Summary
The corneal endothelium (CE) is a single layer of cells lining the posterior face of the cornea providing metabolic functions essential for maintenance of corneal transparency. Adult CE cells lack regenerative potential, and the number of CE cells decreases throughout life. To determine whether endogenous DNA damage contributes to the age-related spontaneous loss of CE, we characterized CE in Ercc1−/Δ mice, which have impaired capacity to repair DNA damage and age prematurely. Eyes from 4.5- to 6-month-old Ercc1−/Δ mice, age-matched wild-type (WT) litter-mates, and old WT mice (24- to 34-month-old) were compared by spectral domain optical coherence tomography and corneal confocal microscopy. Histopathological changes in CE were further identified in paraffin tissue sections, whole-mount immunostaining, and scanning electron and transmission electron microscopy. The CE of old WT mice displayed polymorphism and polymegathism, polyploidy, decreased cell density, increased cell size, increases in Descemet’s thickness, and the presence of posterior projections originating from the CE toward the anterior chamber, similar to changes documented for aging human corneas. Similar changes were observed in young adult Ercc1−/Δ mice CE, demonstrating spontaneous premature aging of the CE of these DNA repair–deficient mice. CD45+ immune cells were associated with the posterior surface of CE from Ercc1−/Δ mice and the tissue expressed increased IL-1α, Cxcl2, and TNFα, proinflammatory proteins associated with senescence-associated secretory phenotype. These data provide strong experimental evidence that DNA damage can promote aging of the CE and that Ercc1−/Δ mice offer a rapid and accurate model to study CE pathogenesis and therapy.
Keywords: aging, cornea, corneal endothelium, DNA repair, genotoxic stress, progeria
Introduction
The cornea is a remarkably transparent tissue that provides protection from environmental exposures and is critical for refracting incoming light. It is composed of three tissue layers (epithelium, stroma, and endothelium) each with distinct properties and functions. The corneal endothelium (CE) is the innermost and single cell layer that functions to maintain proper hydration of the corneal stroma and to allow nutrient and waste exchange with the aqueous humor. Functional CE cells are indispensable for maintaining corneal transparency. However, this single layer of cells is also very fragile and does not regenerate in vivo (Joyce, 2003). Loss of CE cells as a consequence of disease or acute trauma therefore leads to irreversible corneal edema and blindness (Bourne & McLaren, 2004). There is also a steady loss of CE cells in all individuals with aging, at a rate of 0.6% of central cell density per year (Bourne & McLaren, 2004). Currently, corneal transplantation is the only therapeutic option for corneal edema (Bourne & McLaren, 2004). In 2009, > 42 000 individuals in the USA required a corneal transplant, with CE degeneration being a major cause of the need for transplant (Ghosheh et al., 2008). The majority of corneal transplantation recipients are > 60 years old. Hence, discovering the driving force behind spontaneous loss of CE cells with aging is critically important.
Aging-related changes in CE cells include progressive polymegathism (increased cell size to compensate for the loss of cell number), pleomorphism (variability in cell morphology), decreased ability to pump fluid from the corneal stroma, and increased permeability attenuating their function as a barrier (Bourne & McLaren, 2004). In contrast to corneal epithelial cells and keratocytes (Hassell & Birk, 2010), human CE cells have very limited to no proliferative potential in vivo and are without a defined stem cell renewal system (Joyce, 2003). Although limbal stem cells replace the corneal epithelium, there is no evidence that these stem cells replace the CE. Corneal endothelium cells from older donors (> 50 years) have decreased proliferative capacity in vitro compared with cells from younger donors (< 30 years; Senoo & Joyce, 2000). Furthermore, CE cells display numerous features of senescent cells including increased expression of the cyclin-dependent kinase inhibitors p21CIP1 and p16INK4a (Enomoto et al., 2006), senescence-associated β-galactosidase staining (Mimura & Joyce, 2006; Song et al., 2008), and decreased sensitivity to mitogenic stimulation ex vivo (Joyce, 2005). Inhibition of p21 and p16 expression leads to increased CE proliferation ex vivo, demonstrating that these G1/S cell cycle inhibitors contribute to replicative senescence (Joyce & Harris, 2010).
It remains poorly understood why CE lacks regenerative ability. The age-related increase in senescence markers suggests that CE cells may have been exposed to endogenous stimuli that drive senescence. Senescence stimuli include critically short telomeres, DNA damage, and activated oncogenes (Campisi & d’Adda di Fagagna, 2007). Whereas telomeres do not appear to be a driving force behind CE senescence (Konomi & Joyce 2007), DNA damage does accumulate in CE cells as demonstrated by an increase in the presence of 8-hydroxy-2’-deoxyguanosine (8-OHdG), an endogenous oxidative DNA lesion, in corneal buttons from older donors compared with younger (Joyce et al., 2009). Furthermore, γH2AX foci, a marker of DNA double-strand breaks and cellular senescence, are increased in CE from ex vivo older donor corneal tissue (Joyce et al., 2010). Endogenous genotoxins that could drive age-dependent accumulation of oxidative lesions include reactive oxygen species (Haigis & Yankner, 2010), which are a by-product of oxidative phosphorylation. Because CE cells require high metabolic activity for continuous pump function, they may be exposed to greater oxidative stress than other cell types and therefore prone to premature senescence (Joyce, 2003, 2005; Mimura & Joyce, 2006; Joyce et al., 2009, 2010). Exogenous sources of genotoxic stress to CE cells include the UV component of sunlight (Kolozsvari et al., 2002), exposure to DNA-damaging drugs during ocular procedures such as mitomycin C (MMC; Roh et al., 2008), and free radicals generated during routine cataract surgery (Takahashi, 2005).
To determine the effects of endogenous DNA damage on the CE, we characterized corneal tissues of DNA repair–deficient Ercc1−/Δ mice (Niedernhofer et al., 2006). ERCC1-XPF is a highly conserved heterodimeric endonuclease essential in nucleotide excision repair (NER) of helix-distorting DNA lesions, interstrand crosslink repair, and the repair of some double-strand breaks (Niedernhofer et al., 2004; Ahmad et al., 2008). Mice engineered to express reduced levels of this nuclease display numerous symptoms and pathologies associated with advanced age and model a human progeroid syndrome (Weeda et al., 1997; Niedernhofer et al., 2006). Using a genetic mutant defective in nuclear DNA repair allowed us to uniquely focus on the biological impact of endogenous DNA damage. This eliminates variables such as protein, membrane, lipid, and mitochondrial damage, which confound experiments that employ using electrophilic genotoxins in normal hosts.
In this study, we used a combination of imaging and histology to identify significant differences between CE of adult (4.5- to 6-month-old) Ercc1−/Δ and normal littermate mice. Remarkably, many of the observed changes in Ercc1−/Δ mice were also observed in old (24- to 34-month-old) normal mouse CE. These results provide direct experimental evidence that failure to repair endogenous DNA damage can drive aging and degeneration of the CE.
Results
Ercc1−/Δ mice have normal eye development
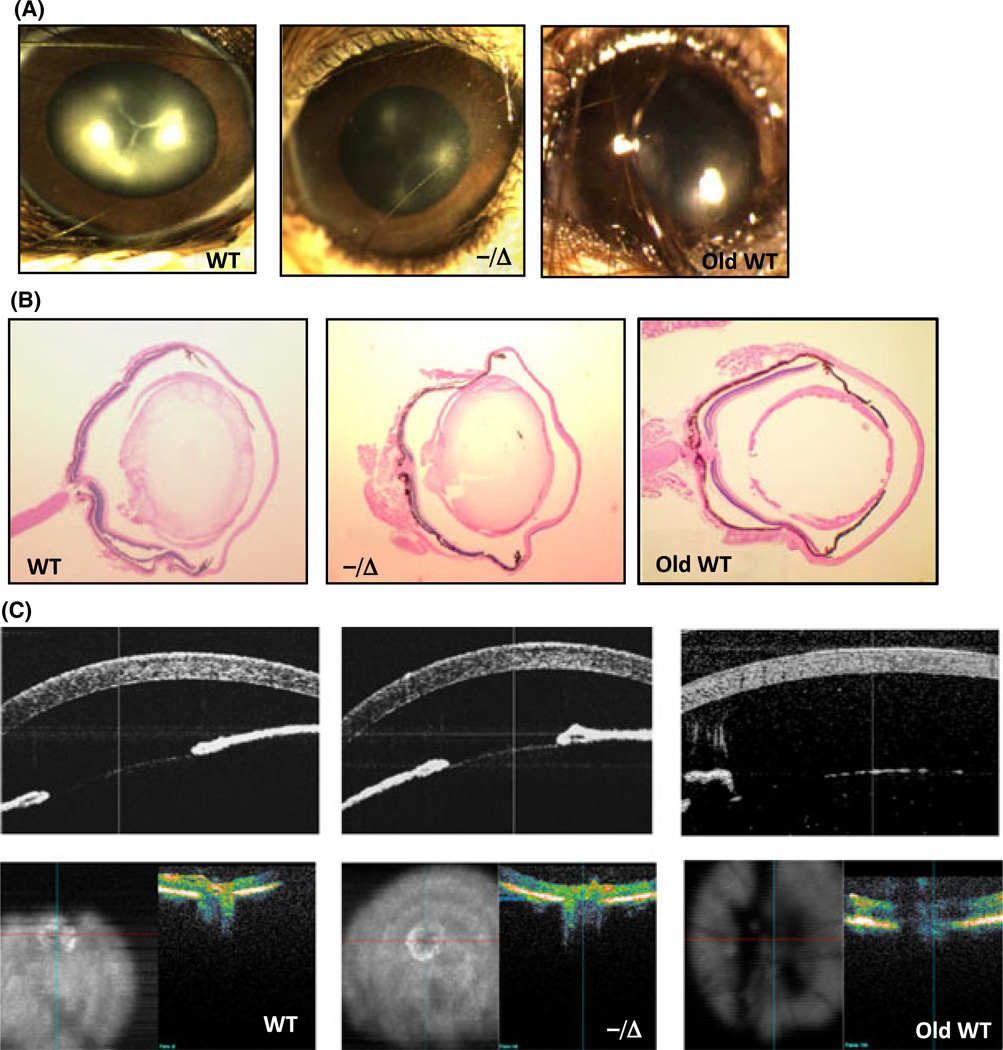
Ercc1−/Δ develop normally, but after reaching sexual maturation (at 8 weeks of age) begin to display symptoms associated with aging, including dystonia, ataxia, loss of muscle mass, kyphosis, and ultimately incontinence (refer to review Gregg et al., 2011). By this age, in contrast to age-matched controls, the Ercc1−/Δ mice have already developed tissue-specific degeneration and pathology similar to natural aging (Vo et al., 2010; Goss et al., 2011; Gregg et al., 2011). We did not detect any ocular developmental or structural defects in Ercc1−/Δ mice based on gross morphology (Fig. 1A), histology (Fig. 1B), or in vivo spectral domain optical coherence tomography (SD-OCT) imaging of the anterior segment and retina/optic nerve head (Fig. 1C). Images of gross morphology demonstrated normal orbits, fully developed iris with symmetric pupils and clear margins, normal conjunctival tissue, and nonvascularized corneas (Fig. 1A). Anesthesia-induced reversible cataracts (lens opacity) occurred in both Ercc1−/Δ and age-matched wild-type (WT) mice, as previously reported (Gabriele et al., 2010), making it difficult to evaluate differences in lens transparency between animals (Fig. 1A). Histological sections revealed the presence of apparently normal ciliary body, lens, retina, and optic nerve structures in Ercc1−/Δ mice (Fig. 1B). These observations support the conclusion that the DNA repair–deficient mice undergo normal ocular development. Therefore, any cellular differences observed between the eyes of WT and age-matched Ercc1−/Δ mice are likely the result of postnatal degenerative processes.
Fig. 1.
Ocular developmental and eye structure is normal in progeroid Ercc1−/Δ mice. (A) Representative images of eyes from age-matched wild-type (WT), Ercc1−/Δ, and old WT mice (34 months) illustrating normal development of ocular structures in the mutant animals. (B) H&E sections of the entire globe from WT, Ercc1−/Δ, and old WT mice (34 months). Sections were taken from the center of the globe as indicated by the entry of the optic nerve head entry at the back of the eye. (C) In vivo spectral domain optical coherence tomography (SD-OCT) imaging of the cornea (top images) and retina/optic nerve head (bottom images) from WT, Ercc1−/Δ, and old WT mice (34 months).
Dystrophic changes in CE from Ercc1−/Δ and naturally old WT mice
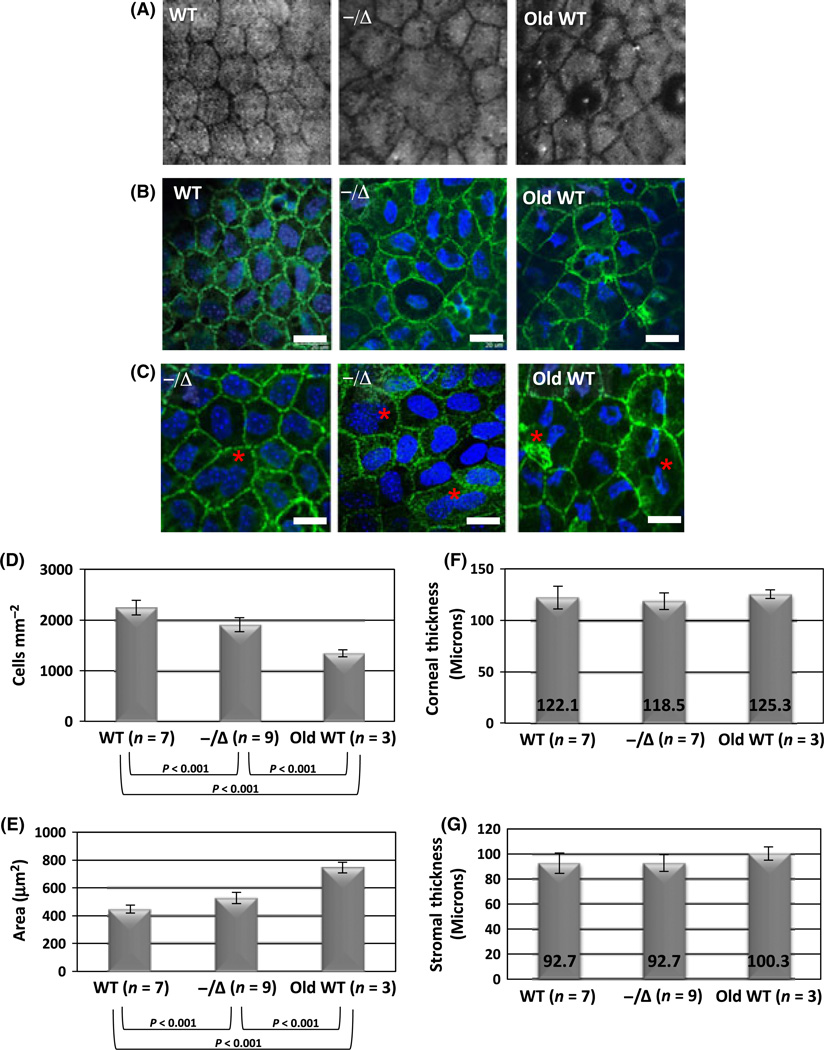
To examine the morphology of the CE layer, we used confocal microscopy and corneal whole-mount staining. Normal adult mice exhibited uniform CE cell shape and size (Fig. 2A, left image). In contrast, Ercc1−/Δ mice displayed dystrophic changes including severe polymorphism and polymegathism (Fig. 2A, middle image). These dystrophic changes were similar to the changes observed in the old WT mice (Fig. 2A, right image). Polymorphism and polymegathism were also observed by corneal whole-mount immunostaining (Fig. 2B) using antibodies to ZO-1 to highlight cell–cell junctions and DAPI to stain nuclei. Large polyploid, binuclear CE cells were also observed in Ercc1−/Δ mice and old WT animals, but not young WT adults (Fig. 2C).
Fig. 2.
Premature onset of aging-related dystrophic changes and loss of cell density in Ercc1−/Δ mouse corneal endothelium (CE). (A) Confocal microscopy images of the CE from a 6-month-old wild-type (WT) mouse, 6-month-old Ercc1−/Δ mouse, and an old WT mouse (24 months). (B) Whole-mount immunostaining of the same specimens with ZO-1 (green) to highlight cell junctions and DAPI (blue) nuclei. Scale bars = 20 µm. (C) Representative images with large polyploid binuclear cells (indicated by red asterisk) in mutant and aged animals. Scale bars = 20 µm. (D, E) Quantitative image analysis of confocal images used to calculate cell density (cells mm−2) and cell size (area µm2) of 4.5-to 6-month-old Ercc1−/Δ mice, normal littermates (WT), and old WT mice (34 months of age). Error bars represent the standard deviation from analysis of left and right eyes from the indicated number of mice (n). P-values are indicated below for intragroup comparison (P < 0.05 for ANOVA, P < 0.001 for post hoc Tukey’s) (F) Thickness of central corneas measured (in µm) from in vivo SD-OCT imaging. (G) Central corneal stromal thickness (µm) measured from confocal images. P> 0.05 using ANOVA indicates no significant differences between groups for both F and G.
Decreased cell density with normal overall corneal thickness in Ercc1−/Δ and naturally old WT mice
Aging-related CE polymorphism and polymegathism indicate compensation for cell loss in the CE cell layer (Bourne & McLaren, 2004). To determine whether this was the case in the Ercc1−/Δ mice, we used images generated by corneal confocal microscopy to measure CE cell density and size in Ercc1−/Δ, WT littermates, and old WT mice. The CE cell density is significantly lower in Ercc1−/Δ mice compared with WT littermate controls (Fig. 2D). Similarly, Ercc1−/Δ mice had significantly increased mean cell size (Fig. 2E). Changes similar to the Ercc1−/Δ mice were found in old WT mice, compared with young WT animals (Fig. 2D,E). This is consistent with prior studies on aged WT C57BL/6 mice (Jun et al., 2006). The dystrophic changes and decreased cell density, however, did not appear to affect overall central corneal thickness measured by SD-OCT (Fig. 2F) or central stromal thickness measured by confocal microscopy (Fig. 2G), as there were no significant differences between progeroid Ercc1−/Δ mice, their young adult WT littermates, or old WT mice.
Increased Descemet’s membrane thickness and abnormal posterior banded collagen in Ercc1−/Δ mice
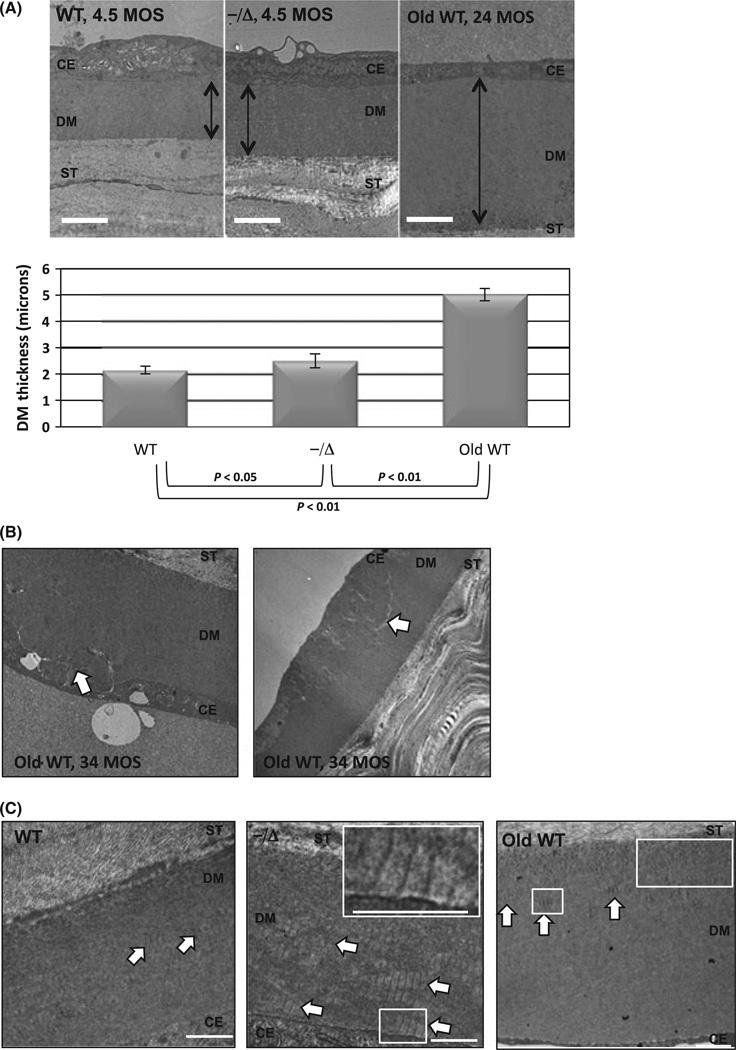
Descemet’s membrane (DM) is continuously secreted by CE cells throughout life, and increased DM thickness is observed in aging humans (Johnson et al., 1982) and mice (Jun et al., 2006). Ultrastructurally, DM is comprised of an anterior banded zone secreted during early development and a posterior nonbanded zone (PNBZ) that accounts for age-dependent DM thickening. In certain disease states affecting the CE, banded collagen, believed to be type VIII, can be observed in the PNBZ (Levy et al., 1996; Zhang et al., 2006). We prepared TEM sections from central cornea to measure central DM thickness and observe changes in DM. In Ercc1−/Δ mice, the DM thickness was significantly increased compared with their WT littermates (Fig. 3A). The DM was also thicker in old WT mice (Fig. 3A, right image) as previously reported (Jun et al., 2006). Interestingly, we also identified focal areas of DM defects in 34-month-old WT mice (Fig. 3B, white arrows) that were not observed in either Ercc1−/Δ mice or their WT littermates. These defects more resembled Hassall-Henle bodies, which are true DM excrescences (guttae) in aging human corneas (Johnson et al., 1982). A closer examination of DM from Ercc1−/Δ mice revealed abnormal deposition of banded collagen (Fig. 3C, middle image, white arrows) near the cell bases indicating recent or active synthesis. This was in contrast to WT littermates where banded collagen was appropriately located more anteriorly (Fig. 3C, left image, white arrows) indicating normal deposition that occurs only early in development. Banded collagen is not continually secreted in the normal aging process (Fig. 3C, right image, white arrows; Jun et al., 2006).
Fig. 3.
Premature thickening and abnormal deposition of Descemet’s membrane (DM) in Ercc1−/Δ mice. (A) Representative TEM images displaying the thickness of DM in wild-type (WT; left), Ercc1−/Δ (center) at 4.5 months of age, and old WT at 24 months (right). The orientation from top to bottom is corneal endothelium cell (CE), DM (double-ended black arrow), posterior corneal stroma (ST). Magnification = 10 0009. Scale bar = 2 µm. The graph below shows the average thickness ± SD from central DM as measured from four WT mice (seven eyes total), five Ercc1−/Δ mice (nine eyes total), and three old WT mice (three eyes total). (B) Representative TEM images displaying defects in the DM of old WT mice (34 months). White arrows indicate areas of abnormal basement membrane. (C) Representative TEM images displaying collagen banding of DM in WT and Ercc1−/Δ at 4.5 months of age and old WT mice at 24 months. The orientation from top to bottom is corneal stroma (ST), DM, and CE cells. White arrows indicate areas of banded collagen. Scale bars indicate 500 nm. Insets in the upper right of Ercc1−/Δ and old WT mice shows magnified area of interest (white box) and scale bar at 500 nm.
Presence of posterior projections in Ercc1−/Δ mice
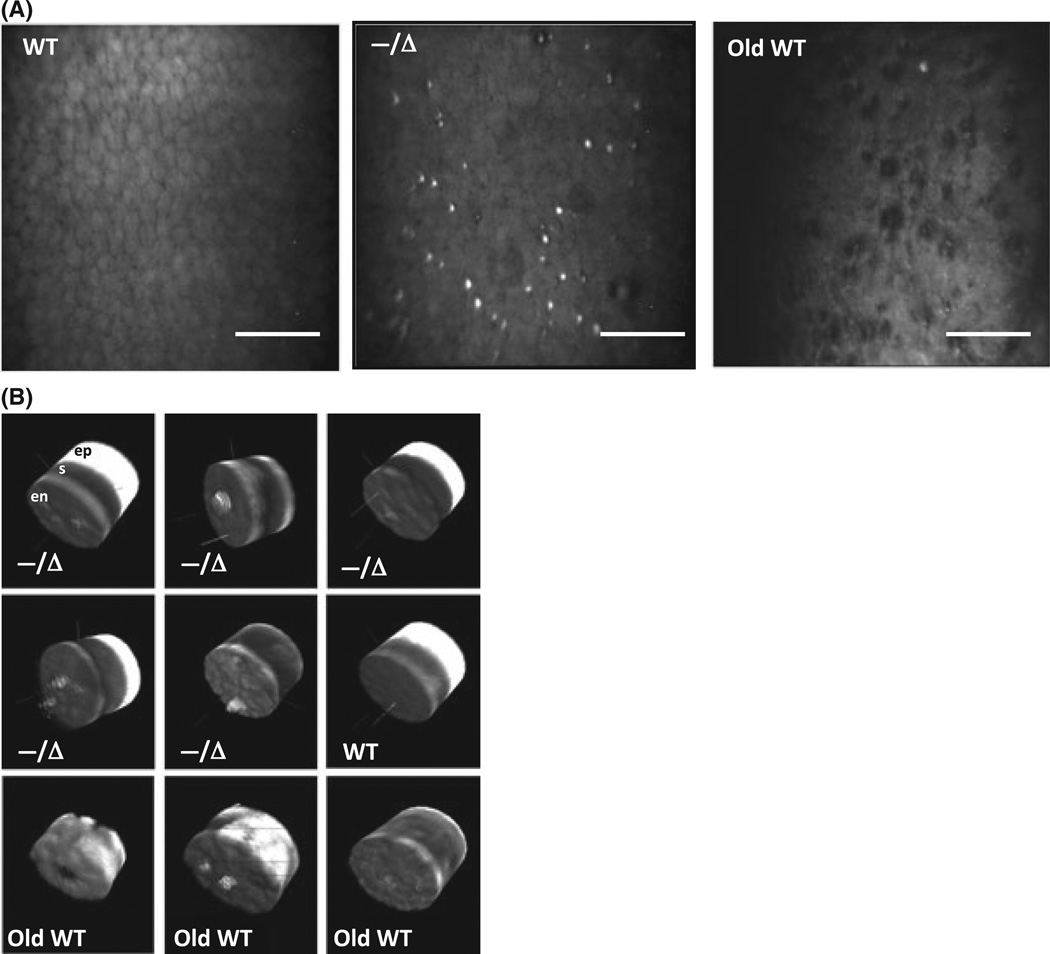
Confocal image z-stacks were used to generate 3D corneal reconstructions to better visualize the CE layer. Figure 4(A) demonstrates the presence of numerous heterogeneous lesions above the surface of the CE monolayer in Ercc1−/Δ mice but not in their age-matched WT littermates. Based on corneal confocal imaging alone, the lesions resembled corneal guttae (projections from DM), which were also observed in old WT CE. These posterior projections had variable size, shape, and reflectivity. The 3D reconstructions revealed that many of the posterior projections in Ercc1−/Δ mice originated from the CE layer and projected into the anterior chamber (Fig. 4B). However, we did not observe focal excrescences arising directly from DM in our Periodic acid– Schiff-stained paraffin (data not shown) or TEM sections (Fig. 3) of Ercc1−/Δ mice. Thus, the posterior projections in Ercc1−/Δ mice are most likely of cellular origin rather than from DM.
Fig. 4.
Posterior projections from Ercc1−/Δ corneal endothelium (CE). (A) Representative confocal images of CE from adult wild-type (WT; left, 6 months), Ercc1−/Δ (middle, 4.5 months), and old WT (34 months) mice. Scale bar = 100 µm. (B) 3D reconstructions of corneas generated from confocal image z-stacks. The three corneal cell layers are labeled in the first image for orientation (en, endothelium; s, stroma; ep, epithelium). The same orientation is used for all images of Ercc1−/Δ (—/Δ), adult WT, and old WT corneas.
Presence of CD45+ cells on Ercc1−/Δ mouse CE, which expresses senescence-associated secretory phenotype
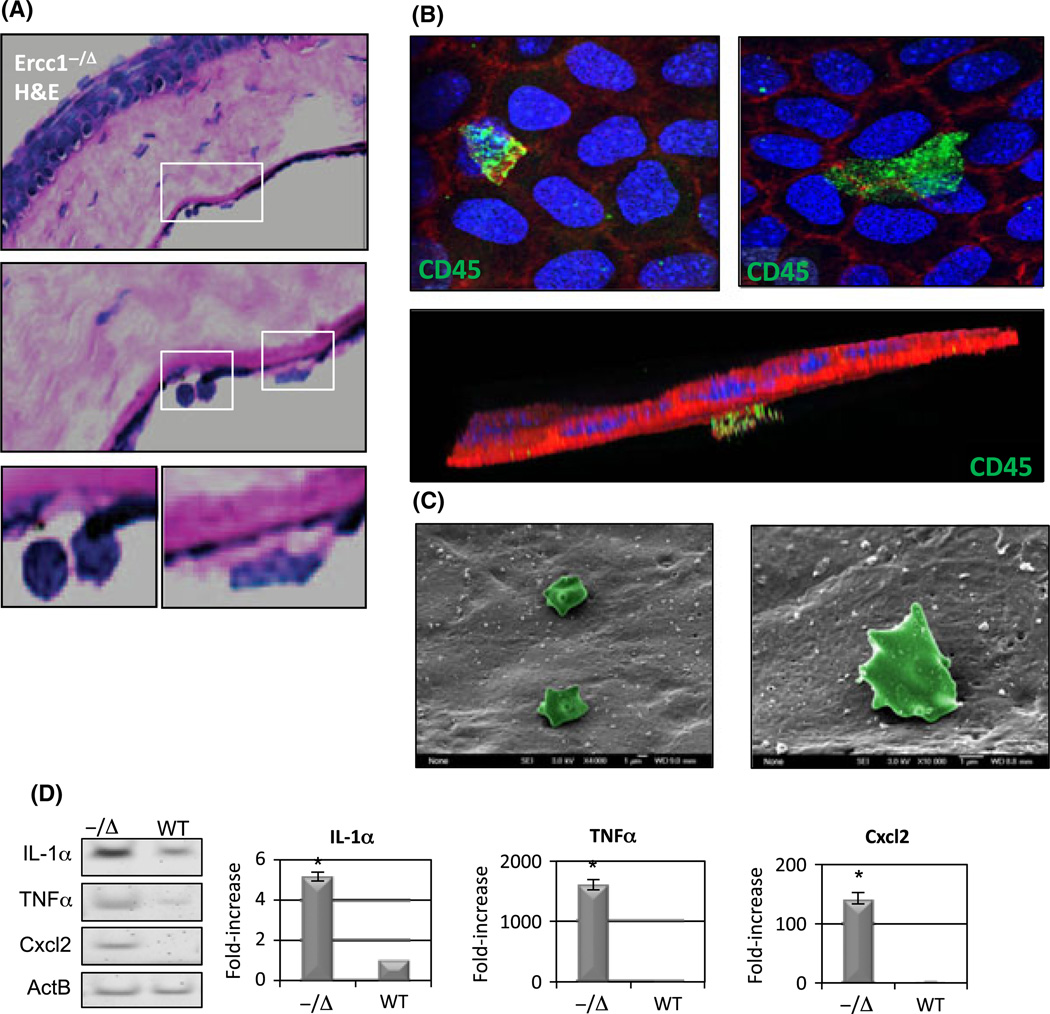
The heterogeneous nature of the posterior projections (Fig. 4) in Ercc1−/Δ CE indicated the possibility that other cells were interfacing with the CE cells of the Ercc1−/Δ mice (Guthoff et al., 2009). We observed occasional leukocytes in proximity to CE in histological sections of Ercc1−/Δ mouse corneas (Fig. 5A), which were not found in their WT littermates. Whole-mount immunostaining using CD45 as a general marker of leukocytes confirmed the presence of immune cells attached to the CE cell surface (Fig. 5B). These CD45+ cells were of various sizes and projected from the CE monolayer into the anterior chamber (Fig. 5B, bottom image projection). Although not specific for cell type, SEM also showed the presence of potential immune cells in proximity of Ercc1−/Δ CE cells (Fig. 5C). These rare cells were not seen in samples from WT littermate or old WT mice. To identify potential chemoattractant properties of CE cells, expression factors associated with senescence-associated secretory phenotype (SASP), which is pro-inflammatory (Orjalo et al., 2009), were measured by RT–PCR in CE cells from Ercc1−/Δ and WT littermates. Expression of IL-1α was increased fivefold in the Ercc1−/Δ CE compared with normal. Similarly, mRNAs for Cxcl2 and TNFα were readily amplified from Ercc1−/Δ CE but were not detected in their WT littermates (Fig. 5D).
Fig. 5.
CD45+ cells in proximity to Ercc1−/Δmouse corneal endothelium (CE), which display senescence-associated secretory phenotype (SASP). (A) H&E sections of Ercc1−/Δ mice CE revealing immune cells in proximity to the CE. (B) Corneal whole-mount sections stained for CD45 (green) to detect leukocytes, phalloidin (red), and DAPI (blue). Lower image is a 3D reconstruction from z-stack images demonstrating CD45+ cell (green) on top of the CE layer facing the anterior chamber. (C) Representative SEM image of putative immune cells (pseudocolored green) in proximity to CE from Ercc1−/Δ mice. (D) Senescence-associated secretory phenotype factor expression in RNA pooled from CE of three Ercc1−/Δ mice compared with three wild-type (WT) mice. Gel shows RT–PCR products (far left) from equal number of cycles, demonstrating relative expression of IL-1α, TNFα, Cxcl2, and control mRNA Actin B. Graphs to the right show qRT–PCR quantification of fold increase in SASP gene expression in CE of Ercc1−/Δ mice compared with WT mice. Cxcl2 did not amplify in WT mice, and thus, the relative value for Cxcl2 in Ercc1−/Δ mice is based on a Ct of 40 cycles for the WT and represents a minimum difference. Graphs show mean from three independent experiments, and the error bars represent the SD Asterisk indicates statistically significant difference from WT (Student’s t-test, P < 0.05).
Corneal endothelium cells are lost by apoptosis in Ercc1−/Δ mice
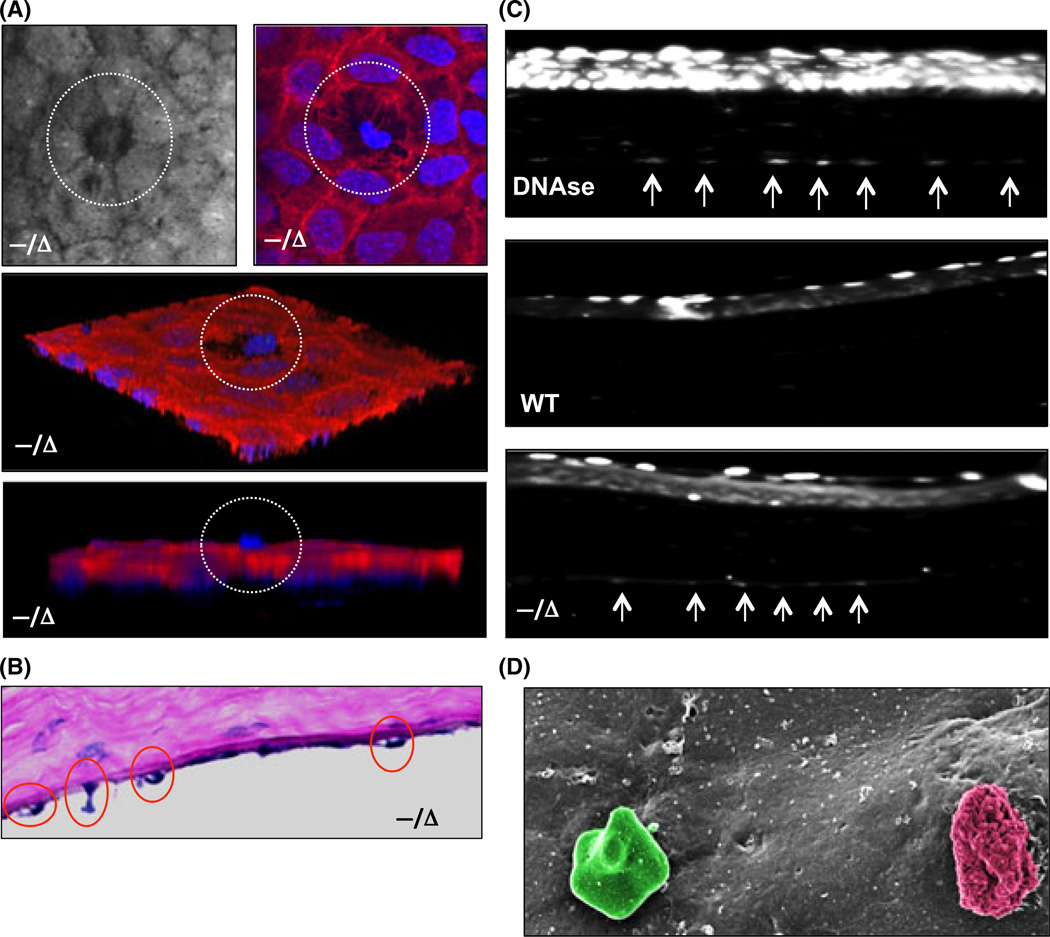
To probe the mechanism by which CE cells are lost in Ercc1−/Δ mice, we performed TUNEL staining of the CE layer. TUNEL staining of the CE layer confirmed the presence of apoptotic cells in Ercc1−/Δ mice but not WT littermates (Fig. 6C). Immunostaining of whole-mount sections for actin (Fig. 6A) and histological sections (Fig. 6B) revealed apoptotic cell extrusion, which is a process that removes dying cells from monolayers while maintaining barrier function (Rosenblatt et al., 2001). SEM captured the presence of a late-stage extruded apoptotic CE cell in the vicinity of a putative leukocyte (Fig. 6D).
Fig. 6.
Apoptosis of corneal endothelium (CE) in Ercc1−/Δ mice. (A) Representative corneal confocal image (upper left) of the CE from an Ercc1−/Δ mouse showing a cell being extruded (white circle). Corneal whole-mount staining (upper right) with phalloidin (red) and DAPI (blue). Lower panels are 3D reconstructions from z-stack images showing highlighted extruded cell in a white circle. (B) H&E section demonstrating extruding CE cells from Ercc1−/Δ mouse cornea. (C) TUNEL assay to detect apoptotic cells on paraffin-embedded corneal sections. The top image is from a positive control using DNAse pretreatment. Arrows indicate TUNEL+ cells in the CE. Wild-type (middle image) demonstrates some apoptosis in the superficial corneal squamous epithelial cell layer, but no TUNEL signal in the CE. The bottom image is cornea from Ercc1−/Δ mice The arrows indicate TUNEL-positive CE cells. (D) SEM of extruding CE cell (pseudocolored red) and putative immune cell (pseudocolored green).
Discussion
In this study, we demonstrated aging-related dystrophic changes in CE from DNA repair–deficient Ercc1−/Δ mice. Remarkably, 4.5- to 6-month-old Ercc1−/Δ mice spontaneously developed degenerative changes typically seen in 2- to 3-year-old WT mice. The prominent parallels between the Ercc1−/Δ and old WT mouse CE are increased polymorphism and polymegathism, polyploidy, decreased cell density, increased Descemet’s thickness, and the presence of posterior projections that are most likely apoptotic CE cells and/or leukocytes. These changes represented marked accelerated aging of the CE in the Ercc1−/Δ mice as a result of their DNA repair deficiency. The severity of the degenerative changes in the Ercc1−/Δ mice during their shortened lifespan did not exceed the old WT mice, however, indicating that additional unknown factors also contribute to CE aging. Nonetheless, our data show that acquiring unrepaired DNA damage can greatly promote aging of the CE.
Many of the dystrophic changes seen in the Ercc1−/Δ and old WT mouse CE signify compensatory responses to cellular damage and loss and are also observed in human CE as a consequence of aging, trauma, disease, or surgery (Bourne & McLaren, 2004). For example, to accommodate for increasing cell loss, the remaining CE cells flatten, increase in size, and alter their morphology to cover the acellular area. The polyploid binuclear cells observed in young adult Ercc1−/Δ and old WT mice are also increased in aged and wounded human CE (Ikebe et al., 1986). Increased polyploidy is positively correlated with exposure to cellular stress, senescence, and aging and is frequently observed in cells of liver, heart, and vascular tissues (Storchova & Pellman, 2004). Increased DM thickness and changes in matrix composition are observed in disease states affecting the CE such as diabetes (Rehany et al., 2000), Fuchs’ corneal endothelial dystrophy (Levy et al., 1996; Zhang et al., 2006), and iridocorneal endothelial syndrome (Levy et al., 1995).
An intriguing finding in our study was the presence of posterior projections in Ercc1−/Δ mouse CE. These projections resembled guttae seen in Fuchs’ corneal endothelial dystrophy, a premature aging disease of the CE (Bergmanson et al., 1999). Fuchs’ endothelial dystrophy is an idiopathic primary degenerative disease of the CE. Like many aging-related degenerative changes, there is growing evidence for oxidative stress and oxidative DNA damage in both the nuclear and mitochondrial genomes of CE cells driving this disease (Jurkunas et al., 2010). However, careful analysis did not reveal any histological evidence that the projections in Ercc1−/Δ CE were excrescences originating from DM characteristic of Fuchs’. Whole-mount staining further distinguished our projections from corneal guttae. Although we observed increased Descemet’s thickness in Ercc1−/Δ mice, focal areas of extracellular matrix-based guttae, as were seen in 34-month-old WT mice, were not detected in the progeroid mutant mice. The posterior projections in the Ercc1−/Δ mice appear instead to be apoptotic cells extruding from the CE monolayer or occasional CD45+ cells on the surface of the CE. We did not further differentiate the CD45+ cells we detected with additional markers; however, believe that they could be a class of leukocytes involved in dead/dying cell removal. Interestingly, there were no histopathological signs of acute or chronic inflammation throughout the Ercc1−/Δ mouse corneal layers. These changes may reflect the response to accelerated cell loss in DNA repair–deficient mice, which allows us to capture these events.
We explored the potential role of senescence-associated secretory factors in attracting immune cells to Ercc1−/Δ mice CE. We observed elevated levels of IL-1α, Cxcl2, and TNFα in Ercc1−/Δ mice CE, consistent with reports that tissues of Ercc1−/Δ mice prematurely display the SASP (Chen et al., 2013). IL-1α has been reported to be the upstream regulator of the SASP in aging and senescent cells (Orjalo et al., 2009). Cxcl2 is a chemokine that triggers leukocyte infiltration, which may explain the presence of CD45+ cells on Ercc1−/Δ CE cells. The aqueous humor is normally anti-inflammatory. Hence, it will be interesting to determine the impact of aging-related SASP on eye tissue function. These data demonstrate for the first time that the CE is vulnerable to DNA damage and that genotoxic stress can promote SASP in the CE.
ERCC1-XPF-deficient mice and humans display accelerated aging of numerous tissues including the skin, liver, kidney, brain, spinal cord, peripheral nerves, bone, and the hematopoietic system (McWhir et al., 1993; Weeda et al., 1997; Niedernhofer et al., 2006; Lawrence et al., 2008; Vo et al., 2010). Because ERCC1-XPF is an essential nuclease involved in numerous DNA repair pathways, a single DNA lesion driving tissue degeneration and premature aging has not been definitely identified. Deficiency in the NER pathway alone does not lead to accelerated aging because NER-deficient Xpa−/- mice display normal lifespan and do not display early onset of aging-related symptoms or pathologies (Dolle et al., 2006; Niedernhofer et al., 2006; Grillari et al., 2007). Thus, it has been hypothesized that DNA interstrand crosslinks, which are extremely cytotoxic and for which ERCC1-XPF is required for repair (Niedernhofer et al., 2004), are the primary lesions that drive the premature aging observed in ERCC1-deficient mice and potentially contribute to normal aging (Weeda et al., 1997; Dolle et al., 2006; Niedernhofer et al., 2006; Grillari et al., 2007). Endogenous interstrand crosslinks may arise as downstream byproducts of chronic oxidative stress and lipid peroxidation, which, for example, enhances the formation of malondialdehyde, a potent DNA crosslinking agent (Niedernhofer et al., 2003; Grillari et al., 2007). Interstrand crosslinks in CE may also be due to exogenous exposures to genotoxins such as the use of MMC used during refractive procedures. Similar to the findings here, we established that therapeutic doses of MMC rapidly induce DNA lesions (Roh et al., 2008).
Evidence from the current study supports a mechanism in which unrepaired nuclear DNA damage leads to CE cell death via apoptosis. This loss of cells in the CE monolayer in turn drives the changes in CE cell morphology. Furthermore, the notion that DNA damage is driving force for these pathological changes is consistent with the elevated expression of senescence markers in CE cells from aged donors (Mimura & Joyce, 2006; Joyce et al., 2009; Song et al., 2008).
In summary, our study provides strong genetic evidence that the CE is sensitive to unrepaired endogenous DNA damage. With this information, future targeted approaches aimed at protecting, preventing, or reversing the effects of DNA damage may help to preserve or even enhance CE integrity. In addition, the study establishes that the Ercc1−/Δ mice are a rapid and accurate model system of aging-related degenerative changes in the CE for testing therapeutic strategies.
Methods
Mice
Ercc1−/Δ were bred in an f1 background by crossing inbred mice heterozygous for each mutant allele in a C57Bl/6J and FVB/n genetic background. This reduces strain-specific pathology yet allows analysis of genetically identical mutant animals. Mice were given a unique identifier by ear punch. Genomic DNA was isolated from ear tissue using Nucleospin 96 RNA Prep (Fisher Scientific, Pittsburgh, PA, USA) and the genotype determined by PCR as previously described (Ahmad et al., 2008). Wild-type littermates were used as young normal controls. Old WT mice were either in the same genetic background or purchased from NIA (CB6F1: BALB/cBy × C57BL/6 mice, 34-month-old males). Animal husbandry and experimental procedures were approved by the University of Pittsburgh IACUC.
Spectral domain optical coherence tomography imaging
Mice were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg, Ketaject; Phoenix Pharmaceuticals, St. Joseph, MI, USA) and xylazine (5 mg/kg, Xyla-ject; Phoenix Pharmaceuticals) and imaged as previously described (Gabriele et al., 2010). Pupils were dilated using Tropicamide (1%; Falcon Pharmaceuticals, Fort Worth, TX, USA). A custom contact lens was applied to the cornea to aid in focusing for retinal images. Mice were secured on a custom stage for imaging of the cornea and retina/optic nerve head. Volumetric images were acquired in both eyes (1.5 × 1.5 × 2.0 mm scans, 250 × 250 A-scans, 1024 pixels in depth, four repeated A-scans) at each location (Bioptigen, Inc., Durham, NC, USA).
Gross imaging
Gross photographs of mouse eyes were taken with a Leica MZFLIII high-resolution stereo fluorescence biomicroscope (Leica Microsystems Inc., Bannockburn, IL, USA). Mice were anesthetized, as described above, and immobilized with a three-point stereotactic mouse restrainer. Each whole cornea was focused, and images were obtained at a magnification of 25×.
Corneal endothelial confocal imaging
Imaging of mouse central CE was accomplished by corneal confocal microscopy (Confoscan3; Nidek Technologies America, New Orleans, LA, USA). Mice were sacrificed by CO2 inhalation followed by cervical dislocation and immediately immobilized on a custom secure platform. A 40× objective optically coupled to the cornea with transparent gel (Viscotears; Novartis Ophthalmics, Duluth, GA, USA) was focused onto the CE surface. The software (NAVIS; Lucent Technologies, Murray Hill, NJ, USA) captured images from the CE through the corneal epithelium every 1.6 µm and stored them as an image stack for the analysis of corneal thickness. Image stacks were inspected individually to determine the stromal–endothelium and stromal–epithelium transitions. Central thickness of the stroma was determined by counting the number of images from the epithelial peak scatter to endothelial peak scatter × 1.6 µm as previously described (Du et al., 2009). Manual cell counting and area determination were performed with ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/). Statistical analyses were carried out by ANOVA and appropriate post hoc tests as indicated using Prism GraphPad software (Prism; GraphPad Software, San Diego, CA, USA). 3D reconstructions of corneas from image stacks were performed with MetaMorph (Molecular Devices, Sunnyvale, CA, USA) to examine corneal endothelial cell layer projections.
Histology and immunohistochemistry
Mouse eyes were enucleated using fine forceps. For paraffin sections, whole globes were injected with 2% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA, USA) and immersed in 2% PFA at 4 °C until processing. Eyes were processed and embedded in paraffin wax. Eight-micrometer-thick serial sections were used for analysis. For corneal whole-mount staining, similar fixation was performed for 48 h at 4 °C in 2% PFA, and then, corneas were dissected from the globes. The corneas were rinsed in PBS and stored at 4 °C in 50% glycerol and 50% PBS (v/v) until further processing. For whole-mount CD45 staining, corneas were washed in PBS and incubated in anti-mouse CD16/CD32 Fc γ III/II (BD Pharmingen, San Jose, CA, USA) at 1:100 for 30 min to block nonspecific binding. Anti-mouse CD45-FITC (BD) was added at 1:100 and incubated overnight at 4 °C. After multiple washes with PBS, phalloidin-546 (Life Technologies Inc., Grand Island, NY, USA) was added at 1:50 together with 4’,6-diamidino-2-phenylindole (DAPI; 0.5 µg/ml; Roche Molecular Biochemicals, Indianapolis, IN, USA) and then incubated for 1 h at room temperature. For ZO-1 staining, tissue was blocked in 10% heat-inactivated goat serum for 1 h at RT followed by overnight incubation with anti-mouse ZO-1 (Invitrogen). Secondary antibody Alexa Fluor 488-conjugated goat anti-mouse (1:2500; Life Technologies Inc.) together with DAPI was added for 1 h at RT. Omission of the primary antibody served as a negative control. The stained whole mounts were placed in aqueous mounting medium (Fisher Scientific, Pittsburgh, PA, USA) on coverslip bottom dishes and examined using an Olympus FluoView FV1000 confocal microscope (Olympus, Tokyo, Japan). Image z-stacks were obtained of whole-mount corneas and reconstructed using MetaMorph.
Scanning electron microscopy
Excised corneas were fixed overnight in 2.5% glutaraldehyde in PBS at 4 °C. Corneas were washed three times in PBS, postfixed for 1 h in aqueous 1% osmium tetroxide, and then washed three times in PBS. Corneas were dehydrated through a graded ethanol series (30–100%), further dehydrated by three additional 15-min washes with absolute ethanol. Next, the corneas were washed in hexamethyldisilazane for 15 min and then removed to air dry. Corneas were then mounted onto aluminum stubs, grounded with silver paint, and sputter coated with 3.5 nm gold/palladium (Cressington Sputter Coater Auto 108; Cressington, Watford, UK). Images were taken using a JEOL JSM-6335F scanning electron microscope (Peabody, MA, USA) at 3 kV.
Transmission electron microscopy and DM thickness
Corneas were fixed in cold 2.5% glutaraldehyde (Taab Chemical, Energy Beam Sciences, East Granby, CT, USA) in 0.1 m PBS (Fisher Scientific), pH 7.3. The corneas were rinsed in PBS, postfixed in 1% osmium tetroxide (Electron Microscopy Sciences) with 0.1% potassium ferricyanide (Fisher Scientific), dehydrated through a graded series of ethanol (30–90% –Reagent Alcohol; Fisher Scientific, and 100% – Ethanol 200 Proof; Pharmco, Fisher Scientific) and embedded in Epon (dodecenyl succinic anhydride, nadic methyl anhydride, Scipoxy 812 resin and dimethylam-inomethyl; Energy Beam Sciences). Using only central corneal regions, semi-thin (300 nm) sections were cut on a Reichart Ultracut, stained with 0.5% Toluidine Blue (Toluidine Blue O and Sodium Borate; Fisher), and examined under the light microscope. Ultrathin sections (65 nm) were stained with 2% uranyl acetate (uranyl acetate dihydrate; Electron Microscopy Sciences, and methanol; Fisher Scientific) and Reynold’s lead citrate (lead nitrate, sodium citrate and sodium hydroxide; Fisher Scientific) and examined on Jeol 1011 transmission electron microscope. Greater than ten measurements of DM thickness per image were taken from multiple images at 5000× magnification using ImageJ software. Averages and standard deviations were calculated from four WT mice (seven eyes total) and five Ercc1−/Δ mice (nine eyes total) and compared using Student’s t-test with a P < 0.05 significance.
TUNEL assay
Paraffin sections were processed for TUNEL staining per the manufacturer’s protocol (Roche, Indianapolis, IN, USA). Briefly, sections were permeabilized in freshly prepared 0.1% Triton X-100 and 0.1% sodium citrate then rinsed in PBS. TUNEL reaction mixture (enzyme and buffer) was added to sections which were allowed to incubate for 60 min at 37 °C in a humidified atmosphere in the dark. The negative control included no enzyme treatment and the positive control included DNAse treatment prior to TUNEL staining. Sections were analyzed by fluorescence microscopy using 488-nm excitation.
Senescence-associated secretory phenotype gene expression
Ercc1−/Δ mice and WT littermate controls were used for SASP analysis. Corneas from three mice of each genotype were excised and pooled, which was necessary due to the small tissue size. Corneal endothelium was dissected from the cornea under a dissecting microscope into RNAlater (Qiagen, Valencia, CA, USA) and stored at 4 °C until use. RNA was isolated from CE using RNeasy Micro kit (Qiagen) and stored at −80 °C. About 15 ng of RNA was converted to cDNA using Ovation WTA Pico V2 kit (NuGEN San Carlos, CA, USA) according to manufacturer’s instructions. qRT–PCR was performed with TaqMan probes and primers (Life Technologies) using reaction conditions per the manufacturer’s instructions for ActB, Cxcl2, IL-1α, and TNFα. Relative expression was calculated using 2−ΔΔCt normalized to housekeeping genes (Du et al., 2009). The mean and standard deviation was calculated from three independent experiments. PCR products were also separated on acrylamide gels (Bio-Rad Hercules, CA, USA), stained with SYBR Safe (Life Sciences), and visualized with UV light.
Acknowledgments
The authors would like to acknowledge Gloria Limetti and Cindy Stone for assistance with histology, Center for Biological Imaging for assistance with SEM/TEM, Kira Lathrop for assistance with image reconstruction and confocal microscopy, Lindsey Folio for assistance with OCT, and Martha Funderburgh for assistance with qRT–PCR. This work was funded by the National Institutes of Health Grants EY016415 (JLF), EY09368 (JLF), P30-EY08098, F30-AG035443 (DSR), ES016114 and −032 (LJN), Research to Prevent Blindness, the Eye and Ear Foundation of Pittsburgh and the Ellison Medical Foundation (AG-NS-0303-05; LJN).
References
- Ahmad A, Robinson AR, Duensing A, van Drunen E, Beverloo HB, Weisberg DB, Hasty P, Hoeijmakers JH, Niedernhofer LJ. ERCC1-XPF endonucle-ase facilitates DNA double-strand break repair. Mol. Cell. Biol. 2008;28:5082–5092. doi: 10.1128/MCB.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmanson JP, Sheldon TM, Goosey JD. Fuchs’ endothelial dystrophy: a fresh look at an aging disease. Ophthalmic Physiol. Opt. 1999;19:210–222. doi: 10.1046/j.1475-1313.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- Bourne WM, McLaren JW. Clinical responses of the corneal endothelium. Exp. Eye Res. 2004;78:561–572. doi: 10.1016/j.exer.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Chen Q, Liu K, Robinson AR, Clauson CL, Blair HC, Robbins PD, Niedernhofer LJ, Ouyang H. DNA damage drives accelerated bone aging via an NF-kappaB-dependent mechanism. J. Bone Miner. Res. 2013;28:1214–1228. doi: 10.1002/jbmr.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle ME, Busuttil RA, Garcia AM, Wijnhoven S, van Drunen E, Niedernhofer LJ, van der Horst G, Hoeijmakers JH, van Steeg H, Vijg J. Increased genomic instability is not a prerequisite for shortened lifespan in DNA repair deficient mice. Mutat. Res. 2006;596:22–35. doi: 10.1016/j.mrfmmm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Du Y, Carlson EC, Funderburgh ML, Birk DE, Pearlman E, Guo N, Kao WW, Funderburgh JL. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27:1635–1642. doi: 10.1002/stem.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto K, Mimura T, Harris DL, Joyce NC. Age differences in cyclin-dependent kinase inhibitor expression and rb hyperphosphorylation in human corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2006;47:4330–4340. doi: 10.1167/iovs.05-1581. [DOI] [PubMed] [Google Scholar]
- Gabriele ML, Ishikawa H, Schuman JS, Bilonick RA, Kim JS, Kagemann L, Wollstein G. Reproducibility of spectral-domain optical coherence tomography total retinal thickness measurements in mice. Invest. Ophthalmol. Vis. Sci. 2010;51:6519–6523. doi: 10.1167/iovs.10-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosheh FR, Cremona FA, Rapuano CJ, Cohen EJ, Ayres BD, Hammersmith KM, Raber IM, Laibson PR. Trends in penetrating keratoplasty in the United States 1980–2005. Int. Ophthalmol. 2008;28:147–153. doi: 10.1007/s10792-007-9177-z. [DOI] [PubMed] [Google Scholar]
- Goss JR, Beer Stolz D, Robinson AR, Zhang BS, Robbins PD, Glorioso JC, Niedernhofer LJ. Premature age-related peripheral neuropathy in a mouse model of progeria. Mech. Ageing Dev. 2011;132:437–442. doi: 10.1016/j.mad.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg SQ, Robinson AER, Niedernhofer LJ. Physiological consequences of defects in ERCC1-XPF DNA repair endonuclease. DNA Repair (Amst) 2011;10:781–791. doi: 10.1016/j.dnarep.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillari J, Katinger H, Voglauer R. Contributions of DNA interstrand cross-links to aging of cells and organisms. Nucleic Acids Res. 2007;35:7566–7576. doi: 10.1093/nar/gkm1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea - a major review. Clin. Experiment. Ophthalmol. 2009;37:100–117. doi: 10.1111/j.1442-9071.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Yankner BA. The aging stress response. Mol. Cell. 2010;40:12. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp. Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe H, Takamatsu T, Itoi M, Fujita S. Age-dependent changes in nuclear DNA content and cell size of presumably normal human corneal endothelium. Exp. Eye Res. 1986;43:251–258. doi: 10.1016/s0014-4835(86)80093-8. [DOI] [PubMed] [Google Scholar]
- Johnson DH, Bourne WM, Campbell RJ. The ultrastructure of Descemet’s membrane. I. Changes with age in normal corneas. Arch. Ophthalmol. 1982;100:1942–1947. doi: 10.1001/archopht.1982.01030040922011. [DOI] [PubMed] [Google Scholar]
- Joyce NC. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003;22:359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- Joyce NC. Cell cycle status in human corneal endothelium. Exp. Eye Res. 2005;81:629–638. doi: 10.1016/j.exer.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Harris DL. Decreasing expression of the G1-phase inhibitors, p21Cip1 and p16INK4a, promotes division of corneal endothelial cells from older donors. Mol. Vis. 2010;16:897–906. [PMC free article] [PubMed] [Google Scholar]
- Joyce NC, Zhu C, Harris DL. Relationship between oxidative stress, DNA damage, and proliferative capacity in human corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2009;50:2116–2122. doi: 10.1167/iovs.08-3007. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Harris DL, Zhu C. Age-related gene response of human corneal endothelium to oxidative stress and DNA damage. Invest. Ophthalmol. Vis. Sci. 2011;52:1641–1649. doi: 10.1167/iovs.10-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun AS, Chakravarti S, Edelhauser HF, Kimos M. Aging changes of mouse corneal endothelium and Descemet’s membrane. Exp. Eye Res. 2006;83:890–896. doi: 10.1016/j.exer.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Jurkunas UV, Bitar MS, Funaki T, Azizi B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am. J. Pathol. 2010;177:2278–2289. doi: 10.2353/ajpath.2010.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolozsvari L, Nogradi A, Hopp B, Bor Z. UV absorbance of the human cornea in the 240- to 400-nm range. Invest. Ophthalmol. Vis. Sci. 2002;43:2165–2168. [PubMed] [Google Scholar]
- Konomi K, Joyce NC. Age and topographical comparison of telomere lengths in human corneal endothelial cells. Mol. Vis. 2007;13:1251–1258. [PubMed] [Google Scholar]
- Lawrence NJ, Sacco JJ, Brownstein DG, Gillingwater TH, Melton DW. A neurological phenotype in mice with DNA repair gene Ercc1 deficiency. DNA Repair (Amst) 2008;7:281–291. doi: 10.1016/j.dnarep.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Levy SG, McCartney AC, Sawada H, Dopping-Hepenstal PJ, Alexander RA, Moss J. Descemet’s membrane in the iridocorneal-endothelial syndrome: morphology and composition. Exp. Eye Res. 1995;61:323–333. doi: 10.1016/s0014-4835(05)80127-7. [DOI] [PubMed] [Google Scholar]
- Levy SG, Moss J, Sawada H, Dopping-Hepenstal PJ, McCartney AC. The composition of wide-spaced collagen in normal and diseased Descemet’s membrane. Curr. Eye Res. 1996;15:45–52. doi: 10.3109/02713689609017610. [DOI] [PubMed] [Google Scholar]
- McWhir J, Selfridge J, Harrison DJ, Squires S, Melton DW. Mice with DNA repair gene (ERCC-1) deficiency have elevated levels of p53, liver nuclear abnormalities and die before weaning. Nat. Genet. 1993;5:217–224. doi: 10.1038/ng1193-217. [DOI] [PubMed] [Google Scholar]
- Mimura T, Joyce NC. Replication competence and senescence in central and peripheral human corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2006;47:1387–1396. doi: 10.1167/iovs.05-1199. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J. Biol. Chem. 2003;278:31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, de Wit J, Jaspers NG, Beverloo HB, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol. Cell. Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somato-troph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl Acad. Sci. USA. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehany U, Ishii Y, Lahav M, Rumelt S. Ultrastructural changes in corneas of diabetic patients: an electron-microscopy study. Cornea. 2000;19:534–538. doi: 10.1097/00003226-200007000-00026. [DOI] [PubMed] [Google Scholar]
- Roh DS, Cook AL, Rhee SS, Joshi A, Kowalski R, Dhaliwal DK, Funderburgh JL. DNA cross-linking, double-strand breaks, and apoptosis in corneal endothelial cells after a single exposure to mitomycin C. Invest. Ophthalmol. Vis. Sci. 2008;49:4837–4843. doi: 10.1167/iovs.08-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest. Ophthalmol. Vis. Sci. 2000;41:660–667. [PubMed] [Google Scholar]
- Song Z, Wang Y, Xie L, Zang X, Yin H. Expression of senescence-related genes in human corneal endothelial cells. Mol. Vis. 2008;14:161–170. [PMC free article] [PubMed] [Google Scholar]
- Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat. Rev. Mol. Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- Takahashi H. Free radical development in phacoemulsification cataract surgery. J. Nippon Med. Sch. 2005;72:4–12. doi: 10.1272/jnms.72.4. [DOI] [PubMed] [Google Scholar]
- Vo N, Seo HY, Robinson A, Sowa G, Bentley D, Taylor L, Studer R, Usas A, Huard J, Alber S, Watkins SC, Lee J, Coehlo P, Wang D, Loppini M, Robbins PD, Niedernhofer LJ, Kang J. Accelerated aging of intervertebral discs in a mouse model of progeria. J. Orthop. Res. 2010;28:1600–1607. doi: 10.1002/jor.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G, Donker I, de Wit J, Morreau H, Janssens R, Vissers CJ, Nigg A, van Steeg H, Bootsma D, Hoeijmakers JH. Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr. Biol. 1997;7:427–439. doi: 10.1016/s0960-9822(06)00190-4. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bell WR, Sundin OH, De La Cruz Z, Stark WJ, Green WR, Gottsch JD. Immunohistochemistry and electron microscopy of early-onset fuchs corneal dystrophy in three cases with the same L450W COL8A2 mutation. Trans. Am. Ophthalmol. Soc. 2006;104:85–97. [PMC free article] [PubMed] [Google Scholar]