Abstract
Quinoa (Chenopodium quinoa Willd.) contains high levels of biologically active phytoecdysteroids, which have been implicated in plant defense from insects, and have shown a range of beneficial pharmacological effects in mammals. We demonstrated that the most prevalent phytoecdysteroid, 20-hydroxyecdysone (20HE), was secreted (leached) from intact quinoa seeds into water during the initial stages of seed germination. Leaching efficiency was optimized by ethanol concentration (70% ethanol), temperature (80°C), time (4 h), and solvent ratio (5 ml/g seed). When compared to extraction of macerated seeds, the leaching procedure released essentially all the 20HE available in the seeds (491 μg/g seed). The optimized quinoa leachate (QL), containing 0.86% 20HE, 1.00% total phytoecdysteroids, 2.59% flavonoid glycosides, 11.9% oil, and 20.4% protein, significantly lowered fasting blood glucose in obese, hyperglycemic mice. Leaching effectively releases and concentrates bioactive phytochemicals from quinoa seeds, providing an efficient means to produce a food-grade mixture that may be useful for anti-diabetic applications.
Keywords: ecdysterone, flavonoids, metabolic syndrome, nutraceutical, phytoecdysteroids
1. Introduction
Quinoa (Chenopodium quinoa Willd., Amaranthaceae), an ancient crop from the Andes Mountains of South America, is rapidly gaining popularity as a functional food and nutraceutical (Vega-Galvez, Miranda, Vergara, Uribe, Puente, & Martinez, 2010). Due to quinoa’s nutritious and stress tolerant properties, the Food and Agriculture Organization of the United Nations launched the International Year of Quinoa in 2013 to promote the worldwide production, consumption, technological development and biodiversity preservation of this crop (FAO, 2012). The focus of quinoa’s health benefits in scientific literature has primarily centered upon its macro- and micro-nutrient profile, especially its complete amino acid content. However, little work has been done to investigate the role of quinoa’s secondary metabolites in human health.
Among agricultural food crops that are regularly sold and consumed in the United States, quinoa is a rich source of phytoecdysteroids (Kumpun, Maria, Crouzet, Evrard-Todeschi, Girault, & Lafont, 2011; Zhu, et al., 2001a). Phytoecdysteroids are polyhydroxylated steroids, structurally related to insect molting hormones, that have been implicated in plant defense by deterring insect herbivory, delaying insect development and causing lethality to insect larvae (Arnault & Slama, 1986; Blackford & Dinan, 1997; Marion-Poll & Descoins, 2002; Rharrabe, Sayan, & Lafont, 2010; Singh & Russell, 1980). Phytoecdysteroids have also shown a wide range of therapeutic effects in mammals, including anabolic, performance enhancing (Gorelick-Feldman, MacLean, Ilic, Poulev, Lila, Cheng, & Raskin, 2008; Slama & Lafont, 1995), anti-osteoporotic (Kapur, Wuttke, Jarry, & Seidlova-Wuttke, 2010; Seidlova-Wuttke, Christel, Kapur, Nguyen, Jarry, & Wuttke, 2010) and wound healing properties (Syrov & Khushbaktova, 1996). These molecules are considered the primary bioactive components of the traditional Chinese and Siberian herbs Ajuga turkestanica, Rhaponticum carthamoides, and Cyanotis vaga (Gorelick-Feldman, et al., 2008; Kokoska & Janovska, 2009; Lafont, 1998). Phytoecdysteroids, extracted from these botanical sources, have been marketed and sold in commercially available health products, such as adaptogens, body building agents, stress reducers, performance enhancers and cosmetics (Bathori, 2002). Recent literature also suggests that the most prevalent phytoecdysteroid, 20-hydroxyecdysone (20HE, also known as ecdysterone or β-ecdysone) may play a role in the treatment and prevention of diabetes and obesity. Administration of 20HE (10 mg/kg body weight for 13 weeks) to diet-induced obese, hyperglycemic mice significantly lowered blood glucose levels, increased insulin sensitivity, decreased body weight and reduced adiposity 41% compared with the control without affecting food consumption (Kizelsztein, Govorko, Komarnytsky, Evans, Wang, Cefalu, & Raskin, 2009).
Diabetes is a metabolic disorder that has reached epidemic proportions worldwide, especially among low and middle-income countries and communities, thereby demanding effective, sustainable and affordable intervention strategies aimed at glycaemic control (Zimmet, Magliano, Herman, & Shaw, 2014). While slow growing, wildcrafted perennial herbs have traditionally been harvested as a source of phytoecdysteroids, cultivated annual edible plants can provide a renewable source of these hypoglycemic molecules. Furthermore, a rapid, efficient, food-grade procedure for concentrating phytoecdysteroids from edible sources may facilitate the development of cost-effective therapeutics that can be integrated into the diet.
The highest concentration of 20HE previously reported in an extract of an edible crop was achieved through a multi-step solvent extraction of spinach leaves (Spinacia oleracea L.) (Gorelick-Feldman, et al., 2008). Quinoa seeds, however, contain 4 – 12 times more 20HE by dry weight (184 – 484 μg/g) (Kumpun et. al., 2011) than spinach leaves (40 μg/g) (Gorelick-Feldman, et al., 2008), and are therefore more attractive for the development of 20HE-enriched botanicals. The elimination of hazardous organic solvents from the extraction procedure is also important for the development of food-grade technologies.
Foucault et al. (2011) has previously reported a method for producing a conventional 20HE-enriched extract (1.5 – 2.0% 20HE w/w) from quinoa seeds. However, this method resulted in a relatively low yield (10 mg extract/g seeds) and necessitated pulverization of the seeds upon boiling, followed by several extractions and drying steps.
We aimed to develop a one-step, non-destructive, high-yield process for concentrating phytoecdysteroids from quinoa seeds. To do so, we investigated the phenomenon of leaching of phytoecdysteroids from intact seeds into solution. We hypothesized that, if phytoecdysteroids play a role in the defense of quinoa seeds from insect herbivory, the molecules would leach from intact seeds into an aqueous environment during the initial stages of seedling germination. Therefore, the maximum secretion of phytoecdysteroids from seeds into water at environmental temperatures was measured, and optimized leaching using ethanol and heat. Furthermore, following on from earlier reports of the anti-diabetic properties of 20HE, we investigated the hypoglycemic activity of quinoa leachate (QL), in a high fat diet-induced, hyperglycemic C57Bl/6J mouse model.
2. Materials and Methods
2.1. Materials
Bolivian-grown red quinoa seeds were purchased from AlterEco, Inc. (San Francisco, CA), containing 11% protein, 10% carbohydrate and 4% fat (Nutrition Facts, AlterEco Red Quinoa). Seeds were polished and washed by the producer to remove the outer layer of bitter saponins before distribution. All chemical reagents were obtained from Sigma (St. Louis, MO), unless otherwise specified. All solvents used for compound isolation were HPLC grade. All water used in the experiments was purified using a Millipore water purification system with a minimum resistivity of 18.2 MΩ cm (Bedford, MA).
2.2. Aqueous leaching of 20HE
To prevent bacterial and fungal contamination, quinoa seeds were first surface-sterilized by immersion in 70% ethanol for 1 minute, followed by 12 minutes in 1.5% sodium hypochlorite, and rinsed three times with sterile water. Seeds were dried overnight on sterile filter paper. Seeds (≈0.25 g) were incubated in 1 ml sterile ddH2O in culture tubes for 24, 48, 72, 96 or 144 h (three replicates per time point) on a shaker at 160 rpm/ min in light and dark conditions at 25 or 37°C. We considered the effect of light versus dark conditions on 20HE leaching due to the possibility that quinoa seeds may synthesize 20HE during the germination process. 20HE leaching was monitored at room temperature (25°C) and elevated temperature (37°C), since heat is an important factor in chemical stability, solubility and seed germination rates. At the end of each incubation time point, the leachate from each sample was filtered through a 0.45 μm syringe filter (Corning, Inc., Corning, NY) into pre-weighed Eppendorf tubes and dried by speed vacuum followed by lyophilization. The dried leachate weights were recorded and each sample was redissolved in water to a concentration of 5 mg/ml for LC-UV-MS injection in 5 μl volumes.
2.3. Optimization of leaching in ethanol
Quinoa seeds (1.6 g) were incubated in 8 ml aqueous ethanol at varying concentrations (0, 25, 33, 40, 50, 60, 70, 80 or 95%) in 15 ml tubes on a shaker at 200 rpm/min in the dark over a range of temperatures (25 – 80°C) and time points (1 – 24 h). Seeds were leached for 24 h at lower temperatures (25 and 37°C) and up to 8 h at higher temperatures (50 and 80°C). Using the optimal solvent, temperature and time conditions, 20 minutes sonication and solvent acidification (1% acetic acid) were also tested for their effects on 20HE leaching. Leachates were filtered through 0.45 μm syringe filters into 8 ml glass vials and dried by speed vacuum followed by lyophilization. The dried leachate weights were determined. Aliquots of each sample were redissolved in their corresponding solvent (0–95% ethanol) at a concentration of 5 mg/ml and injected in the LC-UV-MS in 1–5 μl volumes.
The optimal leaching conditions were determined by three factors: (1) the amount of 20HE released from the seeds into solution (μg 20HE/g seed), (2) the 20HE concentration in final dried leachate (μg 20HE/mg), and (3) the yield, or the amount of leachate obtained per g of seed (mg/g seed).
2.4. Seed extraction
Quinoa seeds were pulverized in a coffee grinder (Krups, Inc.). The seed powder (1.6 g) was mixed with 8 ml 70% ethanol for 4 h at 25 or 80°C, centrifuged at 4,000 rpm for 10 min at 4°C, filtered, dried, and analyzed via LC-UV-MS. In order to compare extraction of macerated seed powder with the one-step process of leaching intact seeds, the seed powder was also extracted at 80°C three times consecutively, using 5 ml of fresh 70% ethanol per g seed powder each time.
2.5. Production and analysis of quinoa leachate (QL) for biological study
Quinoa seeds (1 kg) were rinsed in cold water for 5 min and leached in 5 l of solvent under the optimized conditions (70% ethanol, 80°C, 4 h). The leachate was filtered through a Whatman filter paper, dried and weighed. An aliquot of QL was resuspended in 30% acetonitrile at a concentration of 20 mg/ml, filtered, and injected in the LC-UV-MS in 3 μl volumes for analysis of the total phytoecdysteroid and total flavonoid glycoside content. A separate aliquot of QL (30 g) was used for proximate nutritional and amino acid profile analysis by Eurofins Nutrition Analysis (De Moines, IA) according to standard methods. The concentration of the total phytoecdsyteroids and flavonoid glycosides in QL was subtracted from the crude fat/oil content, which was determined via extraction with diethyl ether and petroleum ether. The carbohydrate content was calculated as 100% minus the sum of the other phytochemical and nutritional components (phytoecdysteroids, flavonoid glycosides, protein, fat/oil, moisture, ash). The remaining QL was used for biological study.
2.6. Isolation and identification of quercetin trisaccharide I (QT-I)
The major flavonoid glycoside present in QL (indicated as peak 3 in Fig. 4. and listed as quercetin trisaccharide I in Table 2) was isolated as follows: QL (14 g) was suspended in water containing 1.0% acetic acid and applied to a pre-conditioned Phenomonex® Strata C18-E (55 μm, 70 Å) solid phase extraction (SPE) cartridge. QL components were eluted into 3 fractions using 3 bed volumes each of water, 70% ethanol and 95% ethanol, successively. Each elution solvent contained 1.0% acetic acid to maintain the flavonoid glycoside stability. The 70% ethanol fraction, determined to contain all the QL flavonoid glycosides by LC-UV-MS analysis, was dried, weighed (1.89 g) and further fractionated using Bench Scale Fast Centrifugal Partition Chromatography (FCPC) Kromatons v. 1.0. with a stationary phase of 4:1 BuOH:EtOAc and a mobile phase of water. Thirteen fractions were collected. QT-I, determined by LC-UV-MS to be concentrated in fraction 6 (FCPC 40 – 65 min, 247 mg), was isolated using preparative reverse phase HPLC (Waters 616 four channel pump with semi-preparative pump heads operated on a Waters 600 Controller; Waters 490E Programmable Multiwavelength Detector set to monitor at 254 nm; Waters 717 Plus Autosampler; Phenomenex® semi-preparative Synergi Hydro C8 column 4μM, 250 × 20 mm; isocratic 15:85 acetonitrile:water containing 0.1% TFA over 60 min, flow rate 10 ml/min), yielding 10.0 mg QT-I (90% pure, tR = 27 min). QT-I was re-purified by RP-HPLC (isocratic 14:86 acetonitrile:water containing 0.1% TFA over 75 min, 10 ml/min), yielding 3.0 mg (99% pure, tR = 50 min). The isolated compound was identified from its mass spectra, 1D and 2D NMR spectra (1H, 13C, COSY, DEPT, HMQC and HMBC) recorded on a Bruker Avance 700 MHz spectrometer (Bruker BioSpin Corporation, Billerica, MA), using CD3OD as the solvent, and by comparison with data reported in the literature (Zhu, Sheng, Li, Lavoie, Karwe, Rosen, & Ho, 2001b).
Figure 4. Quinoa leachate (QL) lowers fasting blood glucose (FBG) in obese, hyperglycemic C57BL/6J mice.
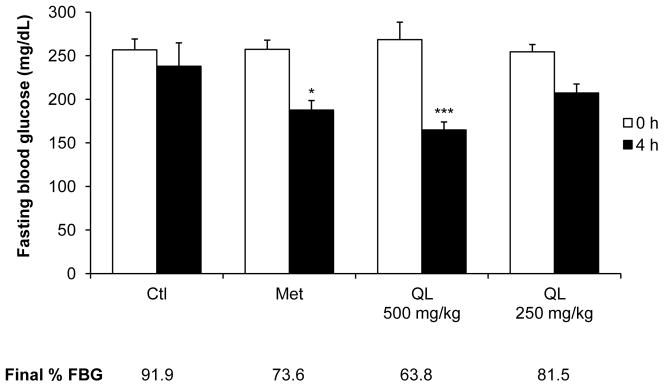
QL was dissolved in 70% Labrasol (vehicle). FBG was determined via tail-nick before and 4 h after administration of vehicle (Ctl), 300 mg/kg Metformin® (Met, positive control), or QL (250 and 500 mg/kg). Data are the mean ± SEM (n=7). Values reported below the x-axis are the results of mean final % FBG for each group. Final % FBG was calculated for individual mice as post-treatment FBG/ pre-treatment FBG x 100. *P < 0.05 and ***P < 0.001 (1-way ANOVA followed by Dunnett’s post hoc test compared to control).
Table 2.
Retention time, putative chemical identification, m/z and concentration of phytochemicals in quinoa leachate (QL).
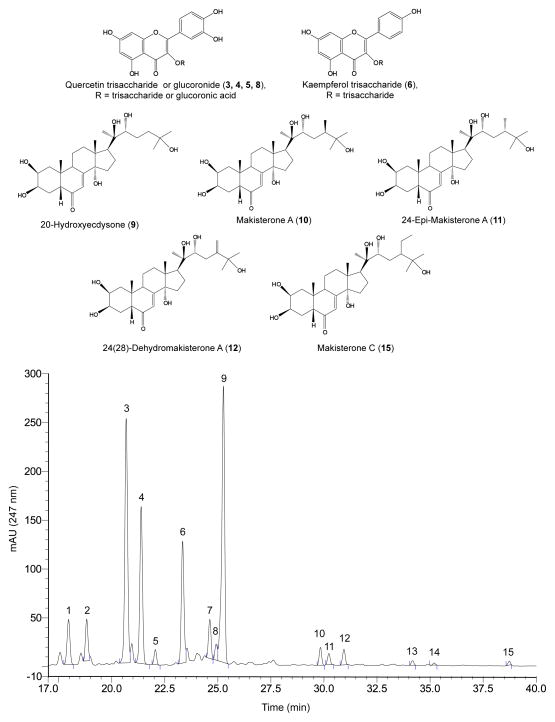
| Peak No.b | tR (min) | Compound | [M+H]+ (m/z) | [M-H]− (m/z) | Fragment (m/z) | Conc. (mg/g) |
|---|---|---|---|---|---|---|
| 1 | 17.97 | Triterpenoid derivative I | 664c | - | ||
| 2 | 18.82 | Triterpenoid derivative II | 664c | - | ||
| 3 | 20.68 | Quercetin trisaccharide Id | 757 | 755 | 611, 303 | 11.68 |
| 4 | 21.39 | Quercetin trisaccharide II | 743 | 741 | 611, 465, 303 | 7.21 |
| 5 | 22.06 | Quercetin trisaccharide III | 743 | 741 | 611, 303 | 0.69 |
| 6 | 23.34 | Kaempferol trisaccharide | 741 | 739 | 595, 449, 287 | 5.59 |
| 7 | 24.62 | Triterpenoid derivative III | 609c | - | ||
| 8 | 24.92 | Quercetin glucoronide | 479 | 303 | 0.72 | |
| 9 | 25.26 | 20-Hydroxyecdysone | 481 | 539c | 463 | 8.60 |
| 10 | 29.83 | Makisterone A | 495 | 477, 459, 441 | 0.46 | |
| 11 | 30.23 | 24-Epi-makisterone A | 495 | 477, 459, 441 | 0.30 | |
| 12 | 30.94 | 24(28)-Dehydromakisterone A | 493 | 475, 457, 439 | 0.45 | |
| 13 | 34.18 | Ecdysteroid | 465 | 464 | 447, 429 | 0.12 |
| 14 | 35.19 | Steroid | 427 | - | ||
| 15 | 38.73 | Makisterone C | 509 | 491 | 0.10 | |
|
| ||||||
| Total phytoecdysteroids | 10.03 | |||||
| Total flavonoid glycosides | 25.89 | |||||
tR= Retention time
Peak number, refer to Fig. 3
The reported [M-H]− represents an acetic acid adduct [M-H+C2H4O2]-.
Quercetin trisaccharide I (QT-I) was identified as quercetin 3-O-(2,6-di-α-L-rhamno-pyranosyl)-β-D-galactopyranoside.
2.7. LC-UV-MS analysis
Standards of 20HE (99% pure, Bosche Scientific, New Brunswick, NJ) and makisterone A (98% pure, A.G. Scientific, Inc., #M1-1080) were dissolved in 70% ethanol at concentrations of 1, 0.1, 0.05 and 0.01 mg/ml and used for chemical identification and quantification by LC-UV-MS at 247 nm with 1 μl injection volumes. QT-I (99% pure, isolated as described above) was dissolved in 95% ethanol at the same concentrations and used as an external standard. Compounds for which no standards were available were putatively identified using their retention time, mass signal for the precursor ion ([M+H]+, [M-H]−) and fragment ions, fragmentation pattern and molecules reported in the literature. 20HE analogs (24-epi-makisterone A, 24(28)-dehydromakisterone A, and makisterone C) were calculated as 20HE equivalents (concentrations of individual molecules were estimated using a standard of 20HE). Concentrations of quercetin and kaempferol glycosides were calculated from the peak areas recorded at 247 nm to the calibration curve obtained with QT-I. Results of flavonoid concentrations were expressed as QT-I equivalents.
Analysis was performed using the Dionex® UltiMate 3000 RSLC ultra-high pressure liquid chromatography system, consisting of a workstation with Dionex’s Chromeleon v. 6.8 software package, solvent rack/degasser SRD-3400, pulseless chromatography pump HPG-3400RS, autosampler WPS-3000RS, column compartment TCC-3000RS and photodiode array detector DAD-3000RS. After the photodiode array detector the eluent flow was guided to a Varian 1200L (Varian Inc., Palo Alto, CA) triple quadrupole mass spectrometer with an electrospray ionization interface (ESI), operated in either positive (5 kV) or negative (-4.5 kV) ionization modes. The drying gas temperature was 280°C and nitrogen was the sheath gas. The mass detector was used in scanning mode from 65 to 1500 atomic mass units (amu). Data from the Varian 1200L mass detector was collected, compiled and analyzed using Varian’s MS Workstation, v. 6.41, SP2. Substances were separated on a Phenomenex® C8 reverse phase column, 150 × 2.0 mm, particle size 3 μm, pore size 100 Å. The mobile phase consists of 2 components: Solvent A (0.5% ACS grade acetic acid in ddH2O, pH 3-3.5) and Solvent B (100% acetonitrile). The mobile phase flow was 0.2 ml/min, and a gradient mode was used for all analyses. The initial conditions of the gradient were 93% A and 7% B; the proportion reached 73% A and 27% B over 40 min; solvent B reached 100% in the next 5 min and was maintained for 2 min; the column was re-equilibrated to initial conditions for 13 min. The total run time was set to 60 min.
2.8. Animal study
An acute hypoglycemic study in diet-induced obese mice was performed as described in Cheng et al. (2012). This study was carried out in accordance with the guidelines approved by the Institutional Animal Care and Use Committee at Rutgers University (Protocol Number: 04-023). Briefly, five-week-old male C57Bl/6J mice were purchased from Jackson Labs, acclimated for 1 wk with ad libitum access to chow (Purina, No. 5014) and water, and maintained on a very high-fat diet (VHFD) containing 60% fat-derived calories (D12492; Research Diets) for an additional 15 wk to induce obesity, insulin resistance and hyperglycemia. The food intake and body weight were monitored weekly. Following a 4 h fasting period, the fasting blood glucose (FBG) was measured using an AlphaTRAK® handheld glucometer (Abbott Labs, Inc.), and mice were randomly divided into FBG-balanced experimental groups. QL was formulated in 70% Labrasol® (Gattefossé Corp., Paramus, NJ). The biological activity of QL was tested using an oral ingestion of 0.25 ml per 50 g body weight of vehicle (70% Labrasol®) or QL (250 and 500 mg/kg) (n=7). Metformin® (300 mg/kg, dissolved in water) was administered as a positive control. FBG was reassessed 4 h post-treatment, and the final % FBG for each mouse was calculated individually using the equation: . The mean final % FBG values for each treatment group were compared to the vehicle by 1-way ANOVA followed by Dunnett’s post hoc test using Prism 6.0 (GraphPad Software, San Diego, CA). P<0.05 was considered significant.
3. Results
3.1. 20HE leaches from germinating seeds
20HE leached from quinoa seeds into water gradually over the course of 144 h, with greater leaching occurring at 37°C (294.0 μg/g seed) compared with at 25°C (156.5 μg/g seed) (Fig. 1). Seedlings germinated under all conditions. The greatest seedling growth occurred in the seeds incubated in the dark at 25°C after 96 hours. Light did not impact the amount of 20HE that was leached from the seeds compared with the seeds germinated in the dark.
Figure 1. 20-Hydroxyecdysone (20HE) leaches from quinoa seeds into water cumulatively over time.
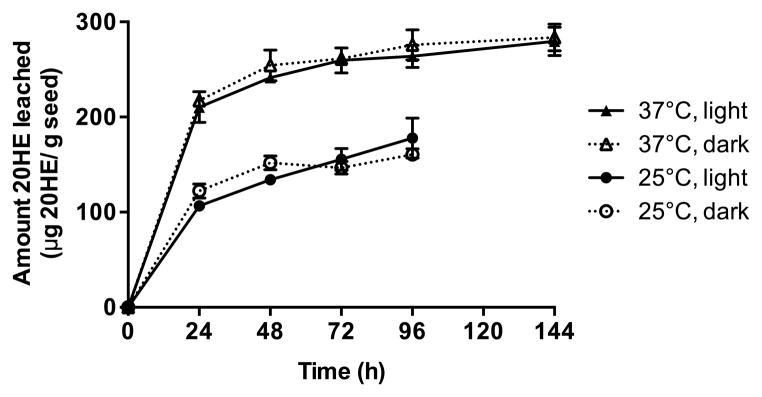
AlterEco Red quinoa seeds were surface sterilized and immersed into sterile water for 24–144 h under light or dark conditions at 25 or 37°C. 20HE content was determined in dried leachates by LC-UV-MS. Data are the mean ± SEM (n=3).
3.2. Optimal leaching conditions
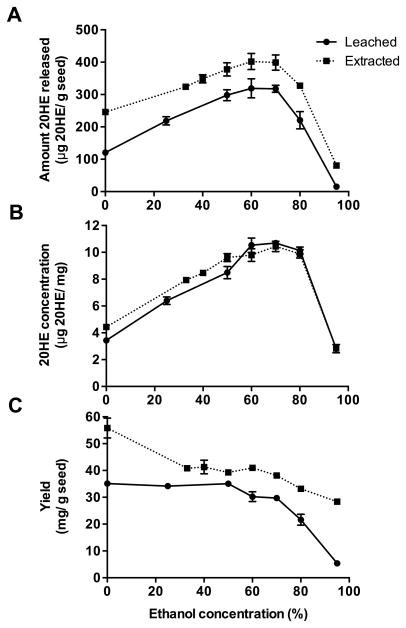
The optimal ethanol concentration for leaching 20HE from quinoa seeds at 25°C was 70% (Fig. 2A). Extracting the ground seeds in 70% ethanol at 25°C released 399 μg 20HE/g seed, whereas leaching intact seeds for 24 h released 317.8 μg 20HE/g seed (about 80% as efficient as extraction). Proportionally less seed material was solubilized into 70% ethanol during leaching compared to extraction. Therefore, the dried extracts and leachates had a similar 20HE content (10.4 and 10.7 μg 20HE/g seed, respectively) (Fig. 2B). The leachate yield, measured as the amount of dried leachate produced from seeds, was generally lower than that of the extract. For example, the leachate yield produced using 70% ethanol was 29.7 mg/g seed compared to 38.1 mg/g for the extract (Fig. 2C). The total amount of 20HE leached, concentration in the dry leachate and leachate yield all decreased dramatically in 95% ethanol.
Figure 2. Leaching versus extraction of 20-hydroxyecdysone (20HE) from quinoa seeds into varying concentrations of ethanol.
Intact quinoa seeds were incubated in ethanol (0–95%) at 25°C for 24 h to produce leachates, or macerated seed material was extracted in ethanol (0–95%) at 25°C for 4 h. The 20HE content was determined in dried leachates and extracts by LC-UV-MS. Three parameters were measured, shown from top to bottom: (A) amount 20HE released from seeds (μg 20HE/g seed), (B) 20HE concentration in the final dry leachate or extract (μg 20HE/mg), and (C) yield of dry leachate or extract (mg/g seed). Data are the mean ± SEM (n=3).
The effect of temperature on the efficiency of 20HE leaching into 70% ethanol as compared to extraction at the same temperature was also studied. At temperatures above 25°C, more seed material, including 20HE, was leached into the solvent (Table 1). Compared with the total 20HE that was obtained from repeated extraction of seeds, leaching intact seeds in 70% ethanol at 80°C for 4 h released the maximum amount of 20HE available in the seeds (491 μg/g) (Table 1). Under these conditions, the leachate yield was 4.5% and the 20HE content of the dried leachate was 1.09%. Leaching was further optimized by time (1–8 h) and solvent to seed ratio (1–5 ml/g seed), demonstrating that 4 h of leaching and 5 ml solvent/g seed were the optimal conditions for leaching most of the 20HE available in seeds and obtaining the highest yield of QL (Supplementary Fig. 1). Sonication and solvent acidification did not affect the amount of 20HE leached from the seeds or the 20HE concentration in the dried leachate (data not shown).
Table 1.
Leaching versus extraction of 20-hydroxyecdysone (20HE) from quinoa seeds at various temperatures.
| Leached | Extracted | ||||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | 25 | 37 | 50 | 80 | 25 | 80 | 80 |
| Time (h) | 24 | 24 | 4 | 4 | 4 | 4 | 4a |
| Amount 20HE released (μg 20HE/g seed) | 318 (±11.0) | 327 (±35.9) | 368 (±4.8) | 491 (±1.0) | 399 (±23.6) | 423 (±1.6) | 449 (±18.8) |
| 20HE concentration (μg 20HE/mg) | 10.7 (±0.2) | 9.2 (±0.9) | 11.4 (±0.2) | 10.9 (±0.1) | 10.4 (±0.4) | 9.3 (±0.4) | 6.2 (±0.2) |
| Yield (mg/g seed) | 29.7 (±0.7) | 35.6 (±0.6) | 32.3 (±0.3) | 45.0 (±0.5) | 38.1 (±0.9) | 45.8 (±1.8) | 72.4 (±1.0) |
Intact quinoa seeds were incubated in 70% ethanol (5 ml/g seed) at increasing temperatures (25, 37, 50, or 80°C) for 4 or 24 h. For comparison, macerated seed powder was extracted in 70% ethanol (5 ml/g seed powder) at 25°C (single extraction) or 80°C (single extraction, or the same seed powder was extracted 3 times consecutively with fresh solvent). Leachates and extracts were dried and analyzed for 20HE content by LC-UV-MS. Data are the mean±SEM (n=3).
Macerated seed material was extracted 3 times consecutively.
3.3. Biochemical analysis of quinoa leachate (QL)
QL was prepared for phytochemical, nutritional, and biological study from 1 kg seed under optimal conditions, yielding 41.0 g QL. The phytochemical content was determined using LC-UV-MS (Table 2). Figure 3 shows the molecular structures and LC-UV chromatogram at 247 nm of the QL phytochemical components. Fifteen peaks corresponding with fifteen different molecules were putatively identified as listed in Table 2, including 4 quercetin glycosides, 1 kaempferol glycoside and 5 phytoecdysteroids (20HE, makisterone A, epi-makisterone A, 24(28)-dehydromakisterone A, and makisterone C). 20HE constituted 0.86% of QL, whereas the total phytoecdysteroid content was 1.00%. The major quercetin trisaccharide in QL (QT-I) was isolated as a yellow powder (99% pure,). Its MS showed a m/z 757 [M+H]+ and m/z 755 [M-H]− as molecular ion peaks, in the positive and negative ionization modes, respectively. The compound was identified as quercetin 3-O-(2,6-di-α-L-rhamno-pyranosyl)-β-D-galactopyranoside according to its MS, MS/MS and NMR spectra, and in comparison with the spectra reported in the literature (Zhu, Sheng, Li, Lavoie, Karwe, Rosen, & Ho, 2001b). The total flavonoid glycoside content, determined as QT-I equivalents, was 2.59% of QL. Other components, determined by proximate nutritional analysis of QL, included carbohydrates (53.6%), protein (20.4%), oil (11.9%), ash (6.97%) and moisture (3.53%). About 0.37% of QL consisted of branched chain amino acids (leucine, isoleucine, and valine) (Supplementary Table 1). LC-MS analysis of QL also revealed trace amounts of saponins (data not shown).
Figure 3. Molecular structures and LC-UV chromatogram at 247 nm of phytochemicals putatively identified in QL.
QL was dissolved in 30% acetonitrile (20 mg/ml) and 3 μl was injected for chemical characterization alongside standards of 20HE, makisterone A and quercetin trisaccharide I via LC-UV using a C8 column with a gradient of 7 to 27% acetonitrile over 40 min. Compounds were putatively identified by UV retention time, MS m/z and fragmentation pattern, and based on quinoa compounds reported in the literature. Numbers indicate peaks corresponding with the compounds listed in Table 2.
3.4 In vivo hypoglycemic effect
Acute oral administration of QL demonstrated a dose dependent decrease in FBG in diet-induced obese, hyperglycemic mice (C57BL/6J) with FBG levels above 200 mg/dL. Relative to the initial FBG levels of individual mice, 500 and 250 mg/kg QL decreased te FBG by an average of 36.2% and 18.5%, respectively. Metformin (300 mg/kg) decreased FBG by 26.4%. Metformin and the higher dose of QL (500 mg/kg) significantly decreased final % FBG compared with the control by 1-way ANOVA followed by Dunnett’s post hoc test (Fig. 4).
4. Discussion
This work demonstrated for the first time that 20HE is leached or secreted from germinating quinoa seeds. At 37°C, approximately 60% of the total 20HE in intact quinoa seeds was leached into water. Phytoecdysteroids are structurally related to insect molting hormones, and have shown a range of effects on insects, including deterrence of leaf herbivory, modulation of insect taste receptors, the delay of larval development and lethality (Arnault & Slama, 1986; Blackford & Dinan, 1997; Marion-Poll, et al., 2002; Rharrabe, et al., 2010; Singh, et al., 1980). A range of soil insects that feed on seeds, seedlings and roots (wireworms, cutworms, thirps, grubs, flea beetles, billbugs and diabrotica beetles) are known predators of other Andean crops, such as maize and potatoes (O’Day, Becker, Keaster, Kabrick, & Steffey, 1998). It is tempting to speculate that phytoecdysteroid leaching plays a role in quinoa’s defense from soil insects during seed germination and seedling production. The co-leaching of 20HE, minor phytoecdysteorids and other secondary metabolites (flavonoids, saponins) may provide quinoa seeds with complimentary, multitarget defense mechanisms against soil pathogens and herbivores.
The fast, efficient method described here for leaching and concentrating 20HE from quinoa seeds enhanced the 20HE content in QL 17.5-fold, compared with the concentration found in the seeds. Post-leached seeds retained their shape, form and colour. The seed by-products were sterilized by 4 h incubation in 70% ethanol, and still contained the majority of their macronutrient content following the leaching process (95.9% of initial seed weight). Post-leached seed material may still be used for human or animal nutrition. Post-leached quinoa seeds can be toasted and consumed directly as “popped quinoa”, incorporated in health food bars and snacks as whole seeds, milled to produce quinoa flakes, or ground to make quinoa flour.
QL significantly decreased the blood glucose levels in a diet-induced obese, hyperglycemic mouse model. Acute studies in this model have been routinely used to demonstrate the in vivo anti-diabetic effects of several pharmacological agents and botanicals (Cheng, Kuhn, Poulev, Rojo, Lila, & Raskin, 2012; Grace, et al., 2009; Kellogg, et al., 2010; Ribnicky, et al., 2009; Rojo, et al., 2012; Roopchand, et al., 2012). The acute hypoglycemic effects of QL, combined with previous reports on the anti-diabetic and anti-obesity effects of chronic 20HE administration, support QL’s potential to treat or prevent hyperglycemia and insulin resistance associated with human metabolic syndrome (Foucault, Mathe, Lafont, Even, Dioh, Veillet, Tome, Huneau, Hermier, & Quignard-Boulange, 2011; Kizelsztein, et al., 2009).
Other components of QL, including flavonoids (quercetin and kaempferol glycosides), fatty acids and amino acids, may potentiate or synergize with 20HE’s pathogen / herbivore-protective affect as well as its anti-diabetic activity. Flavonoids (2.59% of QL) have been widely reported to have antioxidant, anti-diabetic, anti-obesity, anti-hypertensive, anti-cancer and anti-inflammatory properties (Da-Silva, et al. 2007; Jeong, Kang, Choi, Kim, & Kim, 2012; Kelly, 2011; Saragusti, Ortega, Cabrera, Estrin, Marti, & Chiabrando, 2010). Quinoa oil (11.9% of QL) is largely composed of omega-3 and omega-6 fatty acids, which have been shown to improve insulin sensitivity and cardiovascular health (Vega-Galvez, et al., 2010). Meanwhile, amino acids like leucine (0.08% QL) may control blood sugar levels and weight gain in animals (Zhang, Guo, LeBlanc, Loh, Schwartz, & Yu, 2007), while promoting insulin secretion from pancreatic β cells (Yang, Chi, Burkhardt, Guan, & Wolf, 2010).
QL also contains trace amount of saponins. Saponins are sometimes considered toxic due to their hemolytic effect when in direct contact with the blood. However, saponins are known to have low toxicity following oral ingestion, are safe for topical exposure, and are even reported to have beneficial internal effects, including lowering cholesterol, enhancement of mucosal drug absorption, antimicrobial activity and anti-inflammatory effects (Kuljanabhagavad & Wink, 2009; Shi, Arunasalam, Yeung, Kakuda, Mittal, & Jiang, 2004). Most commercially available quinoa seeds are polished and washed to remove most saponins, and the seeds can be rinsed again before leaching to further reduce the saponin content in QL. A 2 g dose of QL would deliver a saponin content that is less than the intake associated with the consumption of 1 serving of quinoa, well within a safe range (Zevallos, Herencia, Chang, Donnelly, Ellis, & Ciclitira, 2014).
Our biochemical characterization corroborates previous reports that quinoa seeds contain relatively high levels of phytoecdysteroids. Zhu et al. (2001a) were the first to isolate and identify phytoecdysteroids from quinoa seeds, reporting 30 μg 20HE/g seed. However, a later study, which screened the 20HE content from various sources of quinoa seeds, found a range of 184 – 484 μg 20HE/g seed (Kumpun et al., 2011). 20HE constituted 61.9% and 88.6% of the total phytoecdysteroids isolated from quinoa seeds in these studies, respectively. In our study, AlterEco red quinoa seeds contained 491.3 μg 20HE/g seed, constituting 85.7% of total phytoecdysteroids.
As noted by Kumpun et al. (2011), 20HE levels in quinoa seeds can vary greatly depending on the source. However, the genetic and environmental factors affecting phytoecdysteroid content in quinoa seeds are currently unknown. Leaching in 70% ethanol may be a simple method to evaluate phytoecdysteroid content among differing quinoa seed sources.
5. Conclusion
We have demonstrated that quinoa seeds leach the majority of their stored phytoecdesteroids, along with several other biologically active compounds, such as flavonoids, into the surrounding medium, possibly as a defense mechanism against soil insects. The leaching process was optimized to concentrate the anti-diabetic quinoa constituents. QL production leaves behind mostly intact quinoa seeds that still contain majority of their macronutrient reserves (protein, starch and oil), which can be recycled as food ingredients. Therefore, quinoa seed leaching may be a way to harness the clinically therapeutic benefits of quinoa phytochemicals while preserving the nutritional benefits of non-leached quinoa components. Our work contributes to the global initiative to promote the use of quinoa seeds in human health and sustainable development.
Supplementary Material
Highlights.
20-Hydroxyecdysone (20HE) and flavonoids can be effectively leached from quinoa seeds into water or aqueous ethanol.
Optimal solvent, temperature, and time conditions for leaching 20HE were established.
The leaching procedure released essentially all 20HE available in the seeds (491 μg/ g seed).
Quinoa leachate (QL) significantly lowered fasting blood glucose in obese, hyperglycemic mice.
Quinoa leaching may be useful for the production of anti-diabetic botanical therapeutics.
Acknowledgments
This study was supported by P50AT002776-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS), which funds the Botanical Research Center. The authors thank Carrie Waterman, Natasha Pogrebnyak, Mirjana Seskar, Tara Shertel, Ruth Dorn, Krishna Patel, and Amjad Saeed for their technical assistance, as well as Lena Struwe, Diana Roopchand, and Diana Ribnicky for editing.
Abbreviations
- ANOVA
analysis of variance
- Ctl
control
- FBG
fasting blood glucose
- 20HE
20-hydroxyecdysone
- LC-UV-MS
liquid chromatography-ultraviolet-mass spectrometry
- Met
metformin
- QL
quinoa leachate
- QT-I
quercetin trisaccharide I
- SEM
standard error of the mean
- tR
retention time
- VHFD
very high fat diet
Footnotes
Conflict of interest
IR and MAL have equity in Nutrasorb LLC, which is involved in quinoa R&D.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnault C, Slama K. Dietary effects of phytoecdysones in the leek-moth, Acrolepiopsis assectella Zell. (Lepidoptera: Acrolepiidae) J Chem Ecol. 1986;12(10):1979–1986. doi: 10.1007/BF01041947. [DOI] [PubMed] [Google Scholar]
- Bathori M. Phytoecdysteroids effects on mammalians, isolation and analysis. Mini Rev Med Chem. 2002;2:285–293. doi: 10.2174/1389557023406269. [DOI] [PubMed] [Google Scholar]
- Blackford MJP, Dinan L. The effects of ingested 20-hydroxyecdysone on the larvae of Aglais urticae, Inachis io, Cynthia cardui (Lepidoptera, Nymphalidae) and Tyria jacobaeae (Lepidoptera, Arctiidae) J Insect Physiol. 1997;43:315–327. doi: 10.1016/s0022-1910(96)00112-6. [DOI] [PubMed] [Google Scholar]
- Cheng DM, Kuhn P, Poulev A, Rojo LE, Lila MA, Raskin I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012;135(4):2994–3002. doi: 10.1016/j.foodchem.2012.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da-Silva WS, Harney JW, Kim BW, Li JM, Bianco SDC, Crescenzi A, Christoffolete MA, Huang SA, Bianco AC. The small polyphenolic molecule kaempferol increases cellular energy expenditure and thyroid hormone activation. Diabetes. 2007;56:767–776. doi: 10.2337/db06-1488. [DOI] [PubMed] [Google Scholar]
- FAO. Master plan for the international year of quinoa: a future sown thousands of years ago. 2012:1–26. [Google Scholar]
- Foucault AS, Mathe V, Lafont R, Even P, Dioh W, Veillet S, Tome D, Huneau JF, Hermier D, Quignard-Boulange A. Quinoa extract enriched in 20-hydroxyecdysone protects mice from diet-induced obesity and modulates adipokines expression. Obesity. 2011;20:270–277. doi: 10.1038/oby.2011.257. [DOI] [PubMed] [Google Scholar]
- Gorelick-Feldman J, MacLean D, Ilic N, Poulev A, Lila MA, Cheng D, Raskin I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J Agric Food Chem. 2008;56(10):3532–3537. doi: 10.1021/jf073059z. [DOI] [PubMed] [Google Scholar]
- Grace MH, Ribnicky DM, Kuhn P, Poulev A, Logendra S, Yousef GG, Raskin I, Lila MA. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16(5):406–415. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SM, Kang MJ, Choi HN, Kim JH, Kim JI. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr Res Pract. 2012;6(3):201–207. doi: 10.4162/nrp.2012.6.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur P, Wuttke W, Jarry H, Seidlova-Wuttke D. Beneficial effects of beta-edysone on the joint, epiphyseal cartilage tissue and trabecular bone in ovariectomized rats. Phytomedicine. 2010;17(5):350–355. doi: 10.1016/j.phymed.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Kellogg J, Wang J, Flint C, Ribnicky D, Kuhn P, Gonzalez de Mejia E, Raskin I, Lila MA. Alaskan wild berry resources and human health under the cloud of climate change. J Agric Food Chem. 2010;58:3884–3900. doi: 10.1021/jf902693r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly GS. Quercetin: monograph. Altern Med Rev. 2011;16(2):172–194. [PubMed] [Google Scholar]
- Kizelsztein P, Govorko D, Komarnytsky S, Evans A, Wang Z, Cefalu WT, Raskin I. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am J Physiol Endocrinol Metab. 2009;296(3):E433–439. doi: 10.1152/ajpendo.90772.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoska L, Janovska D. Chemistry and pharmacology of Rhaponticum carthamoides: a review. Phytochemistry. 2009;70:842–855. doi: 10.1016/j.phytochem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Kuljanabhagavad T, Wink M. Biological activities and chemistry of saponins from Chenopodium quinoa Willd. Phytochem Rev. 2009;8:473–490. [Google Scholar]
- Kumpun S, Maria A, Crouzet S, Evrard-Todeschi N, Girault JP, Lafont R. Ecdysteroids from Chenopodium quinoa Willd., an ancient Andean crop of high nutritional value. Food Chem. 2011;125(4):1226–1234. [Google Scholar]
- Lafont R. Phytoecdysteroids in world flora: diversity, distribution, biosynthesis and evolution. Russian journal of plant physiology: a comprehensive Russian journal on modern phytophysiology. 1998;45(3):276–295. [Google Scholar]
- Marion-Poll F, Descoins C. Taste detection of phytoecdysteroids in larvae of Bombyx mori, Spodoptera littoralis and Ostrinia nubilalis. J Insect Physiol. 2002;48(4):467–476. doi: 10.1016/s0022-1910(02)00068-9. [DOI] [PubMed] [Google Scholar]
- O’Day M, Becker A, Keaster A, Kabrick L, Steffey K. Corn Insect Pests: A Diagnostic Guide. Missouri Manual 166, Illinois Manual C1358. Columbia, Missouri: MU Extension, University of Missouri; 1998. [Google Scholar]
- Rharrabe K, Sayan F, Lafont R. Dietary effects of four phytoecdysteroids on growth and development of the Indian meal moth, Plodia interpunctella. J Insect Sci. 2010;10(13):1–12. doi: 10.1673/031.010.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribnicky DM, Kuhn P, Poulev A, Logendra S, Zuberi A, Cefalu WT, Raskin I. Improved absorption and bioactivity of active compounds from an anti-diabetic extract of Artemisia dracunculus L. Int J Pharm. 2009;370(1–2):87–92. doi: 10.1016/j.ijpharm.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo LE, Ribnicky D, Logendra S, Poulev A, Rojas-Silva P, Kuhn P, Dorn R, Grace MH, Lila MA, Raskin I. In vitro and in vivo anti-diabetic effects of anthocyanins from maqui berry (Aristotelia chilensis) Food Chem. 2012;131(2):387–396. doi: 10.1016/j.foodchem.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopchand DE, Grace MH, Kuhn P, Cheng DM, Plundrich N, Poulev A, Howell A, Fridlender B, Lila MA, Raskin I. Efficient sorption of polyphenols to soybean flour enables natural fortification of foods. Food Chem. 2012;131(4):1193–1200. doi: 10.1016/j.foodchem.2011.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragusti AC, Ortega MG, Cabrera JL, Estrin DA, Marti MA, Chiabrando GA. Inhibitory effect of quercetin on matrix metalloproteinase 9 activity: molecular mechanism and structure-activity relationship of the flavonoid-enzyme intereaction. Eur J Dermatol. 2010;644:138–145. doi: 10.1016/j.ejphar.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Seidlova-Wuttke D, Christel D, Kapur P, Nguyen BT, Jarry H, Wuttke W. Beta-ecdysone has bone protective but no estrogenic effects in ovariectomized rats. Phytomedicine. 2010;17(11):884–889. doi: 10.1016/j.phymed.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Shi J, Arunasalam K, Yeung D, Kakuda Y, Mittal G, Jiang Y. Saponins from edible legumes: chemistry, processing, and health benefits. J Med Food. 2004;7(1):67–78. doi: 10.1089/109662004322984734. [DOI] [PubMed] [Google Scholar]
- Singh P, Russell GB. The dietary effects of 20-hydroxyecdysone on the development of housefly. J Insect Physiol. 1980;26(2):139–142. [Google Scholar]
- Slama K, Lafont R. Insect hormones - ecdysteroids: their presence and actions in vertebrates. Eur J Entomol. 1995;92:355–377. [Google Scholar]
- Syrov VN, Khushbaktova ZA. Wound-healing effects of ecdysteroids. Doklady Akademii Nauk Respubliki Uzbekistana. 1996;12:47–50. [Google Scholar]
- Vega-Galvez A, Miranda M, Vergara J, Uribe E, Puente L, Martinez EA. Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd.). an ancient Andean grain: a review. J Sci Food Agric. 2010;90:2541–2547. doi: 10.1002/jsfa.4158. [DOI] [PubMed] [Google Scholar]
- Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev. 2010;68(5):270–279. doi: 10.1111/j.1753-4887.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevallos VF, Herencia LI, Chang F, Donnelly S, Ellis HJ, Ciclitira PJ. Gastrointestinal effects of eating quinoa (Chenopodium quinoa Willd.) in celiac patients. Am J Gastroenterology. 2014;109:270–278. doi: 10.1038/ajg.2013.431. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu Y. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56(6):1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- Zhu N, Kikuzaki H, Vastano BC, Nakatani N, Karwe MV, Rosen RT, Ho CT. Ecdysteroids of quinoa seeds (Chenopodium quinoa Willd.) J Agric Food Chem. 2001a;49(5):2576–2578. doi: 10.1021/jf0014462. [DOI] [PubMed] [Google Scholar]
- Zhu N, Sheng S, Li D, Lavoie EJ, Karwe MV, Rosen RT, Ho CT. Antioxidative flavonoid glycosides from quinoa seeds (Chenopodium quinoa Willd) J or Food Lipids. 2001b;8(1):37–44. [Google Scholar]
- Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endrocrinol. 2014;2:56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.