Abstract
Background
Orthopedic literature states that fractures of long bones, when associated with traumatic brain injuries, frequently heal with excessive callus and faster than normal. Few studies, however, have reported these phenomena being induced by spinal cord injury (SCI). Our objective is to compare the extent of callus and the rate of healing of long-bone fractures in patients with or without SCI. Subgroup comparisons were performed among the patients with SCI in terms of different levels of SCI.
Methods
The final mean volume of callus formation and the rate of union of nailed fractures of the femur were determined radiologically in 22 femoral fracture patients with SCI (seven cervical, six thoracic, and nine lumbar spine injury) and compared with those in a group of 22 patients with similar types of fractures but without SCI.
Results
The final mean callus volume in the fracture/SCI group was significantly higher than the fracture-only group (P < 0.001). The fractures in the fracture/SCI group united in an average time of 22.86 weeks compared with 25.04 weeks in the fracture-only group (P < 0.05). We observed 84.6% (11 of 13) of patients with cervical and thoracic SCI patients with accelerated fracture healing (cervical 6 of 7, thoracic 5 of 6), but only 44.4% (4 of 9) of patients with lumbar SCI appeared to show this phenomenon (P < 0.05).
Conclusions
These results confirm that SCI may be associated with accelerated fracture healing and enhanced callus formation. Furthermore, our study revealed a trend toward enhanced osteogenesis in cervical or thoracic SCI compared with lumbar SCI.
Keywords: Spinal cord injury, Femoral fracture, Bone healing, Callus
Introduction
Clinical studies have documented increased callus formation and a shorter time to union in patients with central nervous system injuries and an associated long-bone fracture compared with patients with long-bone fractures but without spinal cord injury (SCI).1 These previous studies have mostly focused on traumatic brain injury research,2–5 but few clinical studies have reported on the relationship between the rate of healing of long-bone fractures in patients who also have SCI.6,7 Our previous study showed accelerated fracture healing in a rat fracture/SCI model, which demonstrated that humoral factors may play an important role in the osteo-inductive process.8 However, the different damage levels of SCI on bone and the possible mechanisms involved have not been studied.
The aim of this study was to collect data on 44 consecutively admitted patients with SCI with or without femoral fractures and to investigate the relationship between the healing response in patients with fracture/SCI and those without spinal injury in order to quantify the effect of SCI on bone formation. We also investigated the influence of different spinal cord damage levels on fracture healing.
Methods
Between January 2006 and December 2010, 22 patients with SCI (6 with tetraplegia and 16 with paraplegia) with femoral fractures were treated with intramedullary nailing in the Affiliated People's Hospital at Jiangsu University, China. After the primary diagnostic and therapeutic management, all acute patients with SCI received a bolus of methylprednisolone sodium succinate. The seriously ill patients were transferred to the intensive care unit. The patients’ demographics are shown in Table 1. Their ages ranged from 23 to 70 years (mean 41.95 years). The type and level of the SCI were determined by computerized axial tomography and magnetic resonance. SCI levels varied from C3 to L4 vertebrae, and all patients with SCI showed a complete motor and sensory function disorder and, therefore, were classified as Frankel grade A on admission.
Table 1 .
Demographic and clinical profile of patients with SCI and femoral fracture
| Case | Sex/age | AO/OTA classification | Frankel score classification on admission/discharge | Level of SCI | Procedure | Operation time (minutes) | Volume of callus (cm3) | Time to union (weeks) |
|---|---|---|---|---|---|---|---|---|
| 1 | M/36 | 32-A3 | A/A | C4 | Closed | 56 | 55.18 | 16 |
| 2 | M/62 | 32-C1 | A/A | C5 | Opened | 100 | 25.18 | 27 |
| 3 | F/37 | 32-A2 | A/B | C6 | Closed | 75 | 84.56 | 20 |
| 4 | M/56 | 32-B1 | A/A | C6 | Closed | 90 | 66.45 | 18 |
| 5 | F/29 | 32-A3 | A/A | C6 | Closed | 70 | 67.93 | 22 |
| 6 | F/32 | 32-A2 | A/A | C7 | Closed | 75 | 90.65 | 10 |
| 7 | M/46 | 32-B3 | A/A | C7 | Closed | 85 | 55.42 | 19 |
| 8 | M/45 | 32-C2 | A/A | T8 | Closed | 110 | 22.14 | 34 |
| 9 | M/48 | 32-C2 | A/A | T9 | Opened | 115 | 73.23 | 19 |
| 10 | F/29 | 32-A2 | A/A | T10 | Closed | 71 | 60.14 | 17 |
| 11 | M/70 | 32-A1 | A/A | T11 | Closed | 86 | 59.45 | 23 |
| 12 | M/42 | 32-B3 | A/A | T11 | Closed | 85 | 65.21 | 22 |
| 13 | F/36 | 32-A2 | A/B | T12 | Opened | 105 | 76.48 | 20 |
| 14 | M/33 | 32-B1 | A/A | L1 | Closed | 69 | 18.43 | 36 |
| 15 | F/40 | 32-B2 | A/A | L1 | Closed | 59 | 22.13 | 28 |
| 16 | M/47 | 32-A3 | A/A | L1 | Closed | 62 | 10.32 | 34 |
| 17 | F/23 | 32-B2 | A/B | L2 | Closed | 67 | 45.26 | 20 |
| 18 | M/28 | 32-A3 | A/A | L2 | Closed | 58 | 60.17 | 18 |
| 19 | M/30 | 32-A1 | A/C | L3 | Closed | 83 | 54.2 | 22 |
| 20 | M/51 | 32-B2 | A/C | L3 | Closed | 76 | 62.13 | 20 |
| 21 | M/59 | 32-A3 | A/A | L3 | Closed | 60 | 25.12 | 26 |
| 22 | F/44 | 32-A3 | A/C | L4 | Closed | 63 | 23.24 | 32 |
Another group of 22 consecutively admitted patients, with fracture-only and without SCI, ranged in age from 25 to 72 years (mean 43.27 years). This group had similar fractures to the patients with SCI and was also treated with intramedullary nails (Table 2). We excluded patients with multi-level SCI or with incomplete injury, or with open or multiple fractures. With the majority of patients the nailing was performed using a locked, antegrade, reamed intramedullary nailing technique, but in three of the 22 fractures in the fracture/SCI group the fracture was openly reduced before fixation. In the fracture-only group, two patients required open reduction. If patients lived locally, they were followed up in the same rehabilitation unit; if they lived far away, they were followed up by medical consultation. They were assessed post-operatively, clinically after 1 week, and radiologically after 4 weeks. Ethical approval for this study was granted by the Affiliated People's Hospital at Jiangsu University, China, and all patients or next of kin gave informed consent.
Table 2 .
Demographic and clinical profile of patients with femoral fracture
| Case | Sex/age | AO/OTA classification | Procedure | Operation time (minutes) | Volume of callus (cm3) | Time to union (weeks) |
|---|---|---|---|---|---|---|
| 1 | M/29 | 32-A2 | Closed | 79 | 35.18 | 22 |
| 2 | M/44 | 32-B2 | Closed | 70 | 23.22 | 28 |
| 3 | M/37 | 32-A3 | Closed | 65 | 24.36 | 24 |
| 4 | M/56 | 32-A3 | Closed | 88 | 36.45 | 19 |
| 5 | F/56 | 32-A3 | Closed | 59 | 27.93 | 26 |
| 6 | F/39 | 32-B1 | Closed | 69 | 40.65 | 18 |
| 7 | M/43 | 32-A2 | Closed | 85 | 30.42 | 22 |
| 8 | M/47 | 32-C2 | Opened | 122 | 12.22 | 38 |
| 9 | F/58 | 32-A2 | Closed | 85 | 77.36 | 18 |
| 10 | M/25 | 32-A2 | Closed | 71 | 30.2 | 19 |
| 11 | M/72 | 32-A1 | Closed | 83 | 25.44 | 26 |
| 12 | M/42 | 32-B3 | Closed | 79 | 30.24 | 30 |
| 13 | F/36 | 32-C2 | Opened | 93 | 19.83 | 23 |
| 14 | F/33 | 32-B1 | Closed | 69 | 22.43 | 26 |
| 15 | M/40 | 32-B2 | Closed | 71 | 19.73 | 28 |
| 16 | M/51 | 32-A3 | Closed | 67 | 26.32 | 34 |
| 17 | F/32 | 32-C2 | Closed | 76 | 25.26 | 20 |
| 18 | M/28 | 32-A3 | Closed | 88 | 80.67 | 16 |
| 19 | M/30 | 32-A2 | Closed | 60 | 34.87 | 28 |
| 20 | M/47 | 32-B1 | Closed | 57 | 32.13 | 20 |
| 21 | M/69 | 32-B3 | Closed | 71 | 19.12 | 36 |
| 22 | M/38 | 32-A3 | Closed | 68 | 28.34 | 30 |
Assay for callus proliferation
All patients were initially investigated for their fractures with plain anterior-posterior (AP) and lateral X-rays, which were repeated every 4 weeks until plain radiographs showed bone-bridging of both cortices on the two radiographs. When radiographic union was present, clinical assessment continued for several more weeks, because radiographic union always occurs before clinical union. Only when clinical and radiographic union were both present was it possible to determine that the fracture had united. At this point, we were able to evaluate the time it took for union to occur; we then took the final plain radiographs to calculate the volume of callus. Two radiologists, blinded to the presence or absence of SCI, evaluated time to union. AP and lateral radiographs of fractures were taken of all patients and the volume of callus was calculated using the Perkins volume formula (2πR1(R2 − R1)L, where R1 = femur radius, R2 = callus radius, L = length of callus).9 The final result was the mean callus volume of the AP and lateral positions.
We defined the term “accelerated fracture healing” as callus volume reached the 51.04 cm3 and united time was faster than 22.86 weeks at the same time. (These two data due to the final mean callus volume and average united time in the fracture/SCI group.) In fracture/SCI group, 13 patients’ callus volume reached or exceed 51.04 cm3 and healing time were faster than 22.86 weeks, one patient's healing time was 20 weeks and callus volume (45.26 cm3) was close to mean value, another patient's callus volume was 59.45 cm3 and healing time (23 weeks) was also close to average value, so in this group we considered that 15 patients have displayed this phenomenon (15 of 22). Use the same data in fracture/only group, two patients reached this standard (2 of 22).
Statistics
All data were presented as mean value and standard deviation. Multiple comparisons of the data among the two groups were performed using one-way analysis of variance (ANOVA), t-test and Fisher's exact tests. All statistical analyses were performed using SPSS 12.0 software (SPSS Inc., Chicago, IL, USA). A significance level of P < 0.05 was used for all comparisons.
Results
In the study cohort, there were no neurovascular complications or wound infections. There was no significant difference in the mean operating time between the fracture/SCI group (76.13 ± 14.31 minutes, range 57–122 minutes) and the fracture-only group (78.18 ± 17.35 minutes, range 56–115 minutes) (P = 0.54). Hospitalization ranged from 10 to 20 days post-surgery and no patients were lost at follow-up. At discharge, 16 patients with SCI were still classified as Frankel grade A, 3 as Frankel B and 3 as Frankel C. The final mean callus volume in the combined fracture/SCI group (51.04 ± 23.40 cm3) was significantly higher than in the fracture-only group (31.92 ± 16.59 cm3) (t-test = 3.98, P < 0. 001) (Fig. 1). The fractures in the fracture/SCI group united in an average time of 22.86 ± 6.61 weeks compared with 25.04 ± 6.07 weeks in the fracture-only group (t-test = −2.39, P < 0.05) (Fig. 2). There was accelerated fracture healing in 68.2% (15 of 22) of the patients with fracture/SCI (cervical 6 of 7, thoracic 5 of 6, lumbar 4 of 9), but only 9.1% (2 of 22) in the fracture-only group (Fisher's exact test = 0.000, P < 0.001). The final mean callus volume of patients with cervical and thoracic SCI was significantly higher than in patients with lumbar SCI (Fisher's exact test = 4.43, P = 0.026 < 0.05) (Fig. 3). However, no significance was shown using the one-way ANOVA test on the fracture healing time in these three subgroups (P = 0.079, >0.05). Accelerated fracture healing was observed in 84.6% (11 of 13) of the patients with cervical and thoracic SCI (cervical 6 of 7, thoracic 5 of 6), but in only 44.4% (4 of 9) of the patients with lumbar SCI.
Figure 1 .
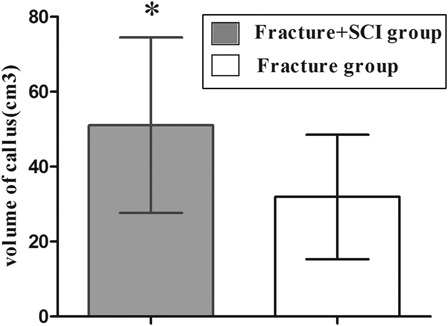
The final mean callus volume in the combined fracture/SCI group was significantly higher than the fracture/only group. *Significant difference between the groups, t test t = 3.98, P < 0.001.
Figure 2 .
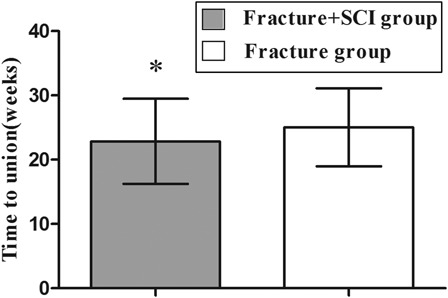
The average healing time of in fracture/SCI group was faster than in the fracture/only group. *Significant difference between the groups, t test t = −2.39, P < 0.05).
Figure 3 .
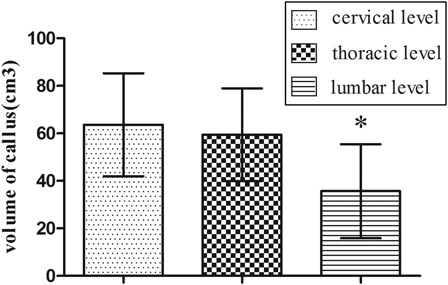
The final mean callus volume of cervical and thoracic spinal injury patients was significantly larger than lumbar spinal injury group. *Significant difference between these groups, one-way ANOVA, F = 4.43, P = 0.026 < 0.05.
Discussion
This study has demonstrated the influence of SCI on femoral fracture healing in clinical patients. Compared with those in the control group, the results showed that in the SCI group there was enhanced fracture healing, which is characterized by a significant increase in callus volume and in average healing time of the fractured femora.
According to reported studies, fracture healing appears to be a complex process. It involves an orderly and intricate succession of events,10 beginning with inflammation, followed by differentiation of fibrous tissue and cartilage, and finally endochondral ossification. Recent investigations indicate that SCI in men may result in various hormonal changes. Some have reported that a large proportion of men with SCI had abnormal pituitary hormone responses to hypothalamic-releasing hormones.11,12 These hormones include serum factors such as growth hormone, transforming growth factor-β, nerve growth factor, insulin-like growth factor II, platelet-derived growth factor, and interleukin-1 (IL-1) and IL-6.13,14 Furthermore, other studies indicate that increased expression of central neurotransmitters may be maintained for an extended period.15,16 These elevated hormones appear to have a large proliferative influence on serum osteo-inductive factors, and therefore, the levels of these humorally released osteo-inductive factors, like osteocalcin and leptin, are increased.8,17 In a study by Li et al.,18 it was reported that elevated leptin expression might also enhance alkaline phosphatase activity, secretion of osteocalcin, and expression of type I collagen mRNA. All these factors, which are involved in de novo bone formation, will act peripherally to induce myeloid precursor cell differentiation and osteoblast proliferation, and will accelerate mineralization of bone at the fracture site. They will also enhance fracture healing and callus formation.19 However, osteo-inductive factors have also been shown to cause harmful heterotopic ossification.17
A few studies have shown results that are contrary to ours. Several clinical studies have reported a high incidence of malunion, delayed union, or non-union sublesional limb fractures in patients with SCI,20,21 suggesting that fracture healing is adversely affected by SCI. Delayed fracture healing was also noted in a further study.22 There is also accumulating evidence that innervation plays an important role in the regulation of local bone turnover. Thus, sensory, autonomic and opioid neuropeptides – well-known regulators of nociception, inflammation, and vasoactivity – have been shown to stimulate bone cells via specific receptors.23 Therefore, denervation after SCI may impair fracture healing, as characterized by a significant decrease in the callus volume and mechanical strength.
These results suggest that there are two main ways in which bone metabolism is altered after SCI: (i) hormone and osteo-inductive factors, which can increase the osteoblast growth factor activity and enhance fracture healing, and (ii) denervation, which may decrease bone turnover and impair fracture healing. Therefore, the bone callus volume and healing time may be related to the balance between bone reabsorption and bone formation caused by the osteo-inductive factors and denervation of the fracture site. Our findings, which showed that the majority of the patients with fracture/SCI had accelerated fracture healing and callus formation, support the hypothesis that SCI enhances fracture healing. Compared with denervation, we consider that osteo-inductive factors play a dominant role during bone healing after SCI, as accelerated fracture healing and enhanced callus formation were observed in the majority of patients with fracture/SCI (15 of 22).
Our investigation also revealed an interesting finding that has never been previously reported: enhanced osteogenesis is mainly observed in patients with cervical (85.7%) and thoracic (83.3%) SCI, and less so in patients with lumbar SCI (44.4%). However, these differences were not statistically significant. We suggest that this may be due to the small number of patients in the study. Although there have been no reports published with similar results to our study, there is evidence in studies of neurogenic heterotopic ossification (NHO) in SCI24–26 from which conclusions similar to ours can be drawn. It has been reported that complete transverse SCI is more commonly associated with NHO than incomplete SCI, and that NHO is less frequently reported in patients with lumbosacral or conus-cauda lesions.27 Complete SCI damage – including diffuse axonal injury, serious contusions, hypoxia, ischemia, and edema – can all lead to wider hypothalamic-pituitary-adrenal axis damage and dysfunction.28 Additionally, the pathophysiology is also more amplified than in incomplete SCI. Therefore, compared with incomplete SCI, complete SCI would lead to more osteo-inductive factor release and to the acceleration of fracture healing.
All patients with SCI in our study showed a complete motor and sensory function disorder on admission, but recovery of SCI below the conus medullaris level was better than that above this level during rehabilitation. Of the 22 patients with SCI in the study, 4 of the 9 patients with lumbar SCI had changed to incomplete status on discharge (1 patient improved from Frankel grade A to B; 3 patients improved from A to C), but only 2 of the 13 cervical and thoracic patients with SCI changed on discharge (Frankel grade A to B). In these patients with partial recovery of SCI, the effect of neural and hormonal factors on bone metabolism decreased gradually, and enhanced osteogenesis was correspondingly weakened. Therefore, the final mean callus volume of the patients with cervical and thoracic SCI was significantly larger than with the lumbar SCI group, although the one-way ANOVA test did not detect a significant effect on fracture healing time in the three subgroups.
Conclusion
In this study, we have demonstrated that SCI is associated with accelerated fracture healing and enhanced callus formation. Our study also observed a trend towards enhanced osteogenesis in cervical and thoracic SCI compared with lumbar SCI. This investigation has several limitations, in particular, the 4-week interval for follow-up may lead to some inaccuracy in determining the exact time to union, but due to the factors of economy and ethics (more cost and larger amount of radiation dosage), it is very difficult for us to do more frequent follow-up. Therefore, similar to other researchers and authors referred to in our paper, we prefer to use clinical assessment every week to reduce the error. Other limitations including the small number of cases, the diversity of the patterns of SCI and lack of comparison with the time interval to surgical treatment of the bone fracture. As the effect of SCI on bone formation and fracture healing is complex, further understanding and identification of the underlying mechanisms and related factors are necessary.
References
- 1.Cipriano CA, Pill SG, Keenan MA. Heterotopic ossification following traumatic brain injury and spinal cord injury. J Am Acad Orthop Surg 2009;17(11):689–97 [DOI] [PubMed] [Google Scholar]
- 2.Giannoudis PV, Mushtaq S, Harwood P, Kambhampati S, Dimoutsos M, Stavrou Z, et al. Accelerated bone healing and excessive callus formation in patients with femoral fracture and head injury. Injury 2006;37Suppl 3:S18–24 [DOI] [PubMed] [Google Scholar]
- 3.Newman RJ, Stone MH, Mukherjee SK. Accelerated fracture union in association with severe head injury. Injury 1987;18(4):241–6 [DOI] [PubMed] [Google Scholar]
- 4.Boes M, Kain M, Kakar S, Nicholls F, Cullinane D, Gerstenfeld L, et al. Osteogenic effects of traumatic brain injury on experimental fracture-healing. J Bone Joint Surg Am 2006;88(4):738–43 [DOI] [PubMed] [Google Scholar]
- 5.Yang TY, Wang TC, Tsai YH, Huang KC. The effects of an injury to the brain on bone healing and callus formation in young adults with fractures of the femoral shaft. J Bone Joint Surg Br 2012;94(2):227–30 [DOI] [PubMed] [Google Scholar]
- 6.Ragnarsson KT, Sell GH. Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil 1981;62(9):418–23 [PubMed] [Google Scholar]
- 7.Ingram RR, Suman RK, Freeman PA. Lower limb fractures in the chronic spinal cord injured patient. Paraplegia 1989;27(2):133–9 [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Tang X, Zhang H, Yuan J, Ding H, Wei Y. Elevated leptin expression in rat model of traumatic spinal cord injury and femoral fracture . J Spin Cord Med 2011;34(5):501–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins R, Skirving AP. Callus formation and the rate of healing of femoral fractures in patients with head injuries. J Bone Joint Surg Br 1987;69(4):521–4 [DOI] [PubMed] [Google Scholar]
- 10.Little DG, Ramachandran M, Schindeler A. The anabolic and catabolic responses in bone repair. J Bone Joint Surg Br 2007;89(4):425–33 [DOI] [PubMed] [Google Scholar]
- 11.Campagnolo DI, Bartlett JA, Chatterton R Jr, Keller SE. Adrenaland pituitary hormone patterns after spinal cord injury. Am J Phys Med Rehabil 1999;78(4):361–6 [DOI] [PubMed] [Google Scholar]
- 12.Wang YH, Huang TS, Lien IN. Hormone changes in men with spinal cord injuries. Am J Phys Med Rehabil 1992;71(6):328–32 [DOI] [PubMed] [Google Scholar]
- 13.Pasinetti GM, Nichols NR, Tocco G, Morgan T, Laping N, Finch CE. Transforming growth factor beta 1 and fibronectin messenger RNA in rat brain: responses to injury and cell-type localization. Neuroscience 1993;54(4):893–907 [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, Yoon SS, Kim YH, Ryu JS. Serial measurement of interleukin-6, transforming growth factor-beta, and S-100 protein in patients with acute stroke. Stroke 1996;27(9):1553–7 [DOI] [PubMed] [Google Scholar]
- 15.Huang TS, Wang YH, Lee SH, Lai JS. Impaired hypothalamuspituitary-adrenal axis in men with spinal cord injuries. Am J Phys Med Rehabil 1998;77(2):108–12 [PubMed] [Google Scholar]
- 16.Olle MM, Pivarnik JM, Klish WJ, Morrow JR Jr. Body composition of sedentary and physically active spinal cord injured individuals estimated from total body electrical conductivity. Arch Phys Med Rehabil 1993;74(7):706–10 [DOI] [PubMed] [Google Scholar]
- 17.Cadosch D, Gautschi OP, Thyer M, Song S, Skirving AP, Filgueira L, et al. Humoral factors enhance fracture-healing and callus formation in patients with traumatic brain injury. J Bone Joint Surg Am 2009;91(2):282–8 [DOI] [PubMed] [Google Scholar]
- 18.Li S, Zhao H, Wei S, An Z, Xie Q, Li X, et al. The effects of leptin on proliferation and function of human osteoblast. Hua Xi Yi Ke Da Xue Xue Bao 2001;32(2):240–2 [PubMed] [Google Scholar]
- 19.Freehafer AA, Yurick R, Mast WA. Para-articular ossification in spinal cord injury. Med Serv J Can 1966;22(7):471–8 [PubMed] [Google Scholar]
- 20.Nottage WM A review of long-bone fractures in patients with spinal cord injuries. Clin Orthop 1981;155:65–70 [PubMed] [Google Scholar]
- 21.Garland DE Clinical observations on fractures and heterotopic ossification in the spinal cord and traumatic brain injured populations. Clin Orthop Relat Res 1988;233:86–101 [PubMed] [Google Scholar]
- 22.Miyamoto T An experimental study on fracture healing in paraplegic rats. Nippon Seikeigeka Gakkai Zasshi 1987;61(10):1135–45 [PubMed] [Google Scholar]
- 23.Ding WG, Jiang SD, Zhang YH, Jiang LS, Dai LY. Bone loss and impaired fracture healing in spinal cord injured mice. Osteoporos Int 2011;22(2):507–15 [DOI] [PubMed] [Google Scholar]
- 24.Knudsen L, Lundberg D, Ericsson G. Myositis ossificans circumscripta in para-/tetraplegics. Scand J Rheum 1982;11(1):27–31 [DOI] [PubMed] [Google Scholar]
- 25.Lal S, Hamilton BB, Heinemann A, Betts HB. Risk factors for heterotopic ossification in spinal cord injury. Arch Phys Med Rehab 1989;70(5):387–90 [PubMed] [Google Scholar]
- 26.Bravo-Payno P, Esclarin A, Arzoz T, Arroyo O, Labarta C. Incidence and risk factors in the appearance of heterotopic ossification in spinal cord injury. Paraplegia 1992;30(10):740–5 [DOI] [PubMed] [Google Scholar]
- 27.Abramson DJ, Kamberg S. Spondylitis, pathological ossification, and calcification associated with spinal cord injury. J Bone Joint Surg 1949;31(2):275–83 [PubMed] [Google Scholar]
- 28.Wilcockson DC, Campbell SJ, Anthony DC, Perry VH. The systemic and local acute phase response following acute brain injury. J Cereb Blood Flow Metab 2002;22(3):318–26 [DOI] [PubMed] [Google Scholar]


