Abstract
Caenorhabditis elegans is a simple genetic organism amenable to large-scale forward and reverse genetic screens and chemical genetic screens. The C. elegans genome includes potential antipsychotic drug (APD) targets conserved in humans, including genes encoding proteins required for neurotransmitter synthesis and for synaptic structure and function. APD exposure produces developmental delay and/or lethality in nematodes in a concentration-dependent manner. These phenotypes are caused, in part, by APD-induced inhibition of pharyngeal pumping1,2. Thus, the developmental phenotype has a neuromuscular basis, making it useful for pharmacogenetic studies of neuroleptics. Here we demonstrate detailed procedures for testing APD effects on nematode development and pharyngeal pumping. For the developmental assay, synchronized embryos are placed on nematode growth medium (NGM) plates containing APDs, and the stages of developing animals are then scored daily. For the pharyngeal pumping rate assay, staged young adult animals are tested on NGM plates containing APDs. The number of pharyngeal pumps per unit time is recorded, and the pumping rate is calculated. These assays can be used for studying many other types of small molecules or even large molecules.
Keywords: Neuroscience, Issue 84, antipsychotic drug, Caenorhabditis elegans, clozapine, developmental delay, lethality, nematode, pharmacogenetics, pharyngeal pumping, schizophrenia
Introduction
Caenorhabditis elegans is a simple genetic organism amenable to large-scale forward and reverse genetic screens and chemical genetic screens. C. elegans is sensitive to a wide spectrum of bioactive compounds and has therefore been used successfully to define the mechanisms of action of a variety of such compounds. For example, bioactive compounds studied using worm pharmacogenetics include acetylcholine receptor agonists (e.g. levamisole, nicotine, morantel, and pyrantel), anesthetics (e.g. halothane), caffeine, cholinesterase inhibitors (e.g. aldicarb, lannate, and trichlorfon), fluoride, GABA-related compounds (e.g. GABA and muscimol), ivermectin, paraquat, phorbol esters, and serotonin-related drugs (e.g. serotonin and imiprimine)3. Furthermore, C. elegans has been used for large-scale small molecule screens, allowing discovery of new bioactive compounds and identification of novel genetic targets4.
The C. elegans genome includes potential antipsychotic drug (APD) targets conserved in humans, including genes encoding proteins required for neurotransmitter synthesis and for synaptic structure and function5. Thus, C. elegans neurogenetics and neurobiology offer methods for discovering novel molecular mechanisms of action of APDs. In nematodes, APD exposure early in development produces developmental delay, and at higher concentrations, lethality2,6. APD exposure during adulthood produces behavioral phenotypes. For example, clozapine exposure inhibits locomotion and pharyngeal pumping and enhances egg laying1,2,7.
APD-induced developmental delay and lethality are powerful phenotypes for large-scale chemical genetic screens. These phenotypes are complex in so far as they likely have more than one cellular and genetic basis. Therefore, such genetic screens are expected to yield a variety of indirect drug targets. However, our laboratory has conducted candidate gene screens and a genome-wide RNAi screen for suppressors of APD-induced developmental delay and lethality and has successfully recovered genes which likely encode direct targets, including dopamine, insulin, and nicotinic acetylcholine receptors2,8. Genetic screens based on APD-induced behaviors in the adult have also led to the identification of novel APD targets, and we are now validating targets from both developmental and behavioral screens in mammals7. Thus, an invertebrate chemical genetic approach to discover novel molecular mechanisms of action of APDs appears to be feasible5,8.
The C. elegans pharynx is an organ that includes 20 neurons, 20 muscle cells, and 20 accessory cells, wrapped by a basement membrane. Similar to the mammalian heart, the pharynx is autonomous and constantly pumps food in from the external environment9. Inhibition of the pharyngeal pumping rate compromises food uptake, and thus mutations or drugs that inhibit pharyngeal pumping cause developmental delay or arrest9. APDs inhibit the pharyngeal pumping rate, accounting in part for their effects on development and viability1,2. Here, we use the atypical APD clozapine as an example to demonstrate drug assays for nematode development and pharyngeal pumping.
Protocol
1. Developmental Delay/Lethality Assay: The Wild-Type (N2) and Two Mutant (Mut1 and Mut2) Strains Are Tested in Three Clozapine Concentrations in a 12-well Plate
On day 1, pour 2 ml NGM medium10 into each well of a 12-well plate (each well with a 2 cm diameter) and allow to harden on the bench at room temperature (RT) overnight. The same day, pick a colony of Escherichia coli OP50 bacteria, infect a bottle of 50 ml LB solution, and culture it in a 37 °C shaker at 220 rpm rotation overnight.
On day 2, transfer 20 µl OP50 bacterial culture onto the center of each NGM well. Incubate the plates in a 37 °C incubator or leave them at RT overnight to allow the bacterial lawn to grow thicker.
Make an 80 mM clozapine stock solution by dissolving clozapine in DMSO (dimethyl sulfoxide) solvent. Then make an 80 µl working solution with the stock solution diluted in 1.7 mM acetic acid based on Table 1. Note: The working solution for a particular drug of interest must be determined by doing a series of preliminary concentration tests to find the most appropriate concentration for distinguishing the wild type from the mutants.
Transfer 80 µl clozapine working solution onto the seeded NGM plate well and swirl the plate to allow the solution to distribute evenly on the surface. Wait for the plate to dry. Use the plate for the assay on the same day or place it in a 20 °C incubator to be used the next day. Note: The working solution is a drug suspension due to the low solubility of clozapine, especially at higher concentration. Therefore, vortex the tube before transferring the solution to the drug plate in order to guarantee that the drug concentration is accurate.
Wash worms off a 3.5 cm plate containing many gravid adults with M9 buffer and then spin them down in a 15 ml tube at 2,000 rpm for 1 min.
Aspirate the supernatant. Add 5 ml bleach solution (NaOH:hypochlorite:ddH2O at 4:1:5) and disrupt the nematodes by gently inverting the tubes.
Observe the animals until half of them are dissolved at around 5 min. Spin down the eggs at 2,000 rpm for 1 min.
Aspirate the supernatant and add 14 ml M9 buffer rapidly.
Spin down the eggs at 2,000 rpm for 1 min and aspirate the supernatant, leaving approximately 100 µl solution. Vortex to suspend the eggs.
Transfer 2 µl eggs suspended in M9 onto a regular NGM plate to test how many eggs are transferred. Adjust the volume of the suspended eggs to ensure that there are approximately 30-35 eggs in each transfer. Transfer 30-35 eggs to each well of the assay plate, and then incubate in a 20 °C incubator for 24 hr.
On the first day of the assay, score the number of eggs hatched. Adjust the total number of hatched animals to 25/well by picking off the extra worms if necessary.
On the following days, observe the animals and score them every 24 hr. Developmental stage is scored based on the size of the animal and the shape of the vulva11. The experiment lasts 5-6 days with the most robust effect typically seen on the third or fourth day.
Input the data into an Excel file and calculate the percentage of animals at each developmental stage for every day of the experiment. Generate graphs with the function '100% Stacked Column' in the '2-D column' menu.
2. Pharyngeal Pumping Assay: Young Adult Animals for the Wild-type and Mutant Strains Are Scored on Clozapine Assay Plates
The assay plate is made following the same protocol used for the developmental delay/lethality assay.
Pick 50 L4 animals for each strain to seeded NGM plates 24 hr before the assay and incubate the worms at 20 °C.
Pick 10 animals to an assay plate well. Then pick 10 animals to the next well every 15-20 min.
After the animals in the first well have been exposed to drug for 30 min, start the assay by observing pharyngeal pumping under a dissection microscope and score the pumps for 20 sec9. Once pharyngeal pumping is scored, pick the worm off the plate.
Representative Results
1. Developmental delay/lethality assay result
A typical result for the developmental delay/lethality assay is demonstrated in Figures 1a and 1b. When wild-type animals in the control group have grown to the gravid adult stage (Figure 1a), wild-type animals exposed to clozapine are still in the young larval stages or are dead (Figure 1b). Figure 1c shows a representative result comparing a suppressor mutant (Mut1) and an enhancer mutant (Mut2) with the wild type at three different clozapine concentrations. At all three clozapine concentrations, the lethality of Mut1 is lower than wild type and the lethality of Mut2 is higher. Surviving Mut1 animals progress to more advanced larval stages than wild type, while surviving Mut2 animals display developmental delay compared to wild type.
2. Pharyngeal pumping rate assay result
Figure 2 shows a representative pharyngeal pumping assay result comparing a suppressor mutant (Mut1) and an enhancer mutant (Mut2) with the wild type at two clozapine concentrations. Clozapine exposure reduces the pharyngeal pumping rates of the wild-type and mutant strains in a concentration-dependent manner. However, the decreased pharyngeal pumping rate of Mut1 is less than that of the wild type, while the decreased pharyngeal pumping rate of Mut2 is greater than that of the wild type.
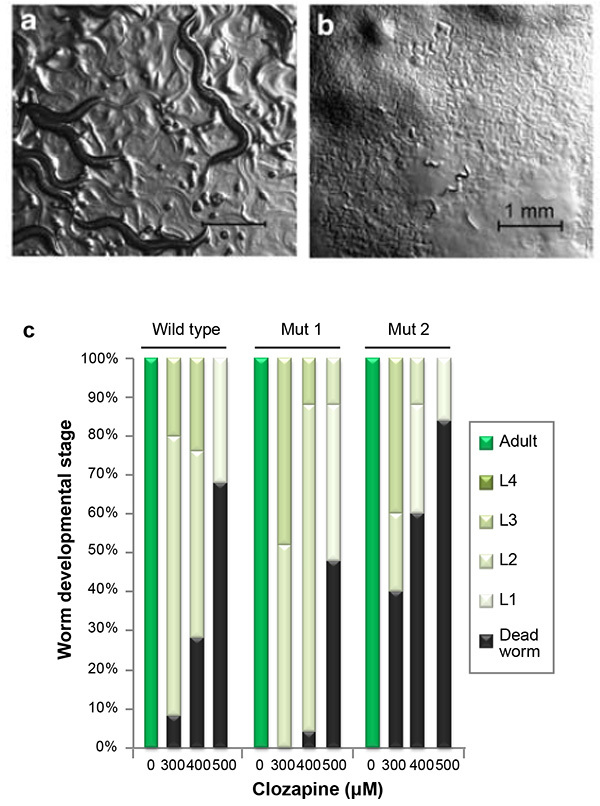 Figure 1. Demonstration of clozapine-induced developmental delay and lethality. Wild-type animals (N2) grown on a control NGM plate (a) and on an NGM plate supplemented with 200 µg/ml (612 µM) clozapine (b). (c) A representative result of the developmental delay/lethality assay showing clozapine's effect on the wild type (N2), a suppressor mutant (Mut1), and an enhancer mutant (Mut2). The images in (a) and (b) are reprinted <http://www.nature.com/npp/journal/v34/n8/full/npp200935a.html> from Neuropsychopharmacology.
34 (8), 1968-1978 (July, 2009). Click here to view larger image.
Figure 1. Demonstration of clozapine-induced developmental delay and lethality. Wild-type animals (N2) grown on a control NGM plate (a) and on an NGM plate supplemented with 200 µg/ml (612 µM) clozapine (b). (c) A representative result of the developmental delay/lethality assay showing clozapine's effect on the wild type (N2), a suppressor mutant (Mut1), and an enhancer mutant (Mut2). The images in (a) and (b) are reprinted <http://www.nature.com/npp/journal/v34/n8/full/npp200935a.html> from Neuropsychopharmacology.
34 (8), 1968-1978 (July, 2009). Click here to view larger image.
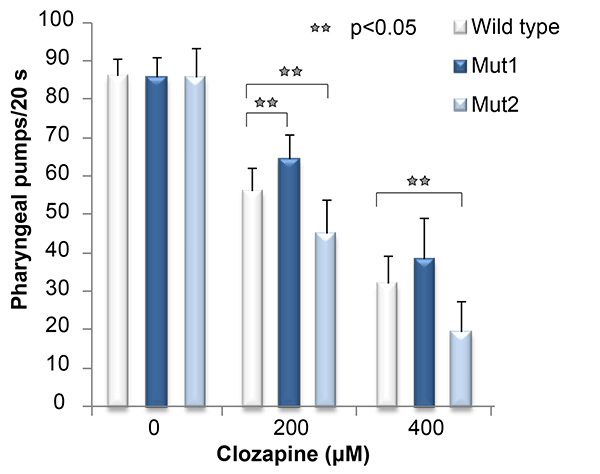 Figure 2. Demonstration of clozapine-induced inhibition of pharyngeal pumping. A typical result for the pharyngeal pumping assay showing clozapine's effect on the wild type (N2), a suppressor mutant (Mut1), and an enhancer mutant (Mut2). The data were analyzed with two-way ANOVA using the statistical software program R. Click here to view larger image.
Figure 2. Demonstration of clozapine-induced inhibition of pharyngeal pumping. A typical result for the pharyngeal pumping assay showing clozapine's effect on the wild type (N2), a suppressor mutant (Mut1), and an enhancer mutant (Mut2). The data were analyzed with two-way ANOVA using the statistical software program R. Click here to view larger image.
Table 1. Preparation of working solution of clozapine. The amount is for 4 wells at each concentration.
| Clozapine concentration | 0 µM | 300 µM | 400 µM | 500 µM |
|---|---|---|---|---|
| HAC solution | 270 µl | 270 µl | 270 µl | 270 µl |
| Clozapine stock solution | - | 30 µl | 40 µl | 50 µl |
| DMSO | 50 µl | 20 µl | 10 µl | 0 µl |
Discussion
Here, we describe methods for testing the effects of APDs on the development and behavior of C. elegans. DMSO or ethanol is used to dissolve clozapine, since the drug is relatively insoluble in water. Because solvents have been reported to affect C. elegans biology12, DMSO-alone or ethanol-alone control groups are essential. The highest concentration of DMSO used in our assays is up to 3%, which does not have an obvious effect on C. elegans development. DMSO can be used for many small molecules, or even some large molecules, e.g. lipid acids.
APD concentrations used in these assays are higher than those given to humans. This is common for most small molecule studies in C. elegans, since high concentrations are required for penetration of the C. elegans cuticle. HPLC studies indicate that the levels of clozapine in C. elegans tissue in the developmental assay are close to those expected in human brains2.
Penetration of the cuticle is a particular concern for studies of large molecules. Mutants with defects in the cuticle barrier, such as acs-20 mutants and Bus (Bacterially Un-Swollen) mutants, offer one approach to circumvent this problem13. Bus mutants have been isolated on the basis of their resistance to infection by the pathogen Microbacterium nematophilum. For example, weak alleles of bus-8 demonstrate that this gene plays a role in production of the cuticle surface. These mutants are resistant to infection, because the bacterium cannot bind to the host surface. Importantly, disruption of cuticle formation also causes increased drug sensitivity in this genetic background14.
The developmental assay described here can be scaled up for large-scale genetic screens by performing the assay in liquid culture. For genome-wide RNA interference (RNAi) screens, for example, the protocol is similar to that described above, but feeding RNAi bacteria is substituted for OP50 bacteria15. The liquid culture assay requires a lower drug concentration than the plate assay (unpublished observation). Our laboratory performed such a genome-wide RNAi screen for suppressors of clozapine-induced developmental delay and obtained 40 suppressors from a screen of ~17,000 C. elegans genes8.
Disclosures
The authors declare that there is not interest conflict involved.
Acknowledgments
The work was supported by an NIH Clinical Scientist Development award K08NS002083, a Shervert Frazier Research Institute Grant, and a NARSAD Young Investigator Award to Edgar A. Buttner.
References
- Donohoe DR, Jarvis RA, Weeks K, Aamodt EJ, Dwyer DS. Behavioral adaptation in C. elegans produced by antipsychotic drugs requires serotonin and is associated with calcium signaling and calcineurin inhibition. Neurosci. Res. 2009;64:280–289. doi: 10.1016/j.neures.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmacharya R, et al. Clozapine interaction with phosphatidyl inositol 3-kinase (PI3K)/insulin-signaling pathway in Caenorhabditis elegans. Neuropsychopharmacology. 2009;34:1968–1978. doi: 10.1038/npp.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand JB, Johnson CD. In: Carnorhabditis elegans Modern Biological Analysis of an Organism. Estein HF, Shakes DC, editors. Vol. 48. Academic Press, Inc.; 1995. pp. 187–204. [Google Scholar]
- Kwok TC, et al. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. 2006;441:91–95. doi: 10.1038/nature04657. [DOI] [PubMed] [Google Scholar]
- Wang X, Sliwoski GR, Buttner EA. The relevance of Caenorhabditis elegans genetics for understanding human psychiatric disease. Harv. Rev. Psychiatry. 2011;19:210–218. doi: 10.3109/10673229.2011.599185. [DOI] [PubMed] [Google Scholar]
- Donohoe DR, Aamodt EJ, Osborn E, Dwyer DS. Antipsychotic drugs disrupt normal development in Caenorhabditis elegans via additional mechanisms besides dopamine and serotonin receptors. Pharmacol. Res. 2006;54:361–372. doi: 10.1016/j.phrs.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmacharya R, et al. Behavioral effects of clozapine: involvement of trace amine pathways in C. elegans and M. musculus. Brain Res. 2011;1393:91–99. doi: 10.1016/j.brainres.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur T, et al. A Genome-Wide RNAi Screen in Caenorhabditis elegans Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, You YJ. C. elegans feeding. WormBook, ed. The C. elegans Research Community, doi:10.1895/wormbook.1.150.1. 2012. Available from: http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Chaudhuri J, Parihar M, Pires-daSilva A. An introduction to worm lab: from culturing worms to mutagenesis. J Vis Exp. 2011;47 doi: 10.3791/2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JA, Fleming JT. In: Carnorhabditis elegans Modern Biological Analysis of an Organism. Estein HF, Shakes DC, editors. Vol. 48. Academic Press, Inc.; 1995. pp. 3–29. [Google Scholar]
- Davis JR, Li Y, Rankin CH. Effects of developmental exposure to ethanol on Caenorhabditis elegans. Alcohol Clin. Exp. Res. 2008;32:853–867. doi: 10.1111/j.1530-0277.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- Kage-Nakadai E, et al. Two very long chain fatty acid acyl-CoA synthetase genes, acs-20 and acs-22, have roles in the cuticle surface barrier in Caenorhabditis elegans. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge FA, Tearle AW, Gravato-Nobre MJ, Schafer WR, Hodgkin J. The C. elegans glycosyltransferase BUS-8 has two distinct and essential roles in epidermal morphogenesis. Dev. Biol. 2008;317:549–559. doi: 10.1016/j.ydbio.2008.02.060. [DOI] [PubMed] [Google Scholar]
- Leung CK, Deonarine A, Strange K, Choe KP. High-throughput screening and biosensing with fluorescent C. elegans strains. J. Vis. Exp. 2011. [DOI] [PMC free article] [PubMed]


