Abstract
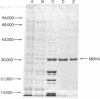
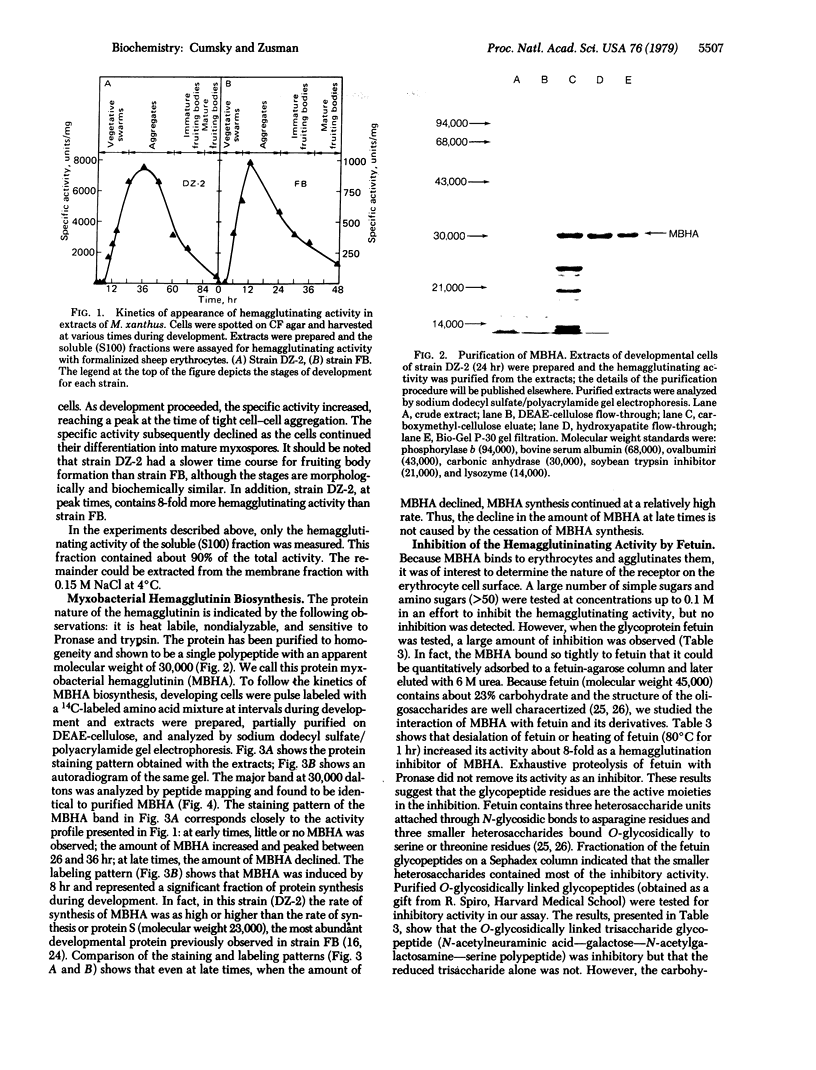
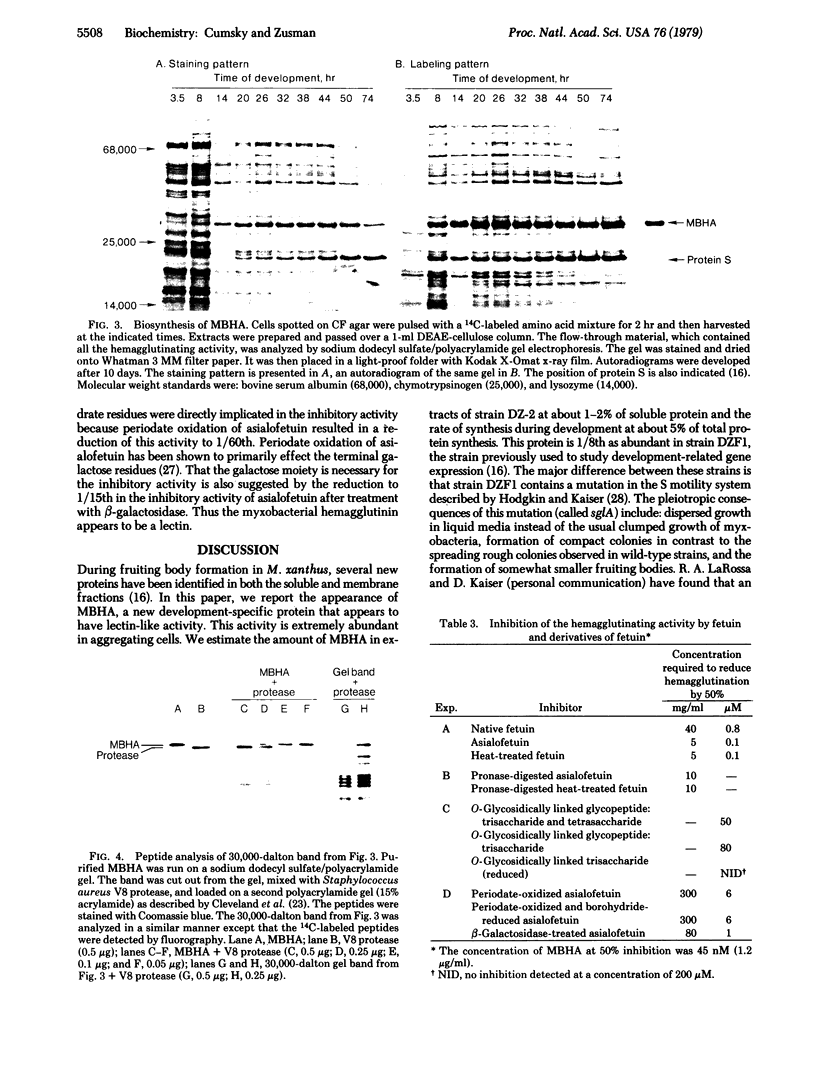
Fruiting body formation in the bacterium Myxococcus xanthus consists of a temporal sequence of cellular aggregation and sporulation. During the period of cellular aggregation, a major new development-specific protein that has lectin-like activity is synthesized. This protein, called myxobacterial hemagglutinin (MBHA), was able to agglutinate sheep or guinea pig erythrocytes but not horse, ox, chicken, or human erythrocytes. MBHA was undetectable in extracts of vegetative cells, cells starved in liquid buffer, or in glycerol-induced cells. However, cells starved on a fruiting medium produced large amounts of MBHA (about 5% of protein synthesis), starting at about 6-8 hr of development. The protein accumulated in the soluble fraction of cells, reaching a peak of 1-2% of total protein at about the time when aggregation was completed. At later times the amount of MBHA present in the soluble fraction declined although synthesis continued. The hemagglutinating activity of MBHA could not be inhibited with simple sugars or aminosugars but could be inhibited with fetuin, a fetal calf serum glycoprotein. The O-glycosidically linked trisaccharide glycopeptide of fetuin was shown to be inhibitory by itself. The penultimate galactose of this glycopeptide was directly implicated in the inhibitory activity, because the inhibition by asialofetuin was reduced to 1/60th by periodate oxidation and to 1/15th after β-galactosidase treatment. MBHA is an abundant biochemical marker of development in M. xanthus. The fact that it is a lectin suggests that it may play a role in cell—cell recognition or agglutination.
Keywords: fruiting body, aggregation, cell—cell recognition, glycoproteins, fetuin
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. T. HEMAGGLUTINATION STUDIES WITH FORMALINIZED ERYTHROCYTES. EFFECT OF BIS-DIAZO-BENZIDINE AND TANNIC ACID TREATMENT ON SENSITIZATION BY SOLUBLE ANTIGEN. J Immunol. 1963 May;90:663–671. [PubMed] [Google Scholar]
- Campos J. M., Geisselsoder J., Zusman D. R. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978 Feb 25;119(2):167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- Campos J. M., Zusman D. R. Regulation of development in Myxococcus xanthus: effect of 3':5'-cyclic AMP, ADP, and nutrition. Proc Natl Acad Sci U S A. 1975 Feb;72(2):518–522. doi: 10.1073/pnas.72.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Podleski T. R. Evidence that a membrane bound lectin mediates fusion of L6 myoblasts. Biochem Biophys Res Commun. 1975 Dec 1;67(3):972–978. doi: 10.1016/0006-291x(75)90770-6. [DOI] [PubMed] [Google Scholar]
- Gilboa-Garber N. Purification and properties of hemagglutinin from Pseudomonas aeruginosa and its reaction with human blood cells. Biochim Biophys Acta. 1972 Jun 26;273(1):165–173. [PubMed] [Google Scholar]
- Hagen D. C., Bretscher A. P., Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978 Jun;64(2):284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Isolation of retina-specific cell-aggregating factor from membranes of embryonic neural retina tissue. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3594–3598. doi: 10.1073/pnas.73.10.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- Inouye M., Inouye S., Zusman D. R. Biosynthesis and self-assembly of protein S, a development-specific protein of Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):209–213. doi: 10.1073/pnas.76.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Inouye S., Zusman D. R. Gene expression during development of Myxococcus xanthus: pattern of protein synthesis. Dev Biol. 1979 Feb;68(2):579–591. doi: 10.1016/0012-1606(79)90228-8. [DOI] [PubMed] [Google Scholar]
- Kobiler D., Barondes S. H. Lectin activity from embryonic chick brain, heart, and liver: changes with development. Dev Biol. 1977 Oct 1;60(1):326–330. doi: 10.1016/0012-1606(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Rosen S. D., Kafka J. A., Simpson D. L., Barondes S. H. Developmentally regulated, carbohydrate-binding protein in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2554–2557. doi: 10.1073/pnas.70.9.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. D., Simpson D. L., Rose J. E., Barondes S. H. Carbohydrate-binding protein from Polysphondylium pallidum implicated in intercellular adhesion. Nature. 1974 Nov 8;252(5479):128, 149-50. doi: 10.1038/252128a0. [DOI] [PubMed] [Google Scholar]
- SPIRO R. G. PERIODATE OXIDATION OF THE GLYCOPROTEIN FETUIN. J Biol Chem. 1964 Feb;239:567–573. [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Studies on fetuin, a glycoprotein of fetal serum. I. Isolation, chemical composition, and physiochemical properties. J Biol Chem. 1960 Oct;235(10):2860–2869. [PubMed] [Google Scholar]
- Teichberg V. I., Silman I., Beitsch D. D., Resheff G. A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1383–1387. doi: 10.1073/pnas.72.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Brackenbury R., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. II. Purification and characterization of a cell adhesion molecule from neural retina. J Biol Chem. 1977 Oct 10;252(19):6841–6845. [PubMed] [Google Scholar]
- de Waard A., Hickman S., Kornfeld S. Isolation and properties of beta-galactoside binding lectins of calf heart and lung. J Biol Chem. 1976 Dec 10;251(23):7581–7587. [PubMed] [Google Scholar]