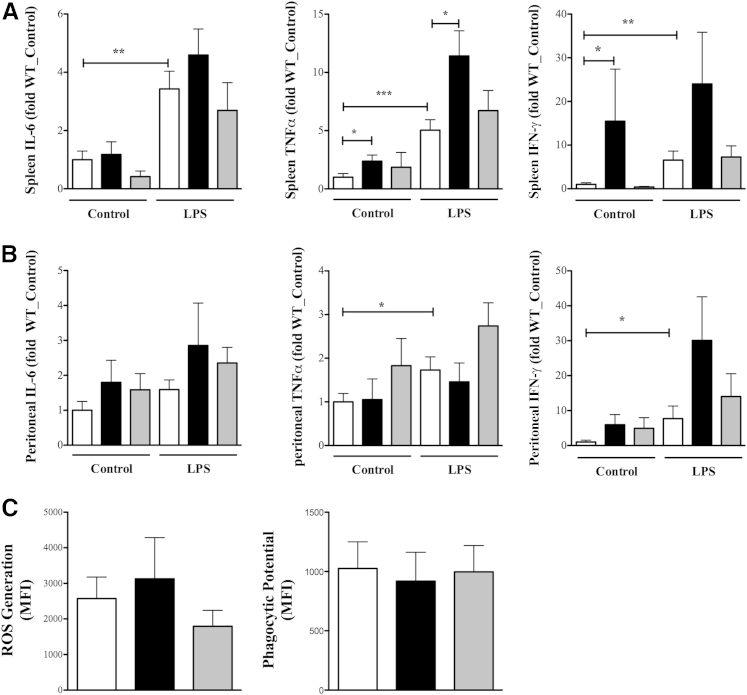
To assess immune suppression, peritoneal and splenic cells from septic mice were stimulated with LPS as secondary stimuli, and the extent of cytokine production determined. Both cell populations responded to further stimuli even though splenic response [IL-6, P < 0.01; TNFα, P < 0.005; interferon-γ (IFN-γ), P < 0.01] was more pronounced as compared to peritoneal cells (IL-6, P = 0.07; TNFα, P < 0.05; IFN-γ, P < 0.05, using a one-tailed t-test) (Figure 6, A and B). In the absence of secondary stimuli (control), KO splenocytes exhibited significantly higher TNFα and IFN-γ production (Figure 6A), whereas peritoneal cells produced higher IFN-γ (Figure 6B) (P = 0.05). Given that the cells had undergone exposure to LPS in vivo to assess immune suppression in response to secondary LPS stimulation, data were converted to fold change over control for each individual animal. There was no statistically significant difference between the three genotypes in regard to the ability of splenic or peritoneal cell populations to respond to the secondary stimuli (Table 3). However, spleen TNFα production was significantly higher in the KO compared to the WT, both without and with secondary stimulation (Figure 6A). To further assess the role of βArr1 in innate cell dysfunction, we determined the phagocytic potential and ROS generation capacity of peritoneal neutrophils from septic mice28 and found neither to be affected by loss of βArr1 in response to a septic insult (Figure 6C). Taken together, these results suggest that innate immune suppression following septic insult was unaffected by the loss of βArr1.
Figure 6.
Role of β-arrestin-1 (βArr1) in sepsis-induced immune-suppression. Wild-type (WT; white bars), βArr1 knockout (β-Arr1−/−; black bars), and βArr1 heterozygous (β-Arr1+/−; gray bars) mice were subjected to 16-guage needle single-puncture surgery, and spleen and peritoneal cells were collected 24 hours after surgery and processed. Cells were then plated and left untreated (control) or stimulated for 18 hours with 100 ng/mL lipopolysaccharide (LPS). A and B: Cytokine levels in the supernatants in splenocytes (A) and peritoneal cells (B) in control and LPS stimuli as determined by ELISA. Data are presented as fold change over WT control. C: Phagocytic potential and reactive oxygen species (ROS) generation in peritoneal cells from septic mice presented as mean fluorescence intensity (MFI) increase over controls. Data were pooled from three independent experiments. n = 8 to 10. Error bars denote SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 using Student's t-test (A and B).
Table 3.
Immune Suppression in Response to Sepsis
| Organs | WT | β-Arr1−/− | β-Arr1+/− |
|---|---|---|---|
| Splenocytes | |||
| IL-6 | 9.7 ± 3.3 | 6.5 ± 2.6 | 51.9 ± 42.6 |
| TNFα | 36.8 ± 19.2 | 15.2 ± 7.6 | 45.9 ± 30.1 |
| IFN-γ | 4.6 ± 2.2 | 1.1 ± 0.2 | 8.9 ± 2.3 |
| Peritoneal cells | |||
| IL-6 | 7.6 ± 5.1 | 10.7 ± 4.8 | 3.8 ± 0.9 |
| TNFα | 5.3 ± 2.1 | 25.2 ± 12.3 | 3.6 ± 0.7 |
| IFN-γ | 37.2 ± 27.3 | 36.3 ± 29.5 | 5.2 ± 2.2 |
Cytokine production by splenocytes and peritoneal cells following ex vivo lipopolysaccharide stimulation shown as fold change over unstimulated cells for each mouse. n = 6 to 8 mice for each genotype with data pooled from two independent experiments.
WT, wild type.
Nonhematopoietic βArr1 Negatively Regulates Inflammation Following Sepsis
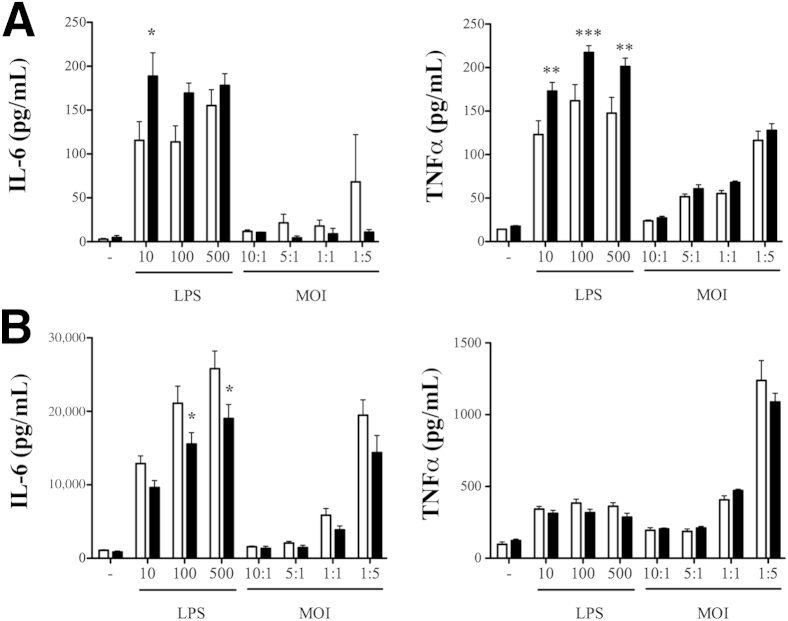
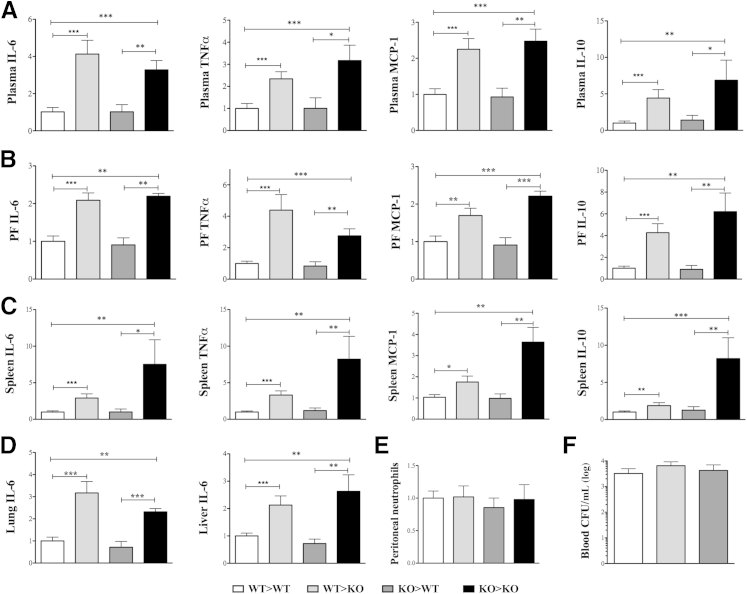
Based on the mortality pattern, we predicted that exacerbated-inflammation was the likely cause of poor outcome in KO animals. In accordance, both systemic and tissue inflammation from septic animals were significantly higher in mice lacking βArr1. To further determine the biochemical mechanisms, we used splenocytes and peritoneal cells from naive mice, and stimulated them with LPS and polymicrobial culture. Interestingly, LPS-induced IL-6 and TNFα levels from the KO splenic cells were significantly higher compared to the WT cells (Figure 7A). This response was quite the opposite in the peritoneal cells from the KO mice with respect to IL-6 production (Figure 7B). There was no difference, however, in cytokine production following polymicrobial stimulation of either population. This suggests that βArr1 plays a cell- and stimuli-specific role in mediating cytokine production in vitro. Given the range of receptors and signalosomes used in polymicrobial sepsis42 and the potential for βArr1 to intercept and regulate downstream effects,9,10,43 we first wanted to test whether the hyperinflammatory phenotype observed in septic KO mice stems from βArr1’s role in the immune cells. To assess this, we generated bone marrow chimeras of WT and βArr1 KO mice with donor and recipient genotypes comprising the hematopoietic or the nonhematopoietic compartments, respectively. The chimeric mice were found to have >92% donor-derived leukocytes in the blood, using flow cytometry to distinguish between 45.1 and 45.2 alleles, except in the case of KO>KO transfers. The four groups of chimeric mice were subjected to CLP and cytokine production, immune cell infiltration (to the site of infection), and then bacterial clearance was determined. Contrary to our expectations, we found that 12 hours after CLP, the nonhematopoietic βArr1 KO mice had significantly elevated levels of IL-6, IL-10, TNFα, and monocyte chemoattractant protein-1 (MCP-1) in plasma, peritoneal fluid, and spleen compared to the WT septic group (Figure 8, A–C). Importantly, levels of these cytokines were similar between the hematopoietic βArr1 KO and the WT septic mice, demonstrating that immune cell-specific βArr1 is not the likely the regulator of sepsis-induced inflammation. Consistent with these systemic effects, lung and liver IL-6 levels were significantly elevated in nonhematopoietic βArr1 KO mice compared to the other groups (Figure 8D). Neither neutrophil infiltration to the initial site of infection (Figure 8E) nor systemic bacterial load (Figure 8F) was affected by the loss of βArr1 in either cellular compartment. Together, these data demonstrate that the nonhematopoietic βArr1 exerts a negative regulatory role in sepsis-induced inflammation in mice.
Figure 7.
Role of β-arrestin-1 in cytokine production in in vitro cell culture models. Spleen and resident peritoneal cells from the three genotypes were collected and processed. Equivalent number of cells were plated and treated with lipopolysaccharide (LPS) and polymicrobial culture at different concentrations and multiplicity of infection (MOI), respectively, for 18 hours. Supernatants were then assayed for IL-6 and TNFα concentrations using ELISA. A and B: Data from splenocytes (A) and peritoneal cells (B). White bars indicate wild-type; black bars, β-Arr1−/−. n = 4 to 5 mice for each genotype. Error bars denote SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 compared to WT as determined by 2-way analysis of variance followed by Bonferroni post test. WT, wild type.
Figure 8.
Nonhematopoietic β-arrestin-1 in sepsis-induced inflammation. Bone marrow chimeras were generated. The four groups of mice were subjected to cecal ligation and puncture (CLP) with a 16-guage needle and 12 hours later euthanized for sample collection. A–D: Cytokine levels as determined by ELISA in plasma (A), peritoneal fluid (B), spleen (C), and lung and liver lysates (D). E and F: Peritoneal neutrophil infiltration (E) and blood bacterial load (F) in septic mice are shown as total count and CFU/mL, respectively. Data in A–D are presented relative to wild type (WT) for each group. The chimeric nomenclature used is donor>recipient such that the chimeric mouse has the donor's hematopoietic cells and recipient's nonhematopoietic cells. Data are pooled from at least two independent experiments, except for the KO>KO group. n = 8 to 21, WT>WT, WT>KO, and KO>WT; n = 5, KO>KO. Error bars denote SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 using Student's t-test. CFU, colony-forming units; KO, knockout.
Discussion
Sepsis is a highly integrative pathophysiological disorder that if left untreated can result in multiple organ damage and mortality. In animal models of sepsis, host inflammatory sequelae, including humoral response, cellular infiltration, lymphoid apoptosis, and consequent immune suppression, have been identified as important factors in determining host susceptibility. The data presented here demonstrate a critical role for βArr1 specifically in sepsis-induced inflammation and mortality, while ruling out the likely role for βArr1 in chemotaxis (to the site of infection), bacterial killing, thymic apoptosis, and immune suppression. Interestingly, the role of βArr1 in polymicrobial sepsis-induced mortality is strikingly opposite to our previous findings on βArr1 in the endotoxemia model of sepsis. It must be noted, however, that in both models, inflammation correlated and could be predictive of susceptibility to disease progression. This difference in outcome in endotoxemia and polymicrobial sepsis has also been observed for β-Arrestin-2 (βArr2)12,44 and IFN alpha-beta receptor (IFNAR) KO mice, which were similarly protected from the former, but susceptible to the latter, model of sepsis.45 This highlights the difference in pathophysiology of endotoxemia and polymicrobial sepsis, with the instigating stimuli being endotoxin in the former versus gut microbes and necrotizing tissue in the latter.
βArr1 is also a potential modulator of other inflammatory diseases, including colitis,14 arthritis,46 and experimental autoimmune encephalomyelitis,16 although its loss is, surprisingly, protective in these models. This suggests that the stimulus and ensuing inflammatory sequelae dictate the role βArr1 plays in modulating the disease, and therefore, understanding its mode of action in these different diseases in the context of instigating stimuli is important. Similar to the role of βArr1, βArr2 (also known as arrestin-3) has also been shown to negatively regulate polymicrobial sepsis, both in surgical CLP as well as a nonsurgical sepsis model.20,44 Whether βArr1 and βArr2 regulate similar or distinct pathways in polymicrobial sepsis will be pursued in future studies.
In various disease models and in vitro studies, βArr1 has been ascribed diverse regulatory roles affecting immune cells. We therefore examined the differences in response to polymicrobial and LPS stimulation, using splenocytes and basal peritoneal cells as in vitro models. Curiously, the role of βArr1 in cytokine production (specifically, IL-6) in response to LPS was found to be different based on the cellular model used, similar to what we had observed earlier with respect to IFN-γ production from CD11b− and CD11b+ splenocytes.12 Because of this perplexity, we further tested whether βArr1 in immune cells is responsible for the observed results in sepsis, and surprisingly, uncovered the dominant-negative regulatory role for βArr1 in the nonhematopoietic cells in sepsis-induced inflammation. Although the identity of these cells remains the subject of future research, our results demonstrate that the role of βArr1 is highly context dependent and therefore highlights major drawbacks in concluding the role of β-arrestins in inflammation based solely on in vitro cell culture studies.
Given the importance of nonimmune cells such as endothelial and neuronal cells in sepsis progression, the role of the nonhematopoietic compartment in sepsis-induced inflammation in general is not surprising. However, because in previous studies the role of βArr1 in modulating inflammation has been extensively studied in, and attributed to, cells of hematopoietic origin (immune cells),9,12,16,43 our results on the role of nonhematopoietic βArr1 in septic inflammation is unexpected and deserves further attention. It should be noted that βArr1 was originally discovered for its role in desensitization of GPCRs3 and more recently has been implicated in biased signaling from GPCRs.47 Many GPCRs, including C5aR,48,49 adenosine receptors,50,51 and adrenergic receptors,52 have all been shown to play a critical role in sepsis pathogenesis. Interestingly, similar to our results, adenosine receptor (A2B) in the nonhematopoietic cells was also found to have a critical role in negatively regulating inflammation in response to sepsis.51 Whether βArr1 is involved in regulating these or other GPCRs in the nonhematopoietic cells in the context of sepsis progression will be examined in future studies.
Taken together in this study, our data provide evidence that βArr1 is a negative regulator of sepsis-induced inflammation and mortality. Even though previous studies have focused on the role of immune cell-specific βArr1 in inflammation, our results demonstrate that βArr1 in the nonhematopoietic cells functions as a negative regulator of sepsis-induced inflammation. Future studies will focus on identifying the appropriate physiological model to understand the biochemical basis by which βArr1 suppresses sepsis-mediated inflammation. These studies will likely open up new avenues for development of therapeutic strategies against this devastating disorder.
Acknowledgments
We thank Drs. Robert J. Lefkowitz (Duke University, Durham, NC) for providing βarr1 knockout mice, Susanne Mohr (and lab members) for assistance in caspase assay execution, and Sandra O’Reilley for assistance with the generation of bone marrow chimeras and the university lab animal resources for taking care of our animals.
Footnotes
Supported by NIH grants HL095637, AR055726, and AR056680 (N.P.), Enteric Research Investigational Network, Cooperative Research Centers (grant U19AI09087; PI L.S. Mansfield), and by a G.D. Edith Hsiung and Margaret E. Kimball Endowed Scholarship (2013; D.S.).
N.Pac. and A.M. contributed equally to this work.
Disclosures: None declared.
Supplemental Data
Role of β-arrestin-1 in systemic cytokine production and cellular infiltration in sepsis. A: Plasma cytokine concentrations in septic (double puncture with a 20-guage needle) and sham mice 6 hours after surgery. White bars indicate wild type; black bars, βArr1 knockout. B: Neutrophil and macrophage infiltration in peritoneal cavity of septic mice shown for the indicated time points (6 hours for sham). Grey bars indicate βarr1 heterozygous mice. Data for septic mice are pooled from three independent experiments. n = 9 to 14 for septic mice, n = 3 for sham. Error bars denote SEM. ∗P < 0.05 using Student's t-test. CLP, cecal ligation and puncture.
Histopathological assessment of organs from septic mice. Histology of liver, kidney, spleen, and lung tissue from septic wild-type (WT) (A) and knockout (KO) (B) mice, 48 hours after surgery. Lung section is shown at three different magnifications, with no difference observed between WT and KO with respect to pulmonary edema, fibrin deposition, or leukocyte infiltration. No significant histopathological changes were observed in the other organs as well. The magnification indicates the objective power, not the total magnification.
References
- 1.Papathanassoglou E.D., Moynihan J.A., Ackerman M.H. Does programmed cell death (apoptosis) play a role in the development of multiple organ dysfunction in critically ill patients? A review and a theoretical framework. Crit Care Med. 2000;28:537–549. doi: 10.1097/00003246-200002000-00042. [DOI] [PubMed] [Google Scholar]
- 2.Shenoy S.K. Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE. 2005;2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- 3.DeFea K.A. Stop that cell! [Beta]-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol. 2007;69:535–560. doi: 10.1146/annurev.physiol.69.022405.154804. [DOI] [PubMed] [Google Scholar]
- 4.Luttrell L., Ferguson S., Daaka Y., Miller W., Maudsley S., Della Rocca G., Lin F.T., Kawakatsu H., Owada K., Luttrell D. [Beta]-Arrestin-dependent formation of β2-adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 5.Violin J.D., DeWire S.M., Yamashita D., Rominger D.H., Nguyen L., Schiller K., Whalen E.J., Gowen M., Lark M.W. Selectively engaging [beta]-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335:572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 6.Tohgo A., Pierce K.L., Choy E.W., Lefkowitz R.J., Luttrell L.M. [Beta]-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 7.Luttrell L.M., Roudabush F.L., Choy E.W., Miller W.E., Field M.E., Pierce K.L., Lefkowitz R.J. Activation and targeting of extracellular signal-regulated kinases by [beta]-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witherow D.S., Garrison T.R., Miller W.E., Lefkowitz R.J. [Beta]-Arrestin inhibits NF-[kappa]B activity by means of its interaction with the NF-[kappa]B inhibitor IkappaBalpha. Proc Natl Acad Sci U S A. 2004;101:8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan H., Luttrell L.M., Tempel G.E., Senn J.J., Halushka P.V., Cook J.A. Beta-arrestins 1 and 2 differentially regulate LPS-induced signaling and pro-inflammatory gene expression. Mol Immunol. 2007;44:3092–3099. doi: 10.1016/j.molimm.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Tang Y., Teng L., Wu Y., Zhao X., Pei G. Association of [beta]-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 11.Parameswaran N., Pao C.S., Leonhard K.S., Kang D.S., Kratz M., Ley S.C., Benovic J.L. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NF B1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J Biol Chem. 2006;281:34159–34170. doi: 10.1074/jbc.M605376200. [DOI] [PubMed] [Google Scholar]
- 12.Porter K.J., Gonipeta B., Parvataneni S., Appledorn D.M., Patial S., Sharma D., Gangur V., Amalfitano A., Parameswaran N. Regulation of lipopolysaccharide-induced inflammatory response and endotoxemia by [beta]-arrestins. J Cell Physiol. 2010;225:406–416. doi: 10.1002/jcp.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li P., Cook J.A., Gilkeson G.S., Luttrell L.M., Wang L., Borg K.T., Halushka P.V., Fan H. Increased expression of beta-arrestin 1 and 2 in murine models of rheumatoid arthritis: isoform specific regulation of inflammation. Mol Immunol. 2011;49:64–74. doi: 10.1016/j.molimm.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee T., Lee E., Irwin R., Lucas P.C., McCabe L.R., Parameswaran N. [Beta]-Arrestin-1 deficiency protects mice from experimental colitis. Am J Pathol. 2013;182:1114–1123. doi: 10.1016/j.ajpath.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchanan F.G., Gorden D.L., Matta P., Shi Q., Matrisian L.M., DuBois R.N. Role of [beta]-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci U S A. 2006;103:1492–1497. doi: 10.1073/pnas.0510562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y., Feng Y., Kang J., Liu C., Li Z., Li D., Cao W., Qiu J., Guo Z., Bi E., Zang L., Lu C., Zhang J.Z., Pei G. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol. 2007;8:817–824. doi: 10.1038/ni1489. [DOI] [PubMed] [Google Scholar]
- 17.Forooghian F., Cheung R.K., Smith W.C., O'Connor P., Dosch H.M. Enolase and arrestin are novel nonmyelin autoantigens in multiple sclerosis. J Clin Immunol. 2007;27:388–396. doi: 10.1007/s10875-007-9091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buras J.A., Holzmann B., Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 19.The National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals: Eighth Edition. [PubMed] [Google Scholar]
- 20.Packiriswamy N., Lee T., Raghavendra P.B., Durairaj H., Wang H., Parameswaran N. G-protein-coupled receptor kinase-5 mediates inflammation but does not regulate cellular infiltration or bacterial load in a polymicrobial sepsis model in mice. J Innate Immun. 2013;5:401–413. doi: 10.1159/000347002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma D., Malik A., Lee E., Britton R.A., Parameswaran N. Gene dosage-dependent negative regulatory role of [beta]-arrestin-2 in polymicrobial infection-induced inflammation. Infect Immun. 2013;81:3035–3044. doi: 10.1128/IAI.00653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent J.A., Mohr S. Inhibition of caspase-1/interleukin-1[beta] signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–230. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- 23.Xiao H., Siddiqui J., Remick D.G. Mechanisms of mortality in early and late sepsis. Infect Immun. 2006;74:5227–5235. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remick D.G., Bolgos G.R., Siddiqui J., Shin J., Nemzek J.A. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Giannoudis P.V., Harwood P.J., Loughenbury P., Van Griensven M., Krettek C., Pape H.C. Correlation between IL-6 levels and the systemic inflammatory response score: can an IL-6 cutoff predict a SIRS state? J Trauma. 2008;65:646–652. doi: 10.1097/TA.0b013e3181820d48. [DOI] [PubMed] [Google Scholar]
- 26.Moitra R., Beal D.R., Belikoff B.G., Remick D.G. Presence of pre-existing antibodies mediate survival in sepsis. Shock. 2012;37:56–62. doi: 10.1097/SHK.0b013e3182356f3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craciun F.L., Schuller E.R., Remick D.G. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J Immunol. 2010;185:6930–6938. doi: 10.4049/jimmunol.1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delano M.J., Thayer T., Gabrilovich S., Kelly-Scumpia K.M., Winfield R.D., Scumpia P.O., Cuenca A.G., Warner E., Wallet S.M., Wallet M.A., O'Malley K.A., Ramphal R., Clare-Salzer M., Efron P.A., Mathews C.E., Moldawer L.L. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186:195–202. doi: 10.4049/jimmunol.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosmann M., Ward P.A. The inflammatory response in sepsis. Trends Immunol. 2013;34:129–136. doi: 10.1016/j.it.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craciun F.L., Iskander K.N., Chiswick E.L., Stepien D.M., Henderson J.M., Remick D.G. Early murine polymicrobial sepsis predominantly causes renal injury. Shock. 2014;41:97–103. doi: 10.1097/SHK.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iskander K.N., Craciun F.L., Stepien D.M., Duffy E.R., Kim J., Moitra R., Vaickus L.J., Osuchowski M.F., Remick D.G. Cecal ligation and puncture-induced murine sepsis does not cause lung injury. Crit Care Med. 2013;41:159–170. doi: 10.1097/CCM.0b013e3182676322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coldewey S.M., Rogazzo M., Collino M., Patel N.S.A., Thiemermann C. Inhibition of I[kappa]B kinase reduces the multiple organ dysfunction caused by sepsis in the mouse. Dis Model Mech. 2013;6:1031–1042. doi: 10.1242/dmm.012435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu J.M., Katsuura Y., Ferrell G.L., Grammas P., Esmon C.T. Endotoxin and thrombin elevate rodent endothelial cell protein C receptor mRNA levels and increase receptor shedding in vivo. Blood. 2000;95:1687–1693. [PubMed] [Google Scholar]
- 34.Dhainaut J.F., Marin N., Mignon A., Vinsonneau C. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med. 2001;29 Suppl:S42–S47. doi: 10.1097/00003246-200107001-00016. [DOI] [PubMed] [Google Scholar]
- 35.Laudes I.J., Guo R.F., Riedemann N.C., Speyer C., Craig R., Sarma J.V., Ward P.A. Disturbed homeostasis of lung intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 during sepsis. Am J Pathol. 2004;164:1435–1445. doi: 10.1016/S0002-9440(10)63230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao M., Wimmer A., Trieu K., DiScipio R.G., Schraufstatter I.U. Arrestin regulates MAPK activation and prevents NADPH oxidase-dependent death of cells expressing CXCR2. J Biol Chem. 2004;279:49259–49267. doi: 10.1074/jbc.M405118200. [DOI] [PubMed] [Google Scholar]
- 37.Gong K., Li Z., Xu M., Du J., Lv Z., Zhang Y. A novel protein kinase A-independent, [beta]-arrestin-1-dependent signaling pathway for p38 mitogen-activated protein kinase activation by [beta]2-adrenergic receptors. J Biol Chem. 2008;283:29028–29036. doi: 10.1074/jbc.M801313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hotchkiss R.S., Nicholson D.W. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 40.Hotchkiss R.S., Chang K.C., Swanson P.E., Tinsley K.W., Hui J.J., Klender P., Xanthoudakis S., Roy S., Black C., Grimm E., Aspiotis R., Han Y., Nicholson D.W., Karl I.E. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 41.Iwata A., de Claro R.A., Morgan-Stevenson V.L., Tupper J.C., Schwartz B.R., Liu L., Zhu X., Jordan K.C., Winn R.K., Harlan J.M. Extracellular administration of BCL2 protein reduces apoptosis and improves survival in a murine model of sepsis. PLoS One. 2011;6:e14729. doi: 10.1371/journal.pone.0014729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 43.Seregin S.S., Appledorn D.M., Patial S., Bujold M., Nance W., Godbehere S., Parameswaran N., Amalfitano A. [beta]-Arrestins modulate Adenovirus-vector-induced innate immune responses: differential regulation by [beta]-arrestin-1 and [beta]-arrestin-2. Virus Res. 2010;147:123–134. doi: 10.1016/j.virusres.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan H., Bitto A., Zingarelli B., Luttrell L.M., Borg K., Halushka P.V., Cook J.A. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–351. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly-Scumpia K.M., Scumpia P.O., Delano M.J., Weinstein J.S., Cuenca A.G., Wynn J.L., Moldawer L.L. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med. 2010;207:319–326. doi: 10.1084/jem.20091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J., Wei B., Guo A., Liu C., Huang S., Du F., Fan W., Bao C., Pei G. Deficiency of [beta]-arrestin1 ameliorates collagen-induced arthritis with impaired TH17 cell differentiation. Proc Natl Acad Sci U S A. 2013;110:7395–7400. doi: 10.1073/pnas.1221608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Violin J.D., Lefkowitz R.J. [beta]-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Ward P.A. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 49.Ward P.A. Functions of C5a receptors. J Mol Med. 2009;87:375–378. doi: 10.1007/s00109-009-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belikoff B.G., Hatfield S., Georgiev P., Ohta A., Lukashev D., Buras J.A., Remick D.G., Sitkovsky M. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J Immunol. 2011;186:2444–2453. doi: 10.4049/jimmunol.1001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Csóka B., Németh Z.H., Rosenberger P., Eltzschig H.K., Spolarics Z., Pacher P., Selmeczy Z., Koscsó B., Himer L., Vizi E.S., Blackburn M.R., Deitch E.A., Haskó G. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Montmollin E., Aboab J., Mansart A., Annane D. Bench-to-bedside review: [beta]-adrenergic modulation in sepsis. Crit Care. 2009;13:230. doi: 10.1186/cc8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Role of β-arrestin-1 in systemic cytokine production and cellular infiltration in sepsis. A: Plasma cytokine concentrations in septic (double puncture with a 20-guage needle) and sham mice 6 hours after surgery. White bars indicate wild type; black bars, βArr1 knockout. B: Neutrophil and macrophage infiltration in peritoneal cavity of septic mice shown for the indicated time points (6 hours for sham). Grey bars indicate βarr1 heterozygous mice. Data for septic mice are pooled from three independent experiments. n = 9 to 14 for septic mice, n = 3 for sham. Error bars denote SEM. ∗P < 0.05 using Student's t-test. CLP, cecal ligation and puncture.
Histopathological assessment of organs from septic mice. Histology of liver, kidney, spleen, and lung tissue from septic wild-type (WT) (A) and knockout (KO) (B) mice, 48 hours after surgery. Lung section is shown at three different magnifications, with no difference observed between WT and KO with respect to pulmonary edema, fibrin deposition, or leukocyte infiltration. No significant histopathological changes were observed in the other organs as well. The magnification indicates the objective power, not the total magnification.