Abstract
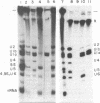
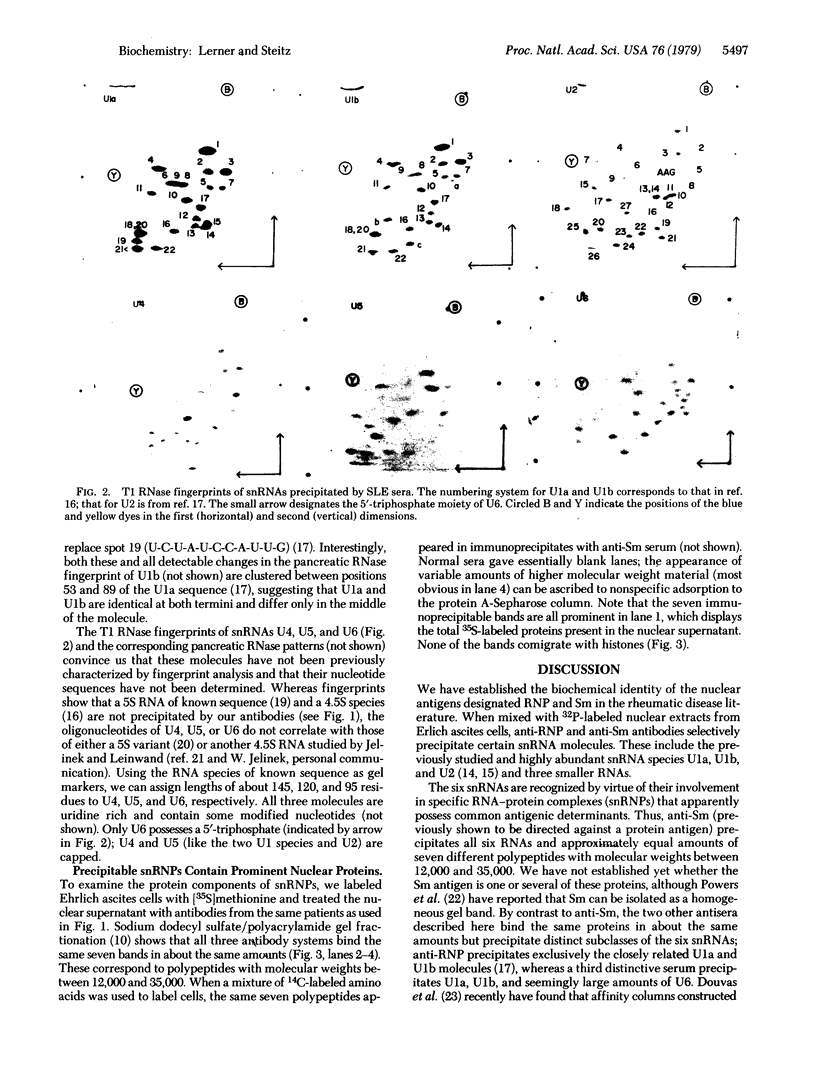
Patients with systemic lupus erythematosus often possess antibodies against two nuclear antigens called Sm and RNP (ribonucleoprotein). We have established the molecular identity of these antigens by analyzing immune precipitates of nuclear extracts from mouse Ehrlich ascites cells labeled with 32P and 35S. Anti-Sm serum selectively precipitates six small nuclear RNA molecules (snRNAs); anti-RNP serum reacts with only two of these; and a third serum, characterized as mostly anti-RNP, precipitates a subset of three snRNA bands. Three of the six RNAs are identified by fingerprint analysis as the previously characterized and highly abundant nucleoplasmic snRNA species U1a (171 nucleotides), U1b, and U2 (196 nucleotides). The other three RNAs (U4, U5, and U6) likewise are uridine rich and contain modified nucleotides, but they are smaller, with lengths of about 145, 120, and 95 residues, respectively. Each of the six snRNAs is complexed with and apparently antigenic by virtue of association with specific proteins. All three sera precipitate an identical complement of seven different polypeptides ranging in molecular weight from 12,000 to 35,000; these proteins are abundant in nuclear extracts, but are neither histones nor the major polypeptides comprising the 30S heterogeneous nuclear RNP particles of mammalian nuclei. Our data argue that each of the six snRNAs exists in a separate small nuclear ribonucleoprotein (snRNP) complex with a total molecular weight of about 175,000. We find that human antisera also precipitate snRNAs from a wide range of vertebrate species and from arthropods. We discuss the antigenic snRNPs in relation to the published literature on snRNAs and nuclear RNPs and consider possible functions of snRNPs in nuclear processes.
Keywords: nuclear ribonucleoprotein, rheumatic disease, RNA processing
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer A. L., Christensen M. E., Walker B. W., LeStourgeon W. M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977 May;11(1):127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- Bolden A., Aucker J., Weissbach A. Synthesis of herpes simplex virus, vaccinia virus, and adenovirus DNA in isolated HeLa cell nuclei. I. Effect of viral-specific antisera and phosphonoacetic acid. J Virol. 1975 Dec;16(6):1584–1592. doi: 10.1128/jvi.16.6.1584-1592.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. F., O'Malley B. W., Robertson M. A., Staden R., Tanaka Y., Brownlee G. G. Nucleotide sequence homology at 12 intron--exon junctions in the chick ovalbumin gene. Nature. 1978 Oct 12;275(5680):510–513. doi: 10.1038/275510a0. [DOI] [PubMed] [Google Scholar]
- Deimel B., Louis C. H., Sekeris C. E. The presence of small molecular weight RNAs in nuclear ribonucleoprotein particles carrying HnRNA. FEBS Lett. 1977 Jan 15;73(1):80–84. [PubMed] [Google Scholar]
- Douvas A. S., Stumph W. E., Reyes P., Tan E. M. Isolation and characterization of nuclear ribonucleoprotein complexes using human anti-nuclear ribonucleoprotein antibodies. J Biol Chem. 1979 May 10;254(9):3608–3616. [PubMed] [Google Scholar]
- Eisenberg R. A., Tan E. M., Dixon F. J. Presence of anti-Sm reactivity in autoimmune mouse strains. J Exp Med. 1978 Feb 1;147(2):582–587. doi: 10.1084/jem.147.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B. G., Weissman S. M. Oligonucleotides produced by digestion of KB cell ribosomal 5 S ribonucleic acid with specific nucleases. J Biol Chem. 1968 Nov 10;243(21):5709–5723. [PubMed] [Google Scholar]
- Guimont-Ducamp C., Sri-Widada J., Jeanteur P. Occurrence of small molecular weight RNAs in Hela nuclear ribonucleoprotein particles containing HnRNA. Biochimie. 1977;59(8-9):755–758. doi: 10.1016/s0300-9084(77)80259-9. [DOI] [PubMed] [Google Scholar]
- Hellung-Larsen P., Frederiksen S. Occurrence and properties of low molecular weight RNA components from cells at different taxonomic levels. Comp Biochem Physiol B. 1977;58(3):273–281. doi: 10.1016/0305-0491(77)90202-4. [DOI] [PubMed] [Google Scholar]
- Howard E. F. Small nuclear RNA molecules in nuclear ribonucleoprotein complexes from mouse erythroleukemia cells. Biochemistry. 1978 Aug 8;17(16):3228–3236. doi: 10.1021/bi00609a009. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Leinwand L. Low molecular weight RNAs hydrogen-bonded to nuclear and cytoplasmic poly(A)-terminated RNA from cultured Chinese hamster ovary cells. Cell. 1978 Sep;15(1):205–214. doi: 10.1016/0092-8674(78)90095-8. [DOI] [PubMed] [Google Scholar]
- Karn J., Vidali G., Boffa L. C., Allfrey V. G. Characterization of the non-histone nuclear proteins associated with rapidly labeled heterogeneous nuclear RNA. J Biol Chem. 1977 Oct 25;252(20):7307–7322. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin T., Billings P., Levey A., Ozarslan S., Quinlan T., Swift H., Urbas L. Some properties of RNA:protein complexes from the nucleus of eukaryotic cells. Cold Spring Harb Symp Quant Biol. 1974;38:921–932. doi: 10.1101/sqb.1974.038.01.094. [DOI] [PubMed] [Google Scholar]
- Mattioli M., Reichlin M. Characterization of a soluble nuclear ribonucleoprotein antigen reactive with SLE sera. J Immunol. 1971 Nov;107(5):1281–1290. [PubMed] [Google Scholar]
- Mattioli M., Reichlin M. Physical association of two nuclear antigens and mutual occurrence of their antibodies: the relationship of the SM and RNAprotein (MO) systems in SLE sera. J Immunol. 1973 May;110(5):1318–1324. [PubMed] [Google Scholar]
- Mory Y., Gefter M. RNA synthesis in isolated nuclei: the use of mercurated nucleotides. Nucleic Acids Res. 1978 Oct;5(10):3899–3912. doi: 10.1093/nar/5.10.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northemann W., Scheurlen M., Gross V., Heinrich P. C. Circular dichroism of ribonucleoprotein complexes from rat liver nuclei. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1130–1137. doi: 10.1016/0006-291x(77)90973-1. [DOI] [PubMed] [Google Scholar]
- Notman D. D., Kurata N., Tan E. M. Profiles of antinuclear antibodies in systemic rheumatic diseases. Ann Intern Med. 1975 Oct;83(4):464–469. doi: 10.7326/0003-4819-83-4-464. [DOI] [PubMed] [Google Scholar]
- Parker M. D. Ribonucleoprotein antibodies: frequency and clinical significance in systemic lupus erythematosus, scleroderma, and mixed connective tissue disease. J Lab Clin Med. 1973 Nov;82(5):769–775. [PubMed] [Google Scholar]
- Pinnas J. L., Northway J. D., Tan E. M. Antinucleolar antibodies in human sera. J Immunol. 1973 Oct;111(4):996–1004. [PubMed] [Google Scholar]
- Provost T. T. Subsets in systemic lupus erythematosus. J Invest Dermatol. 1979 Mar;72(3):110–113. doi: 10.1111/1523-1747.ep12530348. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Ro-Choi T. S., Busch H. Nuclear ribonucleoprotein complexes containing U1 and U2 RNA. Biochemistry. 1975 Oct 7;14(20):4380–4385. doi: 10.1021/bi00691a006. [DOI] [PubMed] [Google Scholar]
- Reddy R., Ro-Choi T. S., Henning D., Busch H. Primary sequence of U-1 nuclear ribonucleic acid of Novikoff hepatoma ascites cells. J Biol Chem. 1974 Oct 25;249(20):6486–6494. [PubMed] [Google Scholar]
- Reichlin M. Problems in differentiating SLE and mixed connective-tissue disease. N Engl J Med. 1976 Nov 18;295(21):1194–1195. doi: 10.1056/NEJM197611182952112. [DOI] [PubMed] [Google Scholar]
- Ro-Choi T. S., Reddy R., Henning D., Busch H. 5S RNA 3 , a new nucleus-specific 5S RNA. Biochem Biophys Res Commun. 1971 Aug 20;44(4):963–972. doi: 10.1016/0006-291x(71)90806-0. [DOI] [PubMed] [Google Scholar]
- Ro-Choi T. S., Redy R., Henning D., Takano T., Taylor C. W., Busch H. Nucleotide sequence of 4.5 S ribonucleic acid of Novikoff hepatoma cell nuclei. J Biol Chem. 1972 May 25;247(10):3205–3222. [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., May C. M., Holman H. R., McDuffie F. C., Hess E. V., Schmid F. R. Association of antibodies to ribonucleoprotein and Sm antigens with mixed connective-tissue disease, systematic lupus erythematosus and other rheumatic diseases. N Engl J Med. 1976 Nov 18;295(21):1149–1154. doi: 10.1056/NEJM197611182952101. [DOI] [PubMed] [Google Scholar]
- Shibata H., Ro-Choi T. S., Reddy R., Choi Y. C., Henning D., Busch H. The primary nucleotide sequence of nuclear U-2 ribonucleic acid. The 5'-terminal portion of the molecule. J Biol Chem. 1975 May 25;250(10):3909–3920. [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Stark B. C., Kole R., Bowman E. J., Altman S. Ribonuclease P: an enzyme with an essential RNA component. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3717–3721. doi: 10.1073/pnas.75.8.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Kunkel H. G. Characteristics of a soluble nuclear antigen precipitating with sera of patients with systemic lupus erythematosus. J Immunol. 1966 Mar;96(3):464–471. [PubMed] [Google Scholar]
- Zieve G., Penman S. Small RNA species of the HeLa cell: metabolism and subcellular localization. Cell. 1976 May;8(1):19–31. doi: 10.1016/0092-8674(76)90181-1. [DOI] [PubMed] [Google Scholar]