Abstract
Objectives
High velocity low amplitude spinal manipulation (HVLA-SM), as performed by manual therapists (eg, doctors of chiropractic and osteopathy) results in mechanical hypoalgesia in clinical settings. This hypoalgesic effect has previously been attributed to alterations in peripheral and/or central pain processing. The objective of this study was to determine whether thrust magnitude of a simulated HVLA-SM alters mechanical trunk response thresholds in wide dynamic range (WDR) and/or nociceptive specific (NS) lateral thalamic neurons.
Methods
Extracellular recordings were carried out in the thalamus of 15 anesthetized Wistar rats. Lateral thalamic neurons having receptive fields which included the lumbar dorsal-lateral trunk were characterized as either WDR (n=22) or NS (n=25). Response thresholds to electronic von Frey (rigid tip) mechanical trunk stimuli were determined in three directions (dorsal-ventral, 45°caudalward, and 45°cranialward) prior to and immediately following the dorsal-ventral delivery of a 100ms HVLA-SM at three thrust magnitudes (control, 55%, 85% body weight; (BW)).
Results
There was a significant difference in mechanical threshold between 85% BW manipulation and control thrust magnitudes in the dorsal-ventral direction in NS neurons (p=.01). No changes were found in WDR neurons at either HVLA-SM thrust magnitude.
Conclusions
This study is the first to investigate the effect of HVLA-SM thrust magnitude on WDR and NS lateral thalamic mechanical response threshold. Our data suggest that at the single lateral thalamic neuron level, there may be a minimal spinal manipulative thrust magnitude required to elicit an increase in trunk mechanical response thresholds.
Keywords: spinal manipulation, thalamus, nociceptive neurons, lumbar vertebrae, chiropractic
Introduction
Spinal manipulation and spinal mobilization are commonly used in clinical practice to alleviate low back pain.1-3 Although the underlying mechanisms remain unknown, these forms of manual therapy have been clinically shown to increase mechanical pressure pain thresholds (i.e. decrease sensitivity) in both symptomatic and asymptomatic subjects.4-12 Cervical spinal manipulation has been shown to result in unilateral as well as bilateral mechanical hypoalgesia.6,7,12,13 Compared to no manual therapy, oscillatory spinal manual therapy at T12 and L4 produced significantly higher paraspinal pain thresholds at T6, L1 and L3 in individuals with rheumatoid arthritis.4 The immediate and widespread hypoalgesia associated with manual therapy treatments has been attributed to alterations in peripheral and/or central pain processing including activation of descending pain inhibitory systems.7,14-16
Increasing evidence from animal models suggests that manual therapy activates the central nervous system and in so doing affects areas well beyond those being treated.14,17,18 Sluka and Wright19 reported in rats that unilateral knee joint mobilization evokes bilateral hypoalgesia, suggesting a widespread centrally-mediated response to joint mobilization. More recently, it was shown that Grade 2 equivalent spinal mobilizations applied manually to the L5 spinous process increases hindpaw mechanical nociceptive thresholds in the awake rat with or without acute inflammation.17 In addition, Song et al.20 reported that instrument delivered HVLA-SM significantly reduces the severity and shortens the duration of pain and hyperalgesia caused by neural inflammation within the intervertebral foramen. These findings from animal models are consistent with the widespread hypoalgesic effects reported clinically following a manual therapeutic intervention. To what extent these hypoalgesic effects are attributable to central mechanisms is undetermined but alterations in convergent supraspinal nociceptive processing likely play a role.
The thalamus is subcortical structure receiving convergent input from all innocuous (dorsal column pathway) and/or nociceptive (spinothalamic pathway) somatosensory receptors stimulated during delivery of a spinal manipulation. The ability of the thalamus to modulate ascending sensory input as well as interact functionally with descending pain modulating structures such as the periaqueductal gray (PAG) is not well understood despite studies showing the existence of direct projections between multiple thalamic nuclei and the PAG.21-23 Recently in humans it was demonstrated that the lateral thalamus and PAG interact reciprocally at short latencies (∼5ms) and that stimulation of either structure relieved pain to various degrees.24 Although more work in this area is required, the authors suggested that the thalamus and PAG influence each other in opposite ways via a fairly direct pathway not involving spinal cord circuitry and thereby being important in pain perception.24 Whether or not such a pathway could contribute to the immediate and widespread hypoalgesia following HVLA-SM is plausible but at this point purely speculative.
Optimization of the biomechanical features that characterize a spinal manipulation such as thrust magnitude, thrust duration, loading direction relative to the patient, tissue pre-load, and anatomical contact site, is thought to be critical to clinical expertise.25-28 A pilot study investigating the relationship between the magnitude of the force applied and hypoalgesia in individuals with lateral epicondylalgia suggested that the amount of applied manual force may determine the extent of hypoalgesia.29 The purpose of the present study was to determine the relationship between HVLA-SM thrust magnitude and trunk mechanical response threshold of lateral thalamic neurons in an animal model. Determination of which (if any) biomechanical characteristics of an HVLA-SM alter neural response characteristics has been the subject of several recent basic investigations.30-34 Together, these studies aim to provide insight into the mechanisms underlying spinal manipulation.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee. Animals were housed individually and exposed to a 12-h light/dark cycle with food and water ad libitum. For terminal electrophysiological recordings, all animals were anesthetized with an intraperitoneal (ip) injection of 50% urethane (1.2g/kg) and maintained with supplements (5% urethane) administered intravenously (iv) as needed.35,36 Depth of anaesthesia was assessed by monitoring pinch withdrawal, corneal reflex, respiration rate and vibrissae movements to maintain an anaesthetic state III-3.35,37 The jugular vein was catheterized and trachea intubated for the purposes of iv infusion and pCO2 monitoring respectively. In addition, oxygen concentration, blood pressure, heart rate, and respiration were monitored by a Mouse Ox system (Starr Life Sciences Corp., Oakmont, PA). Body temperature was monitored with a rectal thermistor and maintained at 37°C with a circulating-water heating pad. The rat's head was secured with the dorsal surface positioned horizontally in a stereotaxic device (Kopf Instruments, Tujunga, CA). A small hole was made in the skull and expanded with bone rongeurs. The exposed dura mater was opened and the extracellular recording electrode was advanced into the thalamus.
Electrophysiology
Single thalamic neurons were recorded extracellularly with DiI (1,1′-dioctabecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate; Invitrogen, Carlsbad, CA)-coated tungsten microelectrodes [6 to 8 MΩ impedance (FHC, Bowdoin, ME)] as previously described35,38,39 (Fig. 1A). Thalamic tracks began 4mm below the surface of the cortex and ended at 7.5mm.40 The tungsten electrode was slowly advanced at a rate of 1-5 μm per step using a motorized micromanipulator (Neurostar, Germany) until spontaneous single unit activity was encountered. If the neuron's receptive field included the dorsal-lateral trunk then spontaneous activity was recorded for at least one minute prior to commencement of mechanical testing using the electronic von Frey anestheiometer. Thalamic electrode tracks were made in parallel columns, 500μm apart, and were located between -2.04mm and -3.30mm caudal to bregma and 1.2mm and 3.8mm lateral to midline.40 Lateral thalamic subnuclei through which the electrode passed included: ventral lateral (VL), ventroposterolateral (VPL), posterior (Po), laterodorsal ventrolateral (LDVL),and laterodorsal dorsal medial (LDDM) (Fig. 1B). Thalamic neuron activity was passed through a high impedance probe (HIP511, Grass, West Warwick, RI) and then amplified (P511K, Grass) and recorded using a PC based data acquisition system (Spike 2, Cambridge Electronic Design, UK).
Fig. 1.
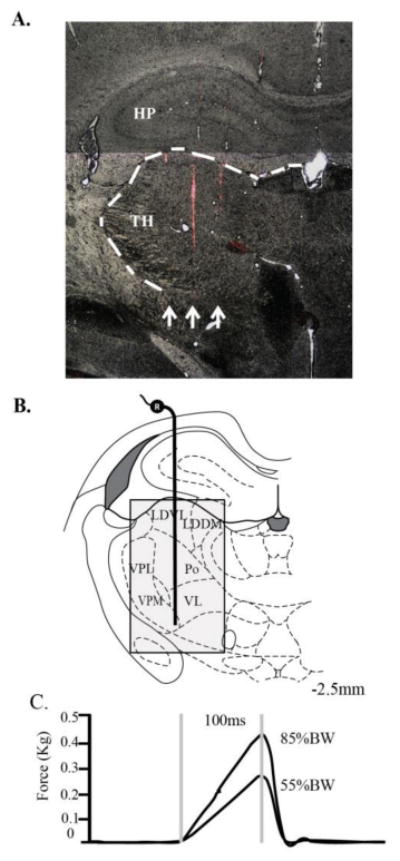
(A). Example of tracks (arrows) from Dil-coated electrodes through the lateral thalamus (TH) and hippocampus (HP) in a coronal brain section at 40x magnification. Of the three Dil electrode tracks shown, the medial & lateral Dil tracks are seen in greater detail in adjacent tissue sections; (B). A diagram illustrating the experimental setup for extracellular thalamic recordings at -2.5mm caudal to bregma. Shading indicates search area which included the laterodorsal dorsal medial (LDDM), laterodorsal ventrolateral (LDVL), ventrolateral (VL), posterior (Po), ventroposteromedial (VPM), and ventroposterolateral (VPL) nuclei; (C). Force profiles for L5 spinal manipulative thrust with 100ms duration at 55% and 85% bodyweight.
The neurons in this study were classified as either wide dynamic range (WDR) responding in a graded fashion to low threshold mechanical (brush stroke) and high mechanical (noxious pinch) or as nociceptive specific (NS) responding only to noxious pinch. Neurons responding solely to low threshold mechanical (brush stroke) trunk stimulation were not considered in this study.
Following recording of baseline thalamic neuronal activity, an electronic von Frey anesthesiometer with a rigid tip adapter (0.79mm2 contact area; IITC Model 2390 www.iitcinc.com) was applied within the receptive field close to the midline of the dorsal trunk (within 2cm of the spine) in three directions: dorsal-ventral, 45°caudalward and 45°cranialward. It was thought that the direction with which the trunk stimuli were applied might differentially affect force transmission to the trunk peripheral mechanoreceptors and thereby impact thalamic response thresholds. The electronic vonFrey stimulus was manually applied in increasing magnitude until either a thalamic response was elicited or 400g had been applied to the trunk. If spontaneous bursting activity was present, mechanical testing occurred during intermittent silent periods or during periods of minimal tonic firing. Thalamic responses to low and/or to high threshold mechanical stimuli were recorded both prior to and following the delivery of a simulated high velocity low amplitude spinal manipulation (HVLA-SM). The directions in which electronic von Frey mechanical stimulus was applied were randomized before HVLA-SM to minimize possible ordering effects. Trunk testing locations were varied between different neurons to reduce potential long-term effects on thalamic neuronal activity such as sensitization or habituation.
Spinal Manipulation
HVLA-SMs were delivered in the dorsal-ventral direction to the L5 vertebra. The lumbar spine was mechanically secured by clamping the L2 spinous process and fixing the iliac crests with hip pins (David Kopf Instruments, Tujunga, CA). A very small skin incision was made over the L5 vertebra through which a pair of adjustable toothed forceps was inserted and rigidly attached to the lateral surfaces of the L5 spinous process. In clinical settings, the HVLA-SM force-time loading profile can be likened to a triangle wave 41,42 with a thrust phase rising to a peak load in less than 150ms.41-43 Clinically, manipulative thrust forces range from 31% to 78% body weight (BW) assuming an average human body weight of 70kg.44,45 Therefore, HVLA-SMs were delivered as a triangle wave with a thrust phase of 100 ms and amplitudes of 55% BW or 85% BW (Fig. 1C). Prior to the delivery of each manipulative thrust, the lumbar spine was positioned neutrally with neither force nor displacement being applied by the motor to the L5 vertebra. The testing order of the three thrust magnitudes [control (i.e. 0% BW), 55% BW, 85% BW] was randomized for each neuron within each direction yielding a randomized complete block design. A period of five minutes lapsed between manipulations which appears to be adequate time to mitigate viscoelastic tissue changes related to HVLA-SM.46
Histology
Following extracellular thalamic recordings, the rat was perfused transcardially with 0.9% saline followed by 4% paraformaldehyde. The brain was removed and stored in a 30% sucrose/10% formalin solution at 4°C until sectioning. Brains were cut with a cryostat (30μm sections) and mounted on microscope slides. Electrode track locations confirmed (Fig. 1A) with postmortem histological reconstructions using with a Nikon Optiphot microscope with EFD-EPI fluorescent attachment and illumination system with adjacent sections stained with cresyl violet as previously established.35,38,39,47-49
Data Analysis
Data analyses were conducted using SAS (version 9.2, SAS Institute, Cary, NC). For each mechanical testing direction, by neuron classification, a one-way analysis of variance (ANOVA) for a randomized complete block design, with neuron as the block, was used to test between thrust magnitudes. Statistical significance was set at .05. Mean changes between pre- and post HVLA-SM mechanical thresholds and 95% confidence intervals under the ANOVA model are given in figures; mean differences between both control and 55% BW with 85% BW and 95% confidence intervals (lower, upper) from the ANOVA model are given in the text.
Results
Electrophysiological activity of 47 thalamic neurons located in lateral thalamic subnuclei which responded to mechanical stimulation applied to the lumbar dorsal-lateral trunk were obtained from 15 adult male Wistar rats (330-540g) (Fig. 2). Of these neurons, 47% (22/47) were classified as WDR neurons and 53% (25/47) were classified as NS neurons. The mean rate of spontaneous activity was 4.64 imp/s (SD 2.58) for WDR neurons and 3.76 imp/s (SD 2.51) for NS neurons. An example of a WDR neuron's response to 45° cranialward and dorsal-ventral mechanical trunk stimulation prior to HVLA-SM is shown in Fig. 3.
Fig. 2.
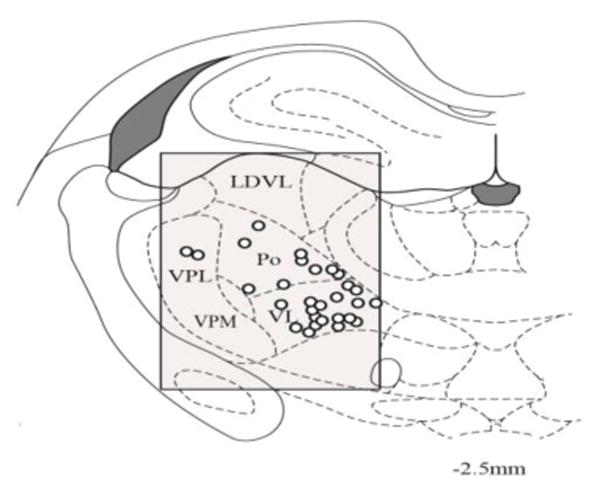
Summary showing the location (open circles) of 29/47 lateral thalamic neurons responding to mechanical stimulation applied to the trunk at -2.5mm caudal from bregma level. The shading indicates the search area. The remaining 18 neurons not shown were located at different thalamic levels.
Fig. 3.
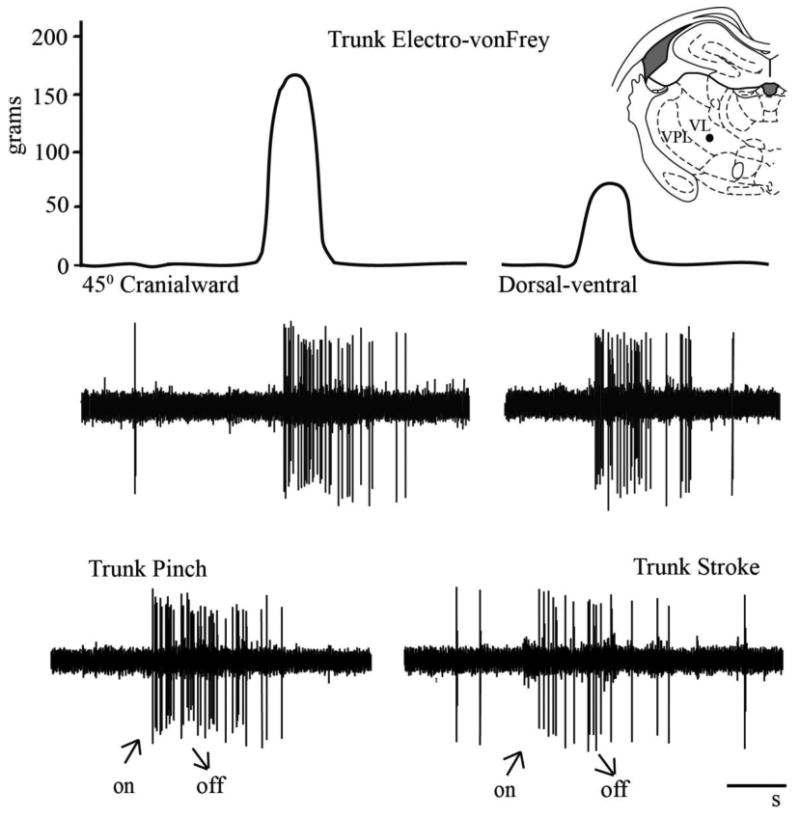
Trunk mechanical stimuli (upper row) responses of a single wide dynamic range thalamic neuron located (●) in the ventral lateral (VL) nucleus at -2.5mm caudal to bregma. Raw electrophysiological recordings (lower rows) of responses to lumbar trunk electronic von Frey stimuli in the 45° cranialward (161g) and dorsal-ventral direction (69g). Note graded response to trunk stroke and trunk pinch. Cal bar = 1s.
Changes in WDR mean trunk mechanical threshold evoked by HVLA-SM were not significantly different among the three thrust magnitudes (control-0%, 55%, 85% BW) during dorsal-ventral testing (F2,42=0.32, p=0.73; Fig. 4A), 45° caudalward (F2,42=0.34, p=0.71; Fig. 4B) nor the 45° cranialward (F2,42=0.85, p=0.44; Fig. 4C) testing directions.
Fig. 4.
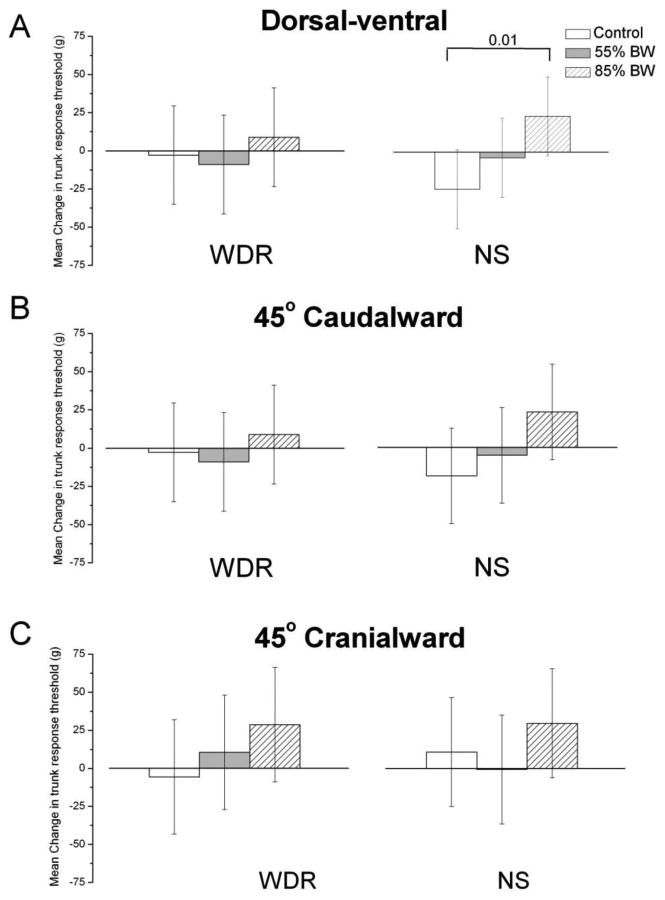
Mean HVLA-SM changes in lumbar trunk electronic von Frey mechanical activation response thresholds (grams) for the dorsal-ventral, 45° caudal and 45° cranial directions of wide dynamic range (WDR) and nociceptive specific (NS) lateral thalamic neurons following time-control, 55% and 85% body weight high velocity low amplitude spinal manipulation thrust duration. Data are reported as means and 95% confidence intervals (lower, upper 95% CI).
Changes in NS neuron mean trunk mechanical threshold evoked by HVLA-SM were significantly different among the three HVLA-SM thrust magnitudes during dorsal-ventral testing (F2,48=3.45, p=0.04; Fig. 4A), but not during the 45° caudalward (F2,48=1.88, p=0.16; Fig. 4B) or 45° cranialward (F2,48=0.74, p=0.48; Fig. 4C) testing directions. During dorsal-ventral testing, the 85% BW mean HVLA-SM thrust magnitude was significantly different from control (53.0g, p=.01, 95% CI: 12.3, 93.6) but not the 55% BW mean HVLA-SM thrust magnitude (30.2g, p=0.14, 95% CI: -10.5, 70.8).
Four WDR neurons that responded to brush stroke prior to the HVLA-SM ceased to respond to this innocuous type of mechanical stimulation following the higher (85% BW) but not lower (55% BW) manipulative thrust magnitude. The remaining 18 WDR neurons which also responded to brush stroke were unaffected by HVLA-SM.
Discussion
In rats, axons from as many as 6000 spinal neurons project to the thalamus.50 Both innocuous and noxious sensory inputs from Aβ, Aδ, and C fibers in cutaneous nerves and group II, III, and IV fibers in muscle nerves converge in the thalamus.51-53 For example, wide dynamic range thalamic cells located in the lateral VPL-VL subnuclei respond to noxious stimulation of muscle and tendon while also responding to innocuous stimulation of the skin.54 The lateral thalamic subnuclei, more specifically the ventral posterior nuclei (VPL, Po) are the primary terminus for spinothalamic inputs from both sides of the spinal cord.55 However in the rat, one must be mindful that an estimated 15-20% of all spinothalamic tract neurons have branches terminating in both medial and lateral thalamic nuclei56,57 indicating a much broader influence on different aspects of the pain matrix. With the thalamus's role of processing innocuous and noxious sensory input along with its diffuse network of connections, it is rational to think that convergent input and reciprocal feedback from pain matrix structures to the thalamus could play a part in the immediate and widespread hypoalgesic effects associated with manual therapy intervention. To our knowledge, this study is the first to investigate the effects of HVLA-SM thrust magnitude on the mechanical thresholds of thalamic neurons.
Relative to control, the 85% BW thrust magnitude HVLA-SM significantly increased mechanical thresholds of lateral thalamic NS neurons to dorsal-ventral mechanical testing of the trunk. Since the mechanical thresholds to 45°caudalward and 45°cranialward testing directions did not significantly differ relative to control, the decrease in trunk mechanical sensitivity likely was not a generalized response. In addition, the majority of WDR neurons (84%) retained their responsiveness to innocuous trunk stimulation following the 85% BW manipulative thrust magnitude suggesting no adverse effects to neural tissue.
The finding that only the higher intensity manipulative stimulus (i.e. 85% BW vs 55% BW or control) decreased the mechanical sensitivity of lateral thalamic neurons to mechanical trunk stimulation coincides with other reports relating graded mechanical or electrical stimulus intensity to the magnitude of central inhibition58,59 but is in contrast to a recent clinical study reporting no differences in pressure pain thresholds in subjects receiving different amplitudes of posterior-anterior lumbar mobilizations.5
Several clinical studies indicate that spinal manipulation alters central processing of mechanical stimuli evidenced by increased pressure pain thresholds and decreased pain sensitivity in asymptomatic and symptomatic subjects following manipulation.6,7,11,60,61 However, questions of mechanism and whether certain biomechanical dose characteristics of spinal manipulation and/or mobilization such as force magnitude, force duration, loading direction (relative to the patient), anatomical contact point, frequency and amplitude of oscillation are required for eliciting hypoalgesia are just beginning to be investigated.5,10,30,32 Krouwel et al.5 recently reported no significant difference in hypoalgesia among subjects receiving different amplitudes of lumbar posterior-anterior mobilizations while Pentelka et al.10 reported that at least four sets of large amplitude lumbar mobilizations (instead of the three sets used traditionally) were required to elicit hypoalgesia. Moss et al.9 suggested that the repetitiveness of movement should be considered as the hypoalgesic stimulus rather than the pressure being used during joint mobilization procedures and this concept appears to be supported by the recent findings of Pentelka et al.10 It may be found that a threshold force is required to elicit hypoalgesic effects during short duration manual procedures such as HVLA-SM but is unnecessary when using slower repetitive interventions such as spinal mobilization.
Limitations
Spinal cord tissue was not harvested nor evaluated histologically. The possibility that an 85% BW thrust magnitude HVLA-SM may have resulted in trauma to underlying neural tissue and thereby influence the outcome is highly unlikely since significant threshold changes were not seen in all mechanical stimuli testing directions following the higher magnitude thrust. This conclusion is further supported by the finding that the 85% BW manipulative thrust failed to eliminate the responsiveness to innocuous trunk stimulation in the vast majority of thalamic neurons. In addition, similar HVLA-SM procedures with thrust magnitudes of 85% - 100% BW have been applied in other animal studies without any indication of neural trauma or underlying tissue damage31,62 and similar forces are applied safely in clinical settings.41-43
Our use of a single application of the electronic von Frey mechanical stimulus to the trunk in a given direction prior to and following a spinal manipulation rather than performing multiple applications (3-10x) as with classical von Frey63 or electronic von Frey64,65 studies may have contributed to the variability we observed in thalamic responses (Fig. 4). The decision to use a single electronic von Frey application to the trunk in any given direction was made to limit potential sensitization and/or tissue damage which can occur from repetitive mechanical stimulations. Mechanically testing in three directions (dorsal-ventral, 45°caudalward, 45°cranialward) prior to and following three thrust magnitudes of (0%-control, 55% BW, 85% BW) equated to 9 mechanical stimulations per neuron. Applying multiple von Frey stimulations per direction would have greatly increased this number of applied mechanical stimulations which may have resulted in sensitization and/or tissue damage. Despite having used single trunk applications of the electronic von Frey in the past,35 future studies should consider decreasing the number of mechanical stimulus directions tested while increasing the number of electronic von Frey applications in a given direction to reduce variability while limiting the potential sensitization or tissue damage.
Trunk mechanical stimuli responses were recorded in multiple lateral thalamic nuclei. Although there is collateral branching of projection fibers among thalamic nuclei along with degrees of functional overlap, it is recognized that several lateral thalamic nuclei included within this study make distinct contributions to somatosensory and/or nociceptive processing. Future studies may wish to concentrate on recording from single thalamic nuclei (i.e. VPL, Po, VL) or those nuclei which are closely related functionally.
It should be noted that whether or not changes in thalamic mechanical response thresholds are related to the clinically reported hypoalgesia following spinal manipulation remains to be determined. It is widely recognized that clinical pain, nociception and pain relief are multifactorial and complex in nature involving multiple spinal and supraspinal centers. Whether HVLA-SM's specific thrust magnitude or any other biomechanical dosage characteristics are required to produce antinociceptive or hypoalgesic related responses in other ascending or descending supraspinal pain processing centers such as the medullary reticular formation or periaqueductal gray remains to be determined.
Conclusion
To our knowledge, this study is the first to investigate the effects of spinal manipulation and its thrust magnitude on the response properties of supraspinal neurons. The results suggest that at the level of a single lateral thalamic neuron, there may be a minimal thrust magnitude required for spinal manipulation to elicit an increase in the response thresholds of NS thalamic neurons to mechanical stimulation of the trunk. Larger studies examining the effects of thrust magnitude and other clinician-controlled mechanical HVLA-SM parameters on thalamic, brainstem, and cortical neurons known to be involved in nociceptive somatosensory processing need to be performed to confirm and extend the present findings and help elucidate the underlying mechanisms of spinal manipulation induced hypoalgesia.
Acknowledgments
The authors wish to thank Amanda Hussman for her assistance with this manuscript.
Funding Sources: This work was supported by grants from the Australian Spinal Research Foundation (LG2010-11) and NIH National Center for Complementary and Alternative Medicine (K01AT005935) to WRR and was conducted in a facility constructed with support from the NIH National Center for Research Resources under Research Facilities Improvement Grant Number C06RR15433. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. No conflicts of interest were reported for this study.
Footnotes
No conflicts of interest were reported for this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
William R. Reed, Palmer Center for Chiropractic Research.
Joel G. Pickar, Palmer Center for Chiropractic Research.
Randall S. Sozio, Palmer Center for Chiropractic Research.
Cynthia R. Long, Palmer Center for Chiropractic Research.
Reference List
- 1.Goertz CM, Pohlman KA, Vining RD, Brantingham JW, Long CR. Patient-centered outcomes of high velocity, low-amplitude and spinal manipulation for low back pain: A systematic review. J Electromyogr Kinesiol. 2012;22:670–91. doi: 10.1016/j.jelekin.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Bronfort G, Haas M, Evans R, Kawchuk G, Dagenais S. Evidence-informed management of chronic low back pain with spinal manipulation and mobilization. Spine J. 2008;8:213–25. doi: 10.1016/j.spinee.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Fritz JM, Whitman JM, Childs JD. Lumbar spine segmental mobility assessment: an examination of validity for determining intervention strategies in patients with low back pain. Arch Phys Med Rehabil. 2005;86:1745–52. doi: 10.1016/j.apmr.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Dhondt W, Willaeys T, Verbruggen LA, Oostendorp RA, Duquet W. Pain threshold in patients with rheumatoid arthritis and effect of manual oscillations. Scand J Rheumatol. 1999;28:88–93. doi: 10.1080/030097499442540. [DOI] [PubMed] [Google Scholar]
- 5.Krouwel O, Hebron C, Willett E. An investigation into the potential hypoalgesic effects of different amplitudes of PA mobilisations on the lumbar spine as measured by pressure pain thresholds (PPT) Man Ther. 2010;15:7–12. doi: 10.1016/j.math.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Maduro deCamargo V, Alburquerque-Sendin F, Berzin F, Stefanelli VC, Rodrigues de Souza DP, Fernandez-de-Las-Penas C. Immediate effects on electromyographic activity and pressure pain thresholds after a cervical manipulation in mechanical neck pain: a randomized controlled trial. J Manipulative Physiol Ther. 2011;34:211–20. doi: 10.1016/j.jmpt.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Vicenzino B, Paungmali A, Buratowski S, Wright A. Specific manipulative therapy treatment for chronic lateral epicondylalgia produces uniquely characteristic hypoalgesia. Man Ther. 2001;6:205–12. doi: 10.1054/math.2001.0411. [DOI] [PubMed] [Google Scholar]
- 8.Willett E, Hebron C, Krouwel O. The initial effects of different rates of lumbar mobilisations on pressure pain thresholds in asymptomatic subjects. Man Ther. 2010;15:173–8. doi: 10.1016/j.math.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Moss P, Sluka K, Wright A. The initial effects of knee joint mobilization on osteoarthritic hyperalgesia. Man Ther. 2007;12:109–18. doi: 10.1016/j.math.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Pentelka L, Hebron C, Shapleski R, Goldshtein I. The effect of increasing sets (within one treatment session) and different set durations (between treatment sessions) of lumbar spine posteroanterior mobilisations on pressure pain thresholds. Man Ther. 2012;17:526–30. doi: 10.1016/j.math.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Wang X, Zhang J, Wang Y. Changes in pressure pain thresholds and basal electromyographical activity after instrument-assisted spinal manipulative therapy in asymptomatic participants: a randomized, controlled trial. J Manipulative Physiol Ther. 2012;35:437–45. doi: 10.1016/j.jmpt.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Srbely JZ, Vernon H, Lee D, Polgar M. Immediate effects of spinal manipulative therapy on regional antinoceptive effects in myofascial tissues in healthy young adults. J Manipulative Physiol Ther. 2013;36:333–41. doi: 10.1016/j.jmpt.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Mansilla-Ferragut P, Fernandez-de-Las PC, Alburquerque-Sendin F, Cleland JA, Bosca-Gandia JJ. Immediate effects of atlanto-occipital joint manipulation on active mouth opening and pressure pain sensitivity in women with mechanical neck pain. J Manipulative Physiol Ther. 2009;32:101–6. doi: 10.1016/j.jmpt.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Skyba DA, Radhakrishnan R, Rohlwing JJ, Wright A, Sluka KA. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABA receptors in the spinal cord. Pain. 2003;106:159–68. doi: 10.1016/s0304-3959(03)00320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright A. Hypoalgesia post-manipulative therapy: a review of a potential neurophysiological mechanism. Man Ther. 1995;1:11–6. doi: 10.1054/math.1995.0244. [DOI] [PubMed] [Google Scholar]
- 16.Wright A. Recent concepts in the neurophysiology of pain. Man Ther. 1999;4:196–202. doi: 10.1054/math.1999.0207. [DOI] [PubMed] [Google Scholar]
- 17.Grayson JE, Barton T, Cabot PJ, Souvlis T. Spinal manual therapy produces rapid onset analgesia in a rodent model. Man Ther. 2012;17:292–7. doi: 10.1016/j.math.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Sluka KA, Skyba DA, Radhakrishnan R, Leeper BJ, Wright A. Joint mobilization reduces hyperalgesia associated with chronic muscle and joint inflammation in rats. J Pain. 2006;7:602–7. doi: 10.1016/j.jpain.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Sluka K, Wright A. Knee joint mobilization reduces secondary mechanical hyperalgesia induced by capsaicin injection into the ankle joint. Eur J Pain. 2001;5:81–7. doi: 10.1053/eujp.2000.0223. [DOI] [PubMed] [Google Scholar]
- 20.Song XJ, Gan Q, Cao JL, Wang ZB, Rupert RL. Spinal manipulation reduces pain and hyperalgesia after lumbar intervertebral foramen inflammation in the rat. J Manipulative Physiol Ther. 2006;29:5–13. doi: 10.1016/j.jmpt.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Krout KE, Loewy AD. Periaqueductal gray matter projections to the midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2000;424:111–41. doi: 10.1002/1096-9861(20000814)424:1<111::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Mantyh PW. Connections of midbrain periaqueductal gray in the monkey, I. Ascending efferent projections. J Neurophysiol. 1983;49:567–81. doi: 10.1152/jn.1983.49.3.567. [DOI] [PubMed] [Google Scholar]
- 23.Rinvik E, Wiberg M. Demonstration of a reciprocal connection between the periaqueductal gray matter and the reticular nucleus of the thalamus. Anat Embryol. 1990;181:577–84. doi: 10.1007/BF00174629. [DOI] [PubMed] [Google Scholar]
- 24.Wu D, Wang S, Stein JF, Aziz TZ, Green AL. Reciprocal interactions between the human thalamus and periaqueductal gray may be important for pain perception. Exp Brain Res. 2014;232:527–34. doi: 10.1007/s00221-013-3761-4. [DOI] [PubMed] [Google Scholar]
- 25.Downie AS, Vemulpad S, Bull PW. Quantifying the high velocity, low amplitude spinal manipulative thrust: a systematic review. J Manipulative Physiol Ther. 2010;33:542–53. doi: 10.1016/j.jmpt.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Nougarou F, Dugas C, Deslauriers C, Page I, Descarreaux M. Physiological responses to spinal manipulative therapy: investigation of the relationship between electromyographic responses and peak force. J Manipulative Physiol Ther. 2013;36:557–63. doi: 10.1016/j.jmpt.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Triano JJ, Gissler T, Forgie M, Milwid D. Maturation in rate of high velocity, low amplitude force development. J Manipulative Physiol Ther. 2011;34:173–80. doi: 10.1016/j.jmpt.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Triano JJ, Descarreaux M, Dugas C. Biomechanics-review of approaches for performance training in spinal manipulation. J Electromyogr Kinesiol. 2012;22:732–9. doi: 10.1016/j.jelekin.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 29.McLean S, Naish R, Reed L, Urry S, Vicenzino B. A pilot study of the manual force levels required to produce manipulation induced hypoalgesia. Clin Biomech. 2002;17:304–8. doi: 10.1016/s0268-0033(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 30.Cao DY, Reed WR, Long CR, Kawchuk GN, Pickar JG. Effects of thrust amplitude and duration of high-velocity, low-amplitude spinal manipulation on lumbar muscle spindle responses to vertebral position and movement. J Manipulative Physiol Ther. 2013;36:68–77. doi: 10.1016/j.jmpt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickar JG, Kang YM. Paraspinal muscle spindle responses to the duration of a spinal manipulation under force control. J Manipulative Physiol Ther. 2006;29:22–31. doi: 10.1016/j.jmpt.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Reed WR, Cao DY, Long CR, Kawchuk GN, Pickar JG. Relationship between biomechanical characteristics of spinal manipulation and neural responses in an animal model: effect of linear control of thrust displacement versus force, thrust amplitude, thrust duration, and thrust rate. Evid Based Complement Altern Med. 2013 doi: 10.1155/2013/492039. http://dx.doi.org/10.1155/2013/492039. [DOI] [PMC free article] [PubMed]
- 33.Reed WR, Long CR, Pickar JG. Effects of unilateral facet fixation and facetectomy on muscle spindle responsiveness during simulated spinal manipulation in an animal model. J Manipulative Physiol Ther. 2013;36:585–94. doi: 10.1016/j.jmpt.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed WR, Long CR, Kawchuk GN, Pickar JG. Neural responses to the mechanical parameters of a high velocity. low amplitude spinal manipulation: Effect of preload parameters. J Manipulative Physiol Ther. 2014;37:68–78. doi: 10.1016/j.jmpt.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed WR, Chadha HK, Hubscher CH. Effects of 17-β-Estradiol on Responses of Viscerosomatic Convergent Thalamic Neurons in the Ovariectomized Female Rat. J Neurophysiol. 2009;102:1062–74. doi: 10.1152/jn.00165.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubscher CH, Johnson RD. Responses of thalamic neurons to input from the male genitalia. J Neurophysiol. 2003;89:2–11. doi: 10.1152/jn.00294.2002. [DOI] [PubMed] [Google Scholar]
- 37.Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anaesthesia. J Neurophysiol. 1999;81:2243–52. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- 38.Chadha HK, Hubscher CH. Convergence of nociceptive information in the forebrain of female rats: reproductive organ response variations with stage of estrus. Exp Neurol. 2008;210:375–87. doi: 10.1016/j.expneurol.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Massey JM, Hubscher CHWMR, Decker JA, Amps J, Silver J, Onifer S. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–14. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th. Burlington, MA: Academic Press; 2007. [Google Scholar]
- 41.Herzog W, Conway PJ, Kawchuk GN, Zhang Y, Hasler EM. Forces exerted during spinal manipulative therapy. Spine. 1993;18:1206–12. doi: 10.1097/00007632-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Triano JJ. Biomechanics of spinal manipulative therapy. Spine J. 2001 Mar;1:121–30. doi: 10.1016/s1529-9430(01)00007-9. [DOI] [PubMed] [Google Scholar]
- 43.Hessell BW, Herzog W, Conway PJW, McEwen MC. Experimental measurement of the force exerted during spinal manipulation using the Thompson technique. J Manipulative Physiol Ther. 1990;13:448–53. [PubMed] [Google Scholar]
- 44.Colloca CJ, Keller TS, Harrison DE, Moore RJ, Gunzburg R, Harrison DD. Spinal manipulation force and duration affect vertebral movement and neuromuscular responses. Clin Biomech. 2006;21:254–62. doi: 10.1016/j.clinbiomech.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Hessell BW, Herzog W, Conway PJ, McEwen MC. Experimental measurement of the force exerted during spinal manipulation using the Thompson technique. J Manipulative Physiol Ther. 1990;13:448–53. [PubMed] [Google Scholar]
- 46.Vaillant M, Edgecombe T, Long CR, Pickar JG, Kawchuk GN. The effect of duration and amplitude of spinal manipulative therapy (SMT) on spinal stiffness. Man Ther. 2012;17:577–83. doi: 10.1016/j.math.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hubscher CH. Estradiol-associated variation in responses of rostral medullary neurons to somatovisceral stimulation. Exp Neurol. 2006;200:227–39. doi: 10.1016/j.expneurol.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Kaddumi EG, Hubscher CH. Changes in rat brainstem responsiveness to somatovisceral inputs following acute bladder irritation. Exp Neurol. 2007;203:349–57. doi: 10.1016/j.expneurol.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Hubscher CH, Reed WR, Kaddumi EG, Armstrong JE, Johnson RD. Select spinal lesions reveal multiple ascending pathways in the rat conveying input from the male genitalia. J Physiol. 2010;588:1073–83. doi: 10.1113/jphysiol.2009.186544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lima D, Coimbra A. Morphological types of spinomesencephalic neurons in the marginal zone (lamina I) of the rat spinal cord, as shown after retrograde labelling with cholera toxin subunit B. J Comp Neurol. 1989;279:327–39. doi: 10.1002/cne.902790212. [DOI] [PubMed] [Google Scholar]
- 51.Foreman RD, Schmidt RF, Willis WDJ. Effects of mechanical and chemical stimulation of fine muscle afferents upon primate spinothalamic tract cells. J Physiol. 1979;286:215–31. doi: 10.1113/jphysiol.1979.sp012615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foreman RD, Hancock M, Willis WDJ. Responses of spinothalamic tract cells in the thoracic spinal cord of the monkey to cutaneous and visceral inputs. Pain. 1981;11:149–62. doi: 10.1016/0304-3959(81)90002-6. [DOI] [PubMed] [Google Scholar]
- 53.Curry MJ, Gordon G. The spinal input to the posterior group in the cat. An electrophysiological investigation. Brain Res. 1972;44:417–37. [PubMed] [Google Scholar]
- 54.Kniffki KD, Mizumura K. Responses of neurons in VPL and VPL-VL region of the cat to algesic stimulation of muscle and tendon. J Neurophysiol. 1983;49:649–61. doi: 10.1152/jn.1983.49.3.649. [DOI] [PubMed] [Google Scholar]
- 55.Gauriau C, Bernard JF. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol. 2004;468:24–56. doi: 10.1002/cne.10873. [DOI] [PubMed] [Google Scholar]
- 56.Kevetter GA, Willis WD. Collaterals of spinothalamic cells in the rat. J Comp Neurol. 1983;215:453–64. doi: 10.1002/cne.902150409. [DOI] [PubMed] [Google Scholar]
- 57.Dickenson AH, Le Bars D. Diffuse noxious inhibitory controls (DNIC) involve trigeminothalamic and spinothalamic neurones in the rat. Exp Brain Res. 1983;49:174–80. doi: 10.1007/BF00238577. [DOI] [PubMed] [Google Scholar]
- 58.Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. II. Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexes. J Neurophysiol. 1991;66:29–39. doi: 10.1152/jn.1991.66.1.29. [DOI] [PubMed] [Google Scholar]
- 59.Zhang JL, Zhang SP, Zhang HQ. Effect of electroacupuncture on thalamic neuronal response to visceral nociception. Eur J Pain. 2009;13:366–72. doi: 10.1016/j.ejpain.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Vernon H. Qualitative review of studies of manipulation-induced hypoalgesia. J Manipulative Physiol Ther. 2000;23:134–8. doi: 10.1016/s0161-4754(00)90084-8. [DOI] [PubMed] [Google Scholar]
- 61.Vicenzino B, Collins D, Benson H, Wright A. An investigation of the interrelationship between manipulative therapy-induced hypoalgesia and sympathoexcitation. J Manipulative Physiol Ther. 1998;21:448–53. [PubMed] [Google Scholar]
- 62.Sung PS, Kang YM, Pickar JG. Effect of spinal manipulation duration on low threshold mechanoreceptors in lumbar paraspinal muscles: a preliminary report. Spine (Phila Pa 1976) 2005;30:115–22. doi: 10.1097/01.brs.0000147800.88242.48. [DOI] [PubMed] [Google Scholar]
- 63.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 64.Martinov T, Mack M, Sykes A, Chatterjea D. Measuring changes in tactile sensitivity in the hindpaw of mice using an electronic vonFrey apparatus. Journal of Visualized Experiments. 2013;82:e51212. doi: 10.3791/51212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moller KA, Johansson B, Berge OG. Assessing mechanical allodynia in the rat paw with a new electronic algometer. J Neurosci Methods. 1998;84:41–7. doi: 10.1016/s0165-0270(98)00083-1. [DOI] [PubMed] [Google Scholar]


