Abstract
Introduction
Accumulating evidence suggests that enhanced inflammatory responses contribute to the pathogenesis of postoperative cognitive dysfunction (POCD). Blood transfusion can trigger an enhancement of acute inflammatory responses. Therefore, we hypothesized that perioperative blood transfusion is associated with a higher risk of POCD in aged patients following total hip replacement surgery.
Material and methods
Patients older than 65 years undergoing elective total hip replacement surgery were enrolled from October 2011 to December 2012. Neurocognitive tests were evaluated at baseline and at 7 d after surgery by a Mini-Mental State Test. Multivariate logistic regression analysis was used to determine risk factors associated with POCD.
Results
Fifty-six patients (27.3%) developed POCD 7 d postoperatively. Patients who developed POCD were older, had a lower education level and preoperative hemoglobin concentration, had more blood loss, and had a lower body weight (p < 0.05). Patients with POCD were more likely to receive red blood cells (RBCs) transfusion (51.8% versus 31.5%; p < 0.05). A multivariable logistic regression model identified older age, lower education level, and perioperative blood transfusion of more than 3 units as independent risk factors for POCD 7 d postoperatively.
Conclusion
Our data suggested that perioperative blood transfusion of more than 3 units of RBCs is an independent risk factor for POCD in aged patients following total hip replacement surgery.
Keywords: Elderly, hip replacement surgery, postoperative cognitive dysfunction, RBCs transfusion
Introduction
Postoperative cognitive dysfunction (POCD) is a significant complication after major surgery in the elderly, exerting detrimental effects on patients’ recovery and future prognosis (1–4). The prevalence of POCD reported in the literature varies from 41%–75% at 7 days to 18%–45% at 3 months postoperatively, a phenomenon attributed to the different diagnosis criteria for POCD, as well as to population and surgery type studied (5). Although its etiology is not fully understood, accumulating evidence suggests that the enhanced inflammatory response contributes significantly to the pathogenesis of POCD (6–8).
Blood transfusion is a major component of the treatment of acute traumatic hemorrhagic shock and can be life-saving (9). Because of limited physiological reserve, elderly patients are particularly vulnerable to the adverse effects of blood loss and anemia after major surgery. Nevertheless, transfusion of allogeneic blood products, including red blood cells (RBCs) and fresh frozen plasma (FFP), can trigger enhanced acute inflammatory responses (10). In support of this notion, it has been demonstrated that blood transfusion is associated with an increase in serum tumor necrosis factor (TNF)-α, interleukin (IL)-β, IL-6, IL-8, and other cytokines (11,12). Therefore, we hypothesized that intraoperative blood transfusion is associated with a higher risk of POCD in patients after major surgery.
Hip replacement surgery is a common procedure in the elderly and is often associated with substantial blood loss that requires blood transfusion (13,14). In the present study, we aimed to investigate the relationship between perioperative blood transfusion and POCD in aged patients after total hip replacement surgery.
Materials and methods
Patients
After obtaining approval by the Ethics Committee of Jinling Hospital, Nanjing University, and written informed patient consent, 313 consecutive patients aged 65 years or more who underwent elective unilateral total hip replacement surgery were enrolled from October 2011 to December 2012. Patients with a Mini-Mental State Examination (MMSE) score <23; a history of alcoholism, drug dependence, psychiatric, or neurological diseases (Alzheimer’s disease, stroke, psychosis, etc.); a severe heart, lung, liver, or kidney disease; unwillingness to comply with the procedures; inability to understand the language (Mandarin Chinese); or a failure in spinal anesthesia who ultimately received general anesthesia were excluded.
Procedures
Hip replacement was performed by the same surgery team, and a cemented double prosthesis was used in all cases through a posterior-lateral approach. No preoperative medication was administered, and patients received no sedatives during surgery. All the patients received postoperative analgesia via a patient-controlled analgesia device for the initial 48 hours after surgery (a constant infusion rate of 2 ml/h with a lock time of 15 min) of 800–1200 mg of tramadol plus 16 mg of ondansetron on the basis of the patients’ age, weight, American Society of Anesthesiologists (ASA) classification, etc. Pethidine was used to treat pain when necessary during the postoperative period.
Anesthesia and monitoring
Spinal anesthesia was performed as previously described (8). Briefly, after a successful puncture in the L2-3 or L3-4 intervertebral space using a 17-gauge Tuohy needle, 1.2–1.4 ml of 0.75% bupivacaine was diluted with the same volume of injection water and was then administered into the spinal space via a 25-G needle using the needle-through-needle technique for spinal anesthesia. Electrocardiogram, pulse oximetry, and invasive blood pressure were continuously monitored during anesthesia.
Ringer’s lactate was administrated before anesthesia induction and during surgery. Blood loss was estimated by quantitative measurement of blood loss (e.g. suction and sponge). When estimated intraoperative blood loss was greater than 500 ml, hydroxyethyl starch (HES) 130/0.4 was infused at the same volume of blood loss. The targeted mean arterial pressure was a minimum of 20 mmHg below the value measured immediately before anesthesia induction. The lowest acceptable mean arterial pressure was 55 mmHg or a systolic blood pressure of 80 mmHg, where a value below aforementioned threshold was treated by an intravenous injection of ephedrine or phenylephrine.
Transfusion protocol
Serum hemoglobin concentrations were measured 1 day before surgery, and 1, 2, 3, and 7 days after surgery. The decision to transfuse was based on the hemoglobin concentration and by immediate arterial blood gas analysis. The blood transfusion strategy was based on the criteria in our hospital, and patients with a hemoglobin concentration < 80 g/l were transfused. Patients with a hemoglobin concentration from 80 to 100 g/l were transfused only if they had relevant symptoms, and those with a hemoglobin concentration >100 g/l were not transfused. The administration of fresh frozen plasma (FFP) and other blood products was at the discretion of the anesthesiologist in charge.
Cognition measurement
Cognitive function was assessed with the Chinese version of the MMSE on a scale of 0 (poor) to 30 (good) in a quiet room preoperatively and 7 d postoperatively. POCD was defined as a decline of 1 SD or more in MMSE score 7 d postoperatively when compared with the preoperative score. Thereafter, the patients were divided into a POCD group and a non-POCD group according to whether POCD occurred 7 d after surgery or not.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 for Windows. Data are presented as means with standard deviations or number, as appropriate. Student’s t test for continuous data and the chi-square test for categorical data were used to compare differences between groups. Repeated-measures analysis of variance with Bonferroni corrections was used to examine visual analog scale scores and hemoglobin concentration. To determine which variables were associated with POCD, standard and stepwise multivariate logistic regressions were performed. Variables that were associated with the study outcome in univariate analysis (p < 0.10) were entered as candidate variables in the multivariate models.
Results
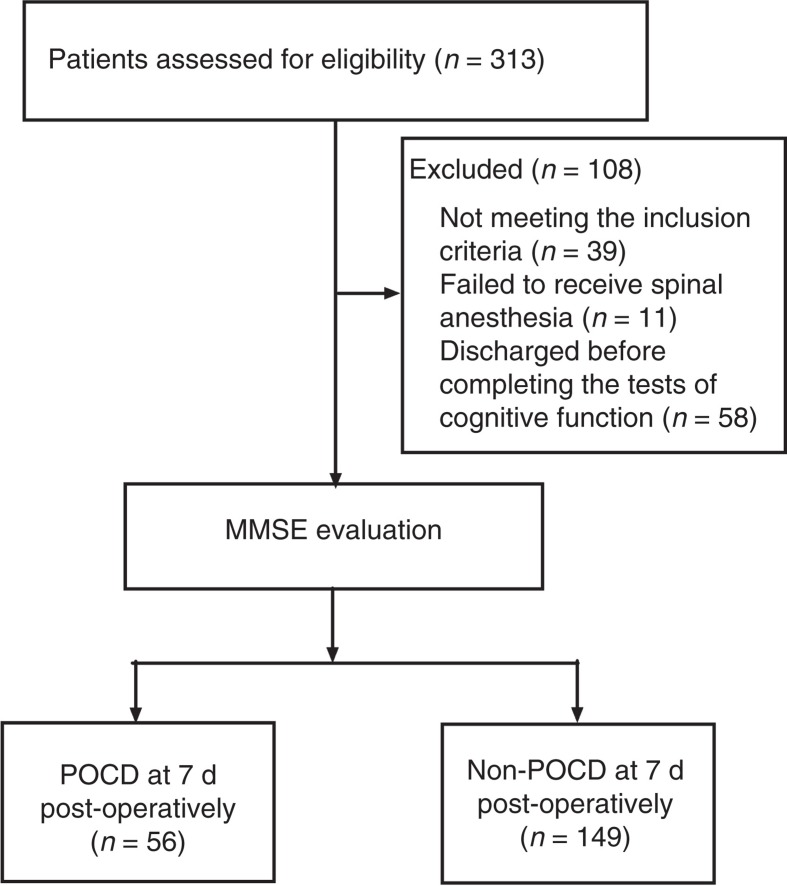
In total 313 patients older than 65 years undergoing elective total hip replacement surgery were enrolled. Thirty-nine patients did not meet the inclusion criteria. Spinal anesthesia was given to 263 patients, and the other 11 patients received general anesthesia. Fifty-eight patients were discharged before completing the tests for cognitive function 7 d postoperatively. Thus, only 205 patients completed the neurocognitive tests (Figure 1).
Figure 1.
The CONSORT diagram showing patients’ flow through the study.
There was no difference in terms of gender, ASA classification, fracture characteristics, episodes of hypertension and intraoperative hypotension, the amount of Ringer’s lactate infusion, length of surgery, postoperative drainage, 7 d postoperative hemoglobin concentration, FFP transfusion, preoperative MMSE scores, postoperative visual analog scale scores, tramadol consumption, or pethidine administration between the POCD and non-POCD groups (all p > 0.05) (Table I). However, patients with POCD received more HES than those without POCD (p = 0.008).
Table I.
Demographic characteristics of the study population.
| Factors | POCD (n = 56) | Non-POCD (n = 149) | p value |
|---|---|---|---|
| Age (y) | 76 ± 6 | 74 ± 6 | 0.007 |
| Gender (male/ female) | 34/ 22 | 97/ 52 | 0.736 |
| Weight (kg) | 56 ± 9 | 63 ± 11 | 0.024 |
| Education (y) | 7.1 ± 2.8 | 8.6 ± 2.8 | 0.001 |
| ASA classification (I/ II/ III) | 3/ 48/ 5 | 7/ 130/ 12 | 0.958 |
| Fracture characteristics (n) | 0.9 | ||
| Femoral neck | 17 | 43 | |
| Intertrochanteric | 30 | 78 | |
| Other | 9 | 28 | |
| History of hypertension (n) | 29 | 71 | 0.640 |
| Intraoperative hypotension (n) | 28 | 65 | 0.435 |
| Ringer’s lactate infusion (mL) | 968 ± 145 | 959 ± 144 | 0.698 |
| HES infusion (mL) | 654 ± 265 | 569 ± 170 | 0.008 |
| Length of surgery (min) | 80 ± 12 | 79 ± 10 | 0.635 |
| Estimated blood loss (mL) | 772 ± 126 | 618 ± 127 | 0.001 |
| Postoperative drainage (mL) | 130 ± 68 | 120 ± 50 | 0.283 |
| Hemoglobin concentration (g/L) | |||
| Preoperative level | 116 ± 13 | 122 ± 12 | 0.006 |
| 7 d after surgery | 101 ± 12 | 99 ± 11 | 0.654 |
| Perioperative RBCs transfusion | 0.012 | ||
| 0 unit (n) | 27 | 102 | |
| 1 unit (n) | 5 | 15 | |
| 2 units (n) | 7 | 14 | |
| 3 units (n) | 9 | 13 | |
| ≥4 units (n) | 8 | 5 | |
| Perioperative FFP transfusion (n) | 9 | 17 | 0.371 |
| MMSE scores | |||
| Before surgery | 28.0 ± 1.2 | 28.1 ± 1.6 | 0.661 |
| 7 d after surgery | 25.7 ± 1.2 | 27.7 ± 1.5 | 0.001 |
| VAS scores | |||
| 1 d after surgery | 3.7 ± 0.9 | 3.8 ± 1.0 | 0.462 |
| 2 d after surgery | 3.5 ± 0.7 | 3.7 ± 0.9 | 0.149 |
| 7 d after surgery | 1.5 ± 0.6 | 1.5 ± 0.5 | 0.765 |
| Tramadol consumption (mg) | 896 ± 129 | 932 ± 143 | 0.109 |
| Pethidine administration (n) | 14 | 56 | 0.09 |
Values are means ± standard deviations, or number as appropriate.
ASA = American Society of Anesthesiologists; FFP = fresh frozen plasma; HES = hydroxyethyl starch; MMSE = Mini-Mental State Examination; POCD = postoperative cognitive dysfunction; RBCs = red blood cells; VAS = visual analog scale.
Fifty-six patients (27.3%) developed POCD 7 d postoperatively. Patients who developed POCD were older, had a lower education level and preoperative hemoglobin concentration, had more blood loss, and had a lower body weight (p < 0.05). Patients with POCD were more likely to have received a RBCs transfusion (51.8% versus 31.5%; p < 0.05) (Table I).
A multivariable logistic regression model identified older age, lower education level, and perioperative blood transfusion of more than 3 units as independent risk factors for POCD 7 d postoperatively (odds ratio 1.57, 95% CI 1.09–2.32; p = 0.045) (Table II).
Table II.
Predictors of POCD identified by multivariable logistic regression.
| Factors | p value | Odds ratio (95% CI) |
|---|---|---|
| Age (y) | 0.005 | 1.08 (1.023–1.14) |
| Weight (kg) | 0.164 | 0.43 (0.12–1.37) |
| Education (y) | 0.002 | 0.83 (0.74–0.93) |
| Estimated blood loss (mL) | 0.067 | 1.23 (0.98–1.56) |
| HES infusion (mL) | 0.12 | 1.35 (0.67–1.78) |
| Preoperative hemoglobin concentration (g/L) | 0.032 | 0.87 (0.72–0.99) |
| Perioperative RBCs transfusion >3 units | 0.045 | 1.57 (1.09–2.32) |
Discussion
Although the underlying mechanisms leading to POCD have not been fully elucidated, several lines of evidence suggest that the enhanced inflammatory response is a key contributor to neurocognitive decline after major surgery (6,8,15). In the present study, we further identified perioperative blood transfusion of more than 3 units as a risk factor for the development of POCD. However, it remains unclear whether this association is because of the adverse effects of the blood transfusions or from the results of the severity of illness in the patients receiving the transfusions.
Blood transfusion is widely used in surgical practice, not only to increase oxygen-carrying capacity, but also to replace acute severe blood loss when maintenance of normovolemia and organ perfusion is necessary (16). Indeed, one recent study has shown that intraoperative blood transfusion is helpful in patients with a preoperative hemoglobin concentration <80 g/l, or in patients with mild to no preoperative anemia when there is substantial blood loss (17). Although anemia is associated with worse outcomes, accumulating evidence suggests that blood transfusion does not improve survivals and even may result in adverse outcomes. It has been demonstrated that blood transfusion is associated with increased susceptibility to infection, immune sensitization, transfusion-related lung injury, exacerbation of stress injury, and postoperative systemic inflammatory response syndrome (10–12,18,19). The pro-inflammatory effects and stress injury may explain why blood transfusion is related to poorer survival after trauma, coronary artery bypass grafting, and in individuals in intensive care (20–22). Although the role of blood transfusion on cognitive function remains less well studied, one study suggested that a liberal transfusion strategy was associated with poorer outcomes on measures of associative verbal fluency, visual memory, and reading than a restrictive transfusion strategy in preterm infants (23). Likewise, another recently published study found that intraoperative blood transfusion is an independent risk factor for the development of delirium during the first postoperative day in older adults undergoing major non-cardiac surgery (24). Our results suggest that perioperative blood transfusion is associated with a higher incidence of POCD 7 d after surgery, indicating more prolonged effects of blood transfusion on adverse neurological outcome. Because we did not measure the time course of inflammatory mediators, the relationship between blood transfusion, inflammatory response, and cognitive function cannot be well established.
The hemoglobin concentration threshold requiring RBCs transfusion has not been well established. The American Society of Anesthesiologists recommends blood transfusion when the hemoglobin concentration is less than 60 g/l and avoidance of blood transfusion when the hemoglobin concentration is above 100 g/l (25). However, we chose a hemoglobin concentration of 80 g/l as the transfusion trigger because our study population constituted aged patients more likely to have serious cardiac diseases and more co-morbidities. However, we cannot rule out that lower perioperative hemoglobin values might decrease cerebral oxygenation, thus causing the development of POCD. Therefore, further studies are needed to address this important issue.
One drawback in the present study is the short duration of follow-up. Second, hemoglobin is a volume-based measurement that can be affected by dehydration or hypervolemia due to different clinical scenarios that probably influence the precision of the estimates. Third, although current transfusion guidelines are mostly based on arbitrary hemoglobin triggers, the use of physiological parameters and clinical variables in addition to hemoglobin values to guide blood transfusion may be more reasonable. Finally, we did not track co-morbidities, acute physiology and chronic health evaluations, or sequential organ failure assessment scores, and further studies are urgently needed to clarify these important confounders.
In summary, we found that perioperative blood transfusion of more than 3 units of RBCs is an independent risk factor for POCD in aged patients following total hip replacement surgery. In light of the potential detrimental effects of blood transfusion, limiting blood transfusion may decrease the risk of POCD in patients undergoing major surgery. However, larger prospective randomized control trials are required to address this important issue.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos. 81271216 and 81300946) and Natural Science Foundation of Jiangsu Province (No. BK2012778), and is attributed to the Department of Anesthesiology, Jinling Hospital, School of Medicine, Nanjing University, Nanjing, China. We thank Dr Anatoly Martynyuk for his revision of the manuscript and Dr Yu-xiu Liu for his suggestions with regard to statistical analysis.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long-term consequences of postoperative cognitive dysfunction . Anesthesiology. 2009;110:548–55. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 2.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery . Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 3.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study . Lancet. 1998;351:6857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 4.Koch S, Forteza A, Lavernia C, Romano JG, Campo-Bustillo I, Campo N, et al. Cerebral fat microembolism and cognitive decline after hip and knee replacement . Stroke. 2007;38:1079–81. doi: 10.1161/01.STR.0000258104.01627.50. [DOI] [PubMed] [Google Scholar]
- 5.Krenk L, Rasmussen LS, Kehlet H. New insights into the pathophysiology of postoperative cognitive dysfunction . Acta Anaesthesiol Scand. 2010;54:951–6. doi: 10.1111/j.1399-6576.2010.02268.x. [DOI] [PubMed] [Google Scholar]
- 6.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, et al. Role of interleukin-1beta in postoperative cognitive dysfunction . Ann Neurol. 2010;68:360–8. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonagh DL, Mathew JP, White WD, Phillips-Bute B, Laskowitz DT, Podgoreanu MV, et al. Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of brain injury . Anesthesiology. 2010;112:852–9. doi: 10.1097/ALN.0b013e3181d31fd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji MH, Yuan HM, Zhang GF, Li XM, Dong L, Li WY, et al. Changes in plasma and cerebrospinal fluid biomarkers in aged patients with early postoperative cognitive dysfunction following total hip-replacement surgery . J Anesth. 2013;27:236–42. doi: 10.1007/s00540-012-1506-3. [DOI] [PubMed] [Google Scholar]
- 9.Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery . Anesthesiology. 2011;114:283–92. doi: 10.1097/ALN.0b013e3182054d06. [DOI] [PubMed] [Google Scholar]
- 10.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions . Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferraris VA, Ballert EQ, Mahan A. The relationship between intraoperative blood transfusion and postoperative systemic inflammatory response syndrome . Am J Surg. 2013;205:457–65. doi: 10.1016/j.amjsurg.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 12.Urner M, Herrmann IK, Buddeberg F, Urner M, Herrmann IK, Buddeberg F, et al. Effects of blood products on inflammatory response in endothelial cells in vitro . PLoS ONE. 2012;7:e33403. doi: 10.1371/journal.pone.0033403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindholm E, Seljeflot I, Aune E, Kirkebøen KA. Proinflammatory cytokines and complement activation in salvaged blood from abdominal aortic aneurism surgery and total hip replacement surgery . Transfusion. 2012;52:1761–9. doi: 10.1111/j.1537-2995.2011.03528.x. [DOI] [PubMed] [Google Scholar]
- 14.Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P, et al. Tranexamic acid in hip fracture surgery: a randomized controlled trial . Br J Anaesth. 2010;104:23–30. doi: 10.1093/bja/aep314. [DOI] [PubMed] [Google Scholar]
- 15.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline . Proc Natl Acad Sci U S A. 2010;107:20518–22. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madjdpour C, Spahn DR, Weiskopf RB. Anemia and perioperative red blood cell transfusion: a matter of tolerance . Crit Care Med. 2006;34:S102–8. doi: 10.1097/01.CCM.0000214317.26717.73. [DOI] [PubMed] [Google Scholar]
- 17.Wu WC, Smith TS, Henderson WG, Eaton CB, Poses RM, Uttley G, et al. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery . Ann Surg. 2010;252:11–17. doi: 10.1097/SLA.0b013e3181e3e43f. [DOI] [PubMed] [Google Scholar]
- 18.Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, Chan CT, Wong PY, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial . Anesthesiology. 2012;116:613–21. doi: 10.1097/ALN.0b013e3182475e39. [DOI] [PubMed] [Google Scholar]
- 19.Zufferey P, Merquiol F, Laporte S, Decousus H, Mismetti P, Auboyer C, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? . Anesthesiology. 2006;105:1034–46. doi: 10.1097/00000542-200611000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Claridge JA, Sawyer RG, Schulman AM, McLemore EC, Young JS. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner . Am Surg. 2002;68:566–72. [PubMed] [Google Scholar]
- 21.Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma . J Trauma. 2003;54:898–905. doi: 10.1097/01.TA.0000060261.10597.5C. [DOI] [PubMed] [Google Scholar]
- 22.Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure . Arch Surg. 1997;132:620–4. [PubMed] [Google Scholar]
- 23.McCoy TE, Conrad AL, Richman LC, Lindgren SD, Nopoulos PC, Bell EF. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion . Child Neuropsychol. 2011;17:347–67. doi: 10.1080/09297049.2010.544647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behrends M, DePalma G, Sands L, Leung J. Association between intraoperative blood transfusions and early postoperative delirium in older adults . J Am Geriatr Soc. 2013;61:365–70. doi: 10.1111/jgs.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies . Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]