Abstract
This research assessed activation in neural substrates involved in implicit associative processes through the imaging (functional magnetic resonance imaging) of an alcohol-Implicit Association Test (IAT) focused on positive outcomes of alcohol use. Comparisons involved 17 heavy and 19 light drinkers, ranging in age from 18 to 22, during compatible and incompatible association task trials. Behaviorally, a significant IAT effect was found with heavy drinkers showing stronger positive implicit associations toward alcohol use than light drinkers. Imaging data revealed heavy drinkers showed greater activity during compatible trials relative to incompatible trials in the left putamen and insula while no significant difference in activity between conditions was found in the light drinkers. Light drinkers showed significantly more activity in the left orbital frontal cortex during both compatible and incompatible trials than heavy drinkers, and the dorsolateral prefrontal cortex was engaged more in both light and heavy drinkers during incompatible trials relative to compatible trials. Further, within-group analyses showed significant amygdala activity along with the putamen and insula among heavy drinkers during compatible trials relative to incompatible trials. These results are consistent with a dual process framework of appetitive behaviors proposing that (1) implicit associations underlying habit are mediated through neural circuitry dependent on the striatum, and (2) controlled behaviors are mediated through neural circuitry more dependent on the prefrontal cortex. This is the first study to evaluate the neural mechanisms elicited by an alcohol-IAT, providing an additional step toward increasing understanding of associative habit processes and their regulatory influence over addictive behaviors.
Keywords: alcohol-IAT, associative processes, dorsal striatum, fMRI, habit, implicit associations
Introduction
Alcohol is the most widely used and abused drug among emerging adults in the United States (National Institute on Alcohol Abuse and Alcoholism (NIAAA) College Drinking 2012). Eighteen- to 22-year-olds exhibit some of the highest rates of use and binge drinking, with full-time college students being more likely to drink heavily and to binge drink than peers not in college full time (SAMHSA 2011). Some of these emerging adults will transition out of heavy alcohol use, while others maintain or exacerbate hazardous levels of use that continue into adulthood (Moss, Chen & Yi 2007). Maturation of the prefrontal cortex and concomitant function affecting behavioral regulation continues into the mid-20s (e.g. Gogtay et al. 2004; Giedd et al. 2009; for reviews, see Spear 2000, 2002; Sowell, Thompson & Toga 2004; Crews & Boettiger 2009), while some key subcortical structures implicated in automatic associative and habit processes (e.g. caudate) have been found to mature earlier in life (for a review, see Giedd 2008). As a result, some frequent drinkers among emerging adults may be especially susceptible to developing alcohol use habits.
The influence of associative memory effects is now well documented in numerous behavioral studies on appetitive behaviors, showing predictive utility across a range of populations and for several drugs of abuse (for reviews, McCusker 2001; Ames, Franken & Coronges 2006; Wiers & Stacy 2006; Rooke, Hine & Thorsteinsson 2008), and appear to be, at least in part, responsible for some of the irrational decision making associated with continued use. Other relevant processes include prefrontal control processes that exert control over automatic associative processes (e.g. see Kahneman 2003). Thus, continued or escalation of alcohol use can be viewed as a strengthening of automatic associative processes, overwhelming or weakening regulatory control processes. Further, homeostatic disturbances associated with urges and craving, and mediated through the insula, could further alter the dynamic balance between the automatic and regulatory control processes in a direction that facilitates automatic/implicit processing and hinders inhibitory control processing (e.g. see Naqvi & Bechara 2009).
Implicit associations and alcohol use
The formation of an alcohol habit may be considered a potent form of reinforced associative learning (for a review of habit learning, see Yin & Knowlton 2006), with continued use resulting in the strengthening of motivationally relevant associative memories (Stacy 1995, 1997). Dopaminergic activity in the striatum reinforces the repetition of behaviors and supports the encoding and processing of proximal stimuli associated with the rewarding event (e.g. Cardinal & Everitt 2004; Everitt & Robbins 2005). Alcohol indirectly activates dopamine systems by stimulating neurons that modulate the release of dopamine through direct effects on other receptors (e.g. gamma-aminobutyric acid receptors). Whether directly or indirectly affecting dopamine systems, rewarding appetitive behaviors, like alcohol use, are mediated by mesolimbic neural systems (Berridge 2001; Robinson & Berridge 2001; Vetulani 2001). As a result, neutral stimuli associated with the behavior (such as drinking) come to represent and can cue the behavior. As associations in memory are strengthened, patterns of associations signal and drive behavior without the necessary involvement of more deliberative or control processes (cf., White 1996; Stacy, Ames & Knowlton 2004; Wiers et al. 2007; Stacy & Wiers 2010). Research on the neurobiology of alcohol and other drug use habit has shown this behavior to be highly sensitive to predictive cues and prior learning experiences, which become encoded into patterns of association. Cues can then trigger an essentially ‘automatic’ pattern of activation in memory that can be described in neural network or connectionist models (e.g. Hopfield & Tank 1986; Queller & Smith 2002).
The brain regions associated with brain reward neural systems overlap with brain regions associated with associative memory and habit systems (see Stacy et al. 2004). These regions are different from those supporting many aspects of controlled cognitive processes and explicit memory (e.g. Squire 1992; White 1996). For example, habit learning has been associated with neural systems of the dorsal striatum (Knowlton, Mangels & Squire 1996; White 1996; Wagner, Maril & Schacter 2000; White & McDonald 2002; Yin et al. 2005; Yin & Knowlton 2006). Through the use of a variety of neuroscientific methods, several types of associative processes and brain regions involved in complex neural circuits have been dissociated (see Yin & Knowlton 2006).
In humans, some of these behavior-related associations can be assessed with validated associative memory assessments that tap into and activate pre-existing associations in memory (see Stacy et al. 2004; Stacy & Wiers 2006; Wiers & Stacy 2006). In addiction research, many studies have reported predictive effects on alcohol use using associative memory assessments (for review, Rooke et al. 2008; Reich, Below & Goldman 2010). One of the most commonly used indirect tests of association in memory is the Implicit Association Test (IAT; for review, Greenwald et al. 2009). The basic assumption of the IAT is that past learning experiences can be represented by the facilitation of information processing of associated concepts as measured by rate of processing. That is, individuals react faster when categorizing strongly associated concepts that share a response key and slower when categorizing concepts that are less likely to be associated and share a response key (Greenwald, McGhee & Schwartz 1998). Behaviorally, the IAT has been found to effectively differentiate substance users from non-users in studies adapting the IAT to evaluate implicit associations in tobacco (e.g. Swanson et al. 2001), alcohol (e.g. Wiers et al. 2002; Jajodia & Earleywine 2003; De Houwer et al. 2004; Wiers et al. 2005; McCarthy & Thompsen 2006; Houben & Wiers 2007, 2008; Thush et al. 2008), marijuana (e.g. Field, Mogg & Bradley 2004; Ames et al. 2007) and cocaine use (Wiers et al. 2007).
A few imaging studies have observed neural correlates of non–addiction-related IATs in the scanner (e.g. racial preference, Beer et al. 2008; flowers/insect pleasantness, Chee et al. 2000; politics, Knutson et al. 2006; gender- and race-related; Knutson et al. 2007; morality, Luo et al. 2006). These studies found incompatible trials correlated with greater frontal activity relative to compatible trials [e.g. increased activity in the ventrolateral, dorsolateral prefrontal cortex (DLPFC) and anterior cingulate: Chee 2000; Luo et al. 2006; left inferior frontal gyrus; Knutson et al. 2006; middle frontal gyrus; Knutson et al. 2007]. During compatible association trials on a racial preference IAT, Beer et al. (2008) found significant activity in the caudate, insular cortex and lateral orbital frontal cortex (OFC). While these studies used the IAT to image behavioral processes involving implicit associations, the present work is the first functional magnetic resonance imaging (fMRI) study of IAT performance that involves an appetitive behavior like alcohol use.
Overview
The goal of the study was to increase our understanding of the neural correlates of cognitive processes involved on an alcohol IAT, an indirect test of strength of alcohol-relevant associations. In general, we aimed to observe whether heavy drinkers and light drinkers showed differences in hemodynamic response in anatomical regions during performance on compatible and incompatible trials of the IAT. We hypothesized that during compatible trials (or trials on which strength of positive implicit associations toward alcohol use should be detected), that relative to incompatible trials and light drinkers, heavy drinkers would show greater activity in regions critical for implicit associative memory processes (and habit learning), namely the dorsal striatum (caudate/putamen) and ventral striatum. Second, because the amygdala is also a part of this neural system (implicated in stimulus-reward associations; e.g. Everitt & Robbins 2005), we hypothesized that implicit associations would engage the amygdala. Third, we hypothesized that the insula would be engaged because this region has been implicated in practiced and more automatic tasks (e.g. Raichle et al. 1994), with recent evidence suggesting that this increased automaticity may be indirect. That is, urges to drink, elicited by cues, are mediated through the insula, which in turn has an effect on increasing activity within neural systems underlying more automatic (habit) behavior (e.g. Naqvi & Bechara 2009).
A different set of hypotheses focuses on incompatible trials, which require more effortful processing given participants are categorizing concepts not generally related. Therefore, we hypothesized as follows: (1) that the incompatible trials would be associated with higher activity in neural regions implicated in executive and inhibitory control processes among all subjects, when compared with compatible trials; and (2) light drinkers would exhibit greater activation in control regions during the incompatible trials relative to heavy drinkers, as a result of perhaps poorer controlled processing among the heavier drinkers. On the basis of some earlier non–addiction-related IAT studies (e.g. Chee et al. 2000; Luo et al. 2006), we anticipated incompatible association trials to elicit greater activation than compatible trials in lateral regions of the orbitofrontal cortex, dorsolateral and adjacent ventrolateral region of the prefrontal cortex, regions implicated in inhibitory control processes (e.g. Aron et al. 2003; Aron, Robbins & Poldrack 2004).
Materials and Methods
Behavioral assessments and imaging took place at the Dana and David Dornsife Cognitive Neuroscience Imaging Center at the University of Southern California (USC) campus. Participants initially were given a practice IAT task (flower/insect target and pleasant/unpleasant attribute categories) to become familiar with the procedure. Subjects were then situated in the scanner where a structural scan was acquired, and then they performed the alcohol-IAT, which took approximately 30 minutes. The fMRI assessment of the alcohol IAT utilized a mixed design. Following scanning, participants completed computerized questionnaires consisting of demographics and behavioral measures, and were given $110 for participation. The practice IAT task, fMRI scan, and computerized questionnaire took a total of 1.5 hours.
Participants
Participants were 36 neurologically normal emerging adults ranging in age from 18 to 22, recruited from USC in Los Angeles, California. Seventeen participants were heavy drinkers (47% female) and 19 were light drinkers (74% female) for between-subject comparisons. There were no significant differences between drinker groups with respect to gender (P = 0.10). The mean age of the heavy drinkers was 20.23 [standard deviation (SD) = 1.2] and the mean age of the light drinkers was 20.78 (SD = 1.1). All participants were right-handed, native English speakers, and free of any history of psychiatric or neurological disorders, or use of psychotropic or other medications that affect the central nervous system. Participants were excluded if they drank alcohol on the day of scanning (see Table 1 for descriptive statistics).
Table 1.
Population descriptive statistics.
| Light drinkers | Heavy drinkers | ||||
|---|---|---|---|---|---|
| N = 19 | N = 17 | ||||
| Mean (SD) | Range | Mean (SD) | Range | P | |
| Age | 20.78 (1.1) | 18–22 | 20.23 (1.2) | 18–22 | 0.17 |
| Audit score | 4.2 (2.04) | 1–7 | 13.38 (.55) | 5–28a | <0.0001 |
| Current drinking days per week | 2.37 (1.06) | 1–5 | 4.24 (1.44) | 3–7 | <.0001 |
| Number of drinks when drinking in past 30 days | 3.00 (0.94) | 2–5 | 6.18 (1.24) | 4–8 | <0.0001 |
| Self-reported binging | None | 100% | <0.0001 | ||
| Post scan Alcohol Urge Questionnaire score | 8.61 (3.20) | 5–20 | 10.12(4.31) | 5–20 | 0.25 |
| Operation span score | 38.81(2.97) | 33–42 | 38.60(4.67) | 25–42 | 0.88 |
Note:
One heavy drinking participant self-reported lower problems with drinking on the Alcohol Use Disorders Identification Test;, however, this individual reported heavy drinking and binging behavior, so this person was kept in the analysis and in the heavy drinking group. SD = standard deviation.
Drinking groups were defined as follows: Heavy drinkers. Male heavy drinkers had to currently consume 15 or more drinks during the week; female heavy drinkers had to currently consume eight or more drinks during the week. All heavy drinkers had to report binging behavior at least twice weekly. A binge for men consisted of five or more drinks at one setting, and a binge for women consisted of four or more drinks at one setting. Scores on the Alcohol Use Disorders Identification Test (AUDIT) were expected to be 8 or above for heavy drinkers.
Light drinkers
Light drinkers were expected to currently drink less than three times during the week and consume two or fewer drinks during any drinking episode, with no reported binging behavior. AUDIT scores were expected to be less than 7.
Questionnaires
Severity and frequency of alcohol consumption was assessed with the AUDIT, an index of consequences and problems experienced from drinking over the past year (alpha range, 0.75–0.94; Saunders et al. 1993; Babor et al. 2001). The AUDIT has been validated across a wide range of populations (for review, Allen et al. 1997), including college students (Fleming, Barry & MacDonald 1991). Binge-drinking was assessed with questions 2 and 3 on the AUDIT, which ask ‘How many drinks containing alcohol do you have on a typical day when you are drinking?’ [response option range from (0) 1 or 2 to (4) 10 or more], and ‘How often do you have six or more drinks on one occasion?’ [response options range from (0) never to (4) daily or almost daily]. Subjective craving/urge was assessed after scans with the Alcohol Urge Questionnaire (AUQ), an 8-item self-report measure that assesses current urge/craving to drink. Response options range from 1 (strongly disagree) to 7 (strongly agree). The AUQ has demonstrated significant positive correlations with an individual's alcohol use severity and exhibits high internal consistency (coefficient alpha = 0.91; Bohn, Krahn & Staehler 1995). In addition, we assessed frequency of alcohol and other drug use over the past 3 years. Past 3 year substance use was assessed with a 12-item rating scale (ranging from ‘never used’ to ‘500+ times’). Participants were asked how many times they used alcohol and other drugs (i.e. marijuana, ecstasy, hallucinogens, methamphetamine, etc.) in the past 3 years.
Working memory capacity was assessed as a proxy measure for general fluid intelligence with the OSPAN (see Engle et al. 1999; Engle 2002; Kane & Engle 2002), a validated automated operation span task (Unsworth et al. 2005; alpha = 0.78; test–retest reliability r = 0.83). The task measures capacity to learn and maintain information in an active state in the presence of interference and demands controlled attention (Kane & Engle 2002). Participants remember a series of three to seven letters presented sequentially on a computer monitor. Between letters, participants solve simple math problems and indicate if an answer to a problem is true or false (e.g. 8/2 + 6 = 10). Math problems serve as distracters requiring control of attention while maintaining letter sequences in short-term memory. A larger number of letters recalled in proper sequence is indicative of higher working memory capacity.
Alcohol-IAT
All participants performed an alcohol-IAT optimized for the scanner. The IAT is a concept categorization task that evaluates the relative strength of associations of contrasted target categories with contrasted attribute categories through rate of processing (Greenwald et al. 1998). In the scanner, participants were instructed as follows: ‘Press the #1 key for items that fit into a category on the top left. Press the #4 key for items that fit into a category on the top right. The categories change from time to time. You will not receive any instructions during the task. Go as fast as you can without making mistakes. Please wait for the task to start automatically.’
The stimuli to be categorized were randomly presented words so that they would more likely activate general meanings at the conceptual level related to alcohol rather than lexical relations between presented words. Subjects observed six different test stimuli in each compatible and incompatible block, with a total of 80 exposures in compatible blocks and 80 in incompatible blocks. Blocks of compatible trials and incompatible trials were counterbalanced and trials within the blocks were randomly ordered. Within each block, trials and fixation points were presented in a designated order, specified using OPTSEQ (Dale 1999) to enhance design efficiency. Fixation point trials served as baseline. Temporal jitter was used in the presentation of the fixation with onset timing ranging from 1.0 to 4.5 seconds, with a mean exposure of 2 seconds, followed by stimuli presentation. Maximum exposure of test stimuli was for 2 seconds. After a participant pressed a response key, the screen would go blank for the remainder of the 2 seconds. Total trial time ranged from 3.0 to 6.5 seconds.
The IAT included the target categories of ‘Alcohol’ words and ‘Mammal’ words. The target and attribute category words were those used by McCarthy & Thompsen (2006) who tested a behavioral version of this IAT among college students. The ‘Alcohol’ words were vodka, rum, whiskey, tequila, beer and gin. The matched ‘Mammal’ words were rabbit, llama, donkey, elephant, sheep and goat. The IAT attribute categories consisted of ‘Positive’ and ‘Neutral’ word categories. The Positive category words were happy, attractive, sexy, relaxed, confident and sociable. The matched Neutral category words were basic, historical, steep, brown, sandy, and stationary. Compatible trials consisted of Alcohol + Positive versus Mammal + Neutral word combinations, while ‘Mammal’ + ‘Positive’ versus ‘Alcohol’ + ‘Neutral’ word combinations were considered incompatible trials.
The IAT included seven blocks: (1) 20 practice trials with target categories only; (2) 20 practice trials with attribute categories only; (3) 20 practice trials for a compatible block with both target and attribute categories; (4) 60 test trials for a compatible block with both target and attribute categories; (5) 20 practice trials with target categories only in reversed positions; (6) 20 practice trials for an incompatible block with both reversed target categories and the attribute categories; and (7) 60 test trials for an incompatible block with both reversed target categories and the attribute categories. IAT incompatible and compatible blocks of trials were counterbalanced across subjects.
Trials for blocks 3 and 4 were included in the fMRI analysis for the compatible trials and blocks 6 and 7 were included for the incompatible trials (or the reverse when counter-balanced). It is standard practice in behavioral studies to include practice blocks 3 and 6 in analyses, and that protocol was followed in the fMRI analyses.
Along with scans, response latencies were obtained and scored according to the algorithm described by Greenwald, Nosek & Banaji (2003) to obtain a D-600 measure. In line with theories of associative strength, individuals should react faster when categorizing strongly associated concepts that share a response key (compatible condition) and slower when categorizing concepts less likely to be associated in memory and share a response key (incompatible condition; Greenwald et al. 1998). Higher D-600 scores reflect a greater difference between compatible and incompatible categorization scores, whereas lower D-600 scores reflect less difference between compatible and incompatible scores (Figs 1 & 2).
Figure 1.
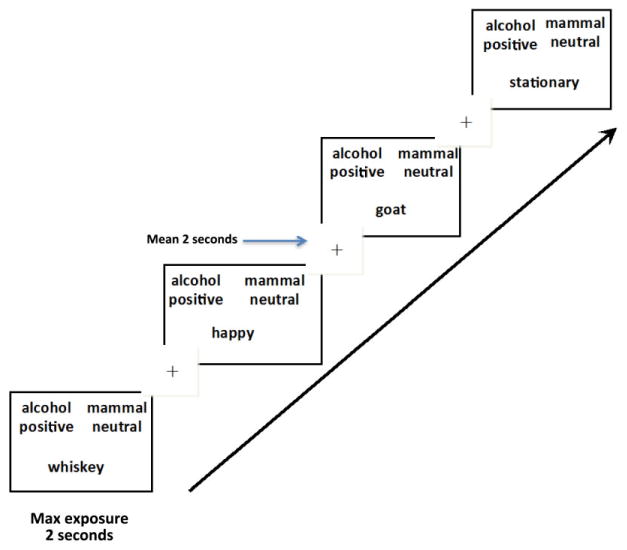
Compatible Association Trials. Temporal layout of 80 compatible trials (figure shows four test trials) or implicit associations toward alcohol use. On compatible trials, individuals are expected to react faster when categorizing strongly associated concepts that share a response key. This should be fairly easy to do for someone with past experience with alcohol. Temporal jitter was used in the presentation of the fixation with onset timing ranging from 1. 0 to 4.5 seconds, with a mean exposure of 2 seconds, followed by stimuli presentation. Maximum exposure of test stimuli was for 2 seconds. After a participant pressed a response key, the screen would go blank for the remainder of the 2 seconds. Total trial time ranged from 3.0 to 6.5 seconds
Figure 2.
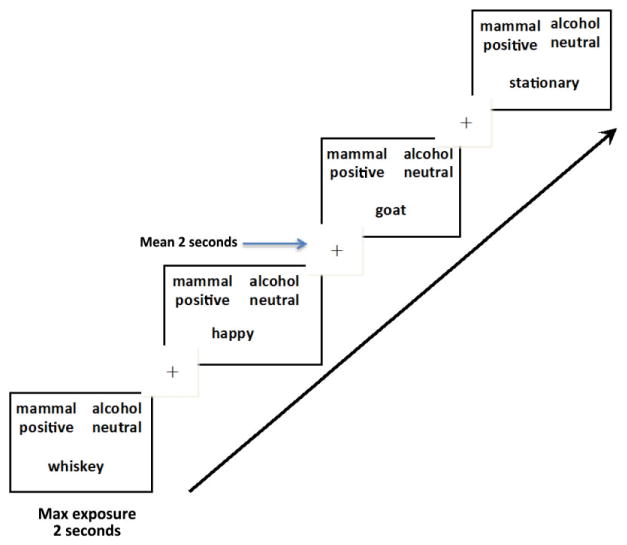
Incompatible Association Trials. Temporal layout of 80 incompatible trials (figure shows four test trials). In this figure, target categories and affective categories are switched. Individuals react slower when categorizing concepts not typically associated that share a response key. These trials require more effortful processing across all subjects. Temporal jitter was used in the presentation of the fixation with onset timing ranging from 1.0 to 4.5 seconds, with a mean exposure of 2 seconds, followed by stimuli presentation. Maximum exposure of test stimuli was for 2 seconds. After a participant pressed a response key, the screen would go blank for the remainder of the 2 seconds. Total trial time ranged from 3.0 to 6.5 seconds
Imaging parameters and data pre-processing
Imaging was performed using a Siemens 3T Magnetom Tim/Trio MR scanner fitted with head coil arrays for parallel imaging to minimize signal loss and image distortion in the orbitofrontal cortex. Participants' responses were collected online using a MRI-compatible button box; the response box consisted of a fiber-optics response pad and four buttons that accept a TR trigger from the scanner. Participants lay supine on the scanner bed and viewed visual stimuli back-projected onto a screen through a mirror attached onto the head coil. Functional images were acquired using a z-shim gradient, single-shot T2*-weighted echo EPI sequence with PACE (prospective acquisition correction). The specific sequence is dedicated to reduce signal loss in the prefrontal and orbitofrontal areas. Scanning parameters were as follows: TR = 2000 ms (whole brain); TE = 25 ms; flip angle = 90°; 64 × 64 matrix size with resolution 3 × 3 mm2; bandwidth:1906 Hz/pixel. Thirty-five 3-mm axial slices were acquired to cover the cerebrum and most of the cerebellum with no gap. An anatomical T1-weighted structural scan was acquired using an MPRAGE sequence (TI = 800 ms; TR = 2530 ms; TE = 3.1 ms; flip angle 10; 208 sagittal slices; 256 × 256 matrix size with spatial resolution as 1 × 1 × 1 mm3).
The fMRI data underwent preprocessing to aid in minimizing non–task-related variability and improve signal detection and sensitivity of statistical analyses. Whole brain analyses were performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (Mathworks Inc.). The functional data were slice acquisition timing corrected, motion corrected, co-registered to the anatomical image, normalized to the standardized template and spatially smoothed with a Gaussian kernel (4 mm full width at half maximum). A high pass filter was applied to the functional data to minimize low-frequency noise such as respiration and cardiac cycles (Friston et al. 2000; Wang et al. 2005). All motion-related translational parameters were within a range of 2 mm along any axis, therefore we did not exclude any scans from the analyses.
Data analysis
Analyses of demographics and behavioral measures were carried out using SAS® software Version 9.3 (SAS Institute Inc 2011). Differences between groups on demographics, alcohol-use frequency and severity, other drug use and the IAT response latencies (D-600 measure) were evaluated using t- and F-tests. Correlations were estimated among individual scores on self-reported drinking behaviors, IAT D-600 measures and working memory capacity. Correlational analyses were used to evaluate hemodynamic response in specified regions of interests—dorsal striatum, insula and OFC—and severity/frequency of alcohol use, binging behavior and alcohol craving. Analyses of behavioral measures were considered significant at P < 0.05.
The fMRI whole brain analyses used the standard two-level general linear model (GLM) model using SPM 8. In the first level, the GLM included two conditions representing two types of stimuli (compatible and incompatible trials), respectively. In analyses of the imaging data, compatible trials consisted of pairs of ‘Alcohol’ + ‘Positive’ and ‘Mammal’ + ‘Neutral’ word combinations; incompatible trials consisted of ‘Mammal’ + ‘Positive’ and ‘Alcohol’ + ‘Neutral’ word combinations, consistent with the standard for behavioral analyses of these trials. Fixation served as baseline. In the second level analysis, a 2 (group: heavy drinkers or light drinkers) × 2 (IAT blocks of trials: compatible or incompatible) full-factor random effects (Friston et al. 1995) model was used. The first-level generated contrast images (compatible and incompatible trials) for heavy drinkers and light drinkers, which were entered into the second-level analysis and modeled with between-and-within-subjects analysis of variance (ANOVA). Restricted maximum likelihood was used to adjust the statistics and degrees of freedom to account for non-sphericity in the ANOVA model. Different contrasts were set to show results. First, the main effects of group and IAT blocks of trials as well as their interaction were tested using F-tests. Then, t-tests were used to identify within-group activation differences between compatible and incompatible trials, as well as group differences within both compatible and incompatible trials, respectively. Voxels were considered significant with a blood-oxygen-level dependence (BOLD) response difference at P < 0.05 [false discovery rate (FDR)-corrected] with more than 30 voxels. Subsequent region of interest (ROI) analyses were performed on extracted percent signal change in each region for each participant under each condition separately. ROI analyses were used for display purposes only.
Results
Behavioral findings
A significant IAT effect for reaction times was found during task performance in the scanner with heavy drinkers showing stronger positive implicit associations toward alcohol than lighter drinkers (t = 2.35, P = 0.022). The mean D-600 measure for the heavy drinkers was 0.276 (SD = 0.20; range = −0.080 to 0.725), and the mean D-600 measure for the light drinkers was 0.030 (SD = 0.039.; range = −0.602 to 0.670). There was no significant difference in error rates on the IAT between the heavy (M = 0.030, SD = 0.019) and the light (M = 0.028, SD = 0.014) drinkers (t = −0.26, P = 0.80). The overall error rate on the task was small at 2.92%.
There was a main effect for block type, F(1, 33) = 8.74, P < 0.001, with reaction times being slower during incompatible blocks, as expected. In addition, there was a practice effect with reaction times faster during the critical test trials than during practice trials, F(1, 33) = 22.55, P < 0.001. Practice effects are common in the IAT (Nosek, Greenwald & Banaji 2005).
Drinker status correlated with past 3 year alcohol use (P < 0.005). Heavy drinkers reported drinking on average 151 to 200 times (mean 4.7, SD = 1.53) and light drinkers reported drinking on average 51–100 times in the past 3 years (mean = 3.11, SD = 1.56). Heavy drinkers reported more marijuana use (mean = 2.82, SD = 1.9) than light drinkers (mean = 1.6, SD = 0.7, P = 0.02). There were no significant differences in cigarette use or other illicit drug use between drinking groups. Self-reported illicit drug use was minimal with almost all subjects reporting never having used methamphetamine, cocaine, tranquilizers, ecstasy, hallucinogens, opiates or inhalants. There were no significant differences between groups in working memory capacity (P = 0.88).
The IAT D600 measure significantly correlated with binging behavior and current number of drinking days (Ps = 0.02), and trended toward significance with current number of drinks per week (P = 0.06). Significant correlations were also found between alcohol urges and working memory capacity in the sample. Higher self-reported urges for alcohol were associated with lower working capacity. Similarly, higher self-reported problems with alcohol assessed with the AUDIT were associated with lower working memory capacity (see Table 2).
Table 2.
Correlations between selected variables.
| Variable | IAT (d600 score) | Working memory capacity (OSPAN score) | ||
|---|---|---|---|---|
| r | P | R | P | |
| Alcohol urge | 0.10 | 0.56 | −0.41 | 0.02 |
| Audit score | 0.37 | 0.03 | −0.39 | 0.03 |
| Binging | 0.37 | 0.02 | −0.03 | 0.88 |
| Current # of drinking days weekly | 0.39 | 0.02 | −0.16 | 0.38 |
| Current # of drinks weekly | 0.32 | 0.06 | −0.15 | 0.42 |
IAT = Implicit Association Test; OSPAN = operation span task.
No significant correlations were found between neural response in the dorsal striatum, insula or OFC and severity of alcohol use (assessed with the AUDIT), number of days drinking during the week, number of drinks during a drinking occasion, binging behavior, or alcohol craving on compatible or incompatible trials.
fMRI findings
Condition effects
Compatible > incompatible association trials
Table 3 provides whole brain analysis peak activity t statistics for condition effects for heavy drinkers during compatible trials relative to incompatible trials. Heavy drinkers showed significantly greater activity in regions of the dorsal striatum, amygdala and insula during compatible trials relative to incompatible trials.
Table 3.
Condition effects for heavy drinkers.
| L/R | Region | N of voxels | Coordinates | t value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Compatible > incompatible trials | ||||||
| L | Putamen/amygdala | 36 | −28 | −18 | −4 | 3.93 |
| R | Insula | 152 | 38 | 14 | 2 | 3.76 |
| Incompatible > compatible trials | ||||||
| None | ||||||
P < 0.05 FDR-corrected, >30 voxels.
Table 4 provides condition effects for light drinkers. During compatible trials relative to incompatible trials, light drinkers showed no significantly greater regional activity.
Table 4.
Condition effects for light drinkers.
| L/R | Region | N of voxels | Coordinates | t-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Compatible > incompatible trials | ||||||
| None | ||||||
| Incompatible > compatible trials | ||||||
| L | DLPFC | 349 | −48 | 24 | 20 | 4.21 |
| R | DLPFC | 45 | 58 | 16 | 16 | 3.55 |
P < 0.05 FDR-corrected, >30 voxels. DLPFC = dorsolateral prefrontal cortex; FDR = false discovery rate.
Incompatible > compatible association trials
We observed no significantly greater regional activity among heavy drinkers during incompatible trials relative to compatible trials. Among the light drinkers, we observed greater bilateral activity in the DLPFC (see Table 4).
Group effects
Heavy > light drinkers
During compatible trials, we observed significant activity among heavy drinkers (compared with light drinkers) in the left and right insula and bilateral anterior cingulate cortex (see Table 5). During incompatible trials, there was no significantly greater activity among heavy drinkers relative to light drinkers.
Table 5.
Group effects during compatible association trials.
| L/R | Region | N of voxels | Coordinates | t-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Heavy > light drinkers | ||||||
| L | Insula | 60 | −48 | 16 | −4 | 4.27 |
| L/R | ACC | 65 | 0 | 8 | 38 | 4.24 |
| R | Insula | 64 | 38 | 16 | 2 | 3.98 |
| Light > heavy drinkers | ||||||
| None | ||||||
P < 0.05 FDR-corrected, >30 voxels. ACC = anterior cingulate cortex; FDR = false discovery rate .
Light > heavy drinkers
Among light drinkers relative to heavy drinkers on compatible trials, there was no significantly greater activity observed (see Table 5). Activity in the right insula and left amygdala correlated with incompatible task performance among lighter drinkers (Table 6).
Table 6.
Group effects during incompatible association trials
| L/R | Region | N of voxels | Coordinates | t-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Heavy > light drinkers | ||||||
| None | ||||||
| Light > heavy drinkers | ||||||
| R | Insula | 63 | 46 | 6 | −6 | 4.12 |
| L | Amygdala | 81 | −28 | −4 | −24 | 3.80 |
P < 0.05 FDR-corrected, >30 voxels.
Full-factor analyses
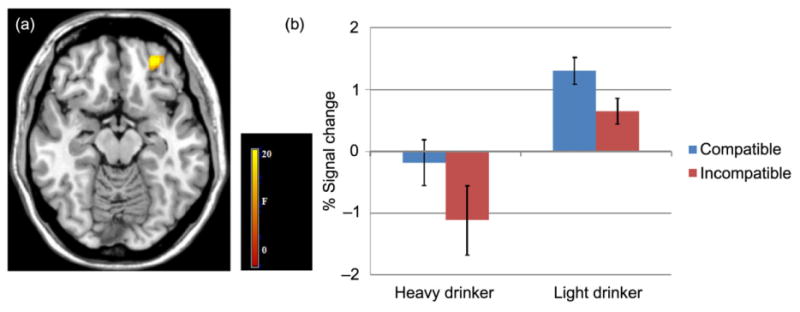
Findings for the 2 × 2 full-factor analyses were as follows: (1) a significant main effect for group was observed in the left OFC. Light drinkers showed significantly more activity in the left OFC during both compatible and incompatible association trials (see Fig. 3); (2) a significant main effect for condition was observed in the DLPFC. The DLPFC was engaged more in both light and heavy drinkers during incompatible association trials (see Fig. 4); and (3) a significant group by condition interaction was found in some hypothesized regions (see Fig. 5, all Ps < 0.05, FDR-corrected). That is, the effect of group on BOLD response varied depending on the compatible and incompatible conditions, described later (see Table 7).
Figure 3.
Group effects during compatible and incompatible association trials. (a) functional magnetic resonance imaging results suggest the left orbital frontal cortex (OFC) showed a significant effect of group; z = −14. (b) Bar graphs show percent (%) signal change for the left OFC activity during compatible and incompatible trials for heavy and light drinkers. Error bars denote within-subject error. Significance set at P < 0.05 FDR-corrected, voxels >30
Figure 4.
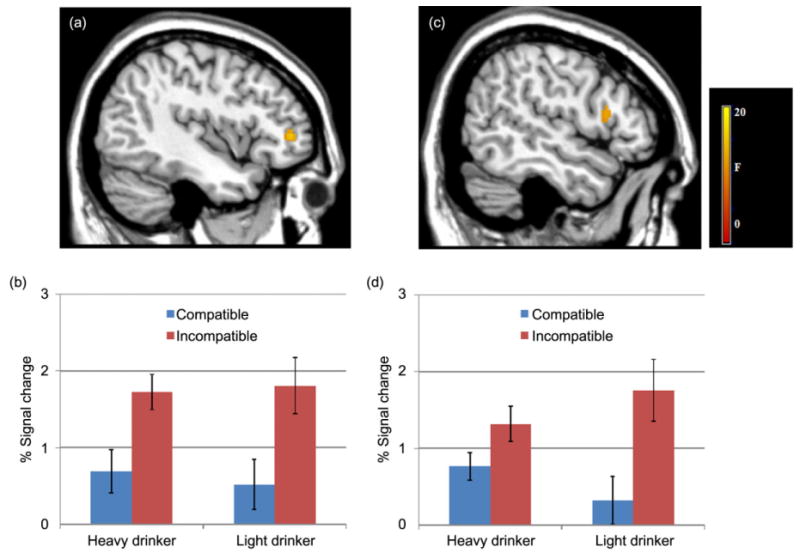
Condition effects during compatible and incompatible association trials. Functional magnetic resonance imaging results suggest that both the left (x = −44; a) and right (x = 52; c) dorsolateral prefrontal cortex (DLPFC) showed significant effects of condition. Bar graphs show percent (%) signal change for the left (b) and right (d) DLPFC activity during compatible and incompatible trials for heavy and light drinkers. Error bars denote within-subject error. Significance set at P < 0.05 FDR-corrected, voxels >30
Figure 5.
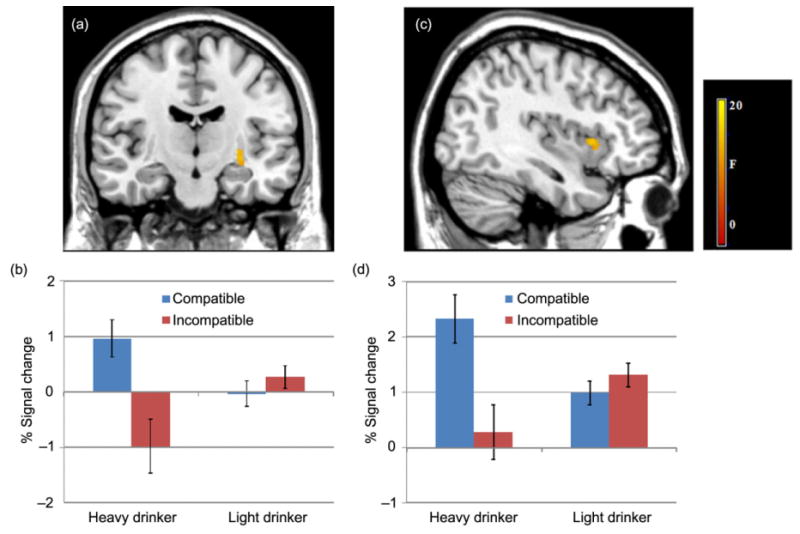
Groups by condition interaction effects. Interaction effects during task trials by drinking groups for the left putamen (y = −14; a) and right insula (x = 38; c). Bar graphs show the percent signal change for the left putamen (b) and right insula (d) activity during compatible and incompatible trials for heavy and light drinkers. Significance set at P < 0.05 FDR-corrected, voxels >30
Table 7.
Full factor analysis results.
| L/R | Region | N of voxels | Coordinates | F-value | ||
|---|---|---|---|---|---|---|
| x | Y | Z | ||||
| Main effect of group | ||||||
| L | OFC | 61 | −28 | 48 | −14 | 23.23 |
| Main effect of condition | ||||||
| L | DLPFC | 44 | −42 | 44 | 4 | 16.23 |
| R | DLPFC | 51 | 54 | 16 | 16 | 13.62 |
| Interaction between group and condition | ||||||
| L | Putamen | 43 | −30 | −20 | −4 | 14.92 |
| R | Insula | 54 | 34 | 16 | 2 | 12.84 |
P < 0.05 FDR-corrected, >30 voxels. DLPFC = dorsolateral prefrontal cortex; FDR = false discovery rate; OFC = orbital frontal cortex.
Interaction effects
Significant group by condition interactions in the left putamen and right insula were observed (see Fig. 5). The left putamen showed significantly greater activity among heavy drinkers during performance on compatible trials relative to incompatible trials, but there was no significant difference among light drinkers with respect to activity across conditions (see Fig. 5). The right insula showed a similar pattern of activity; heavy drinkers showed significantly greater activity during compatible trials relative to incompatible trials with no significant difference between conditions among light drinkers.
Discussion
The results of this study are consistent with a dual-process framework of appetitive behaviors and addiction proposing that (1) implicit memory associations for alcohol-related stimuli, particularly in heavier drinkers, engage neural circuitry in the dorsal striatum; and (2) regulatory and self-control mechanisms, particularly in lighter drinkers, engage neural circuitry in sectors of the prefrontal cortex. Further, based on clinical findings, we expected categorization of positive alcohol-related associations to trigger strong urges to drink, and consequently elicit greater activity in the insula in heavy drinkers (relative to light drinkers; e.g. Naqvi & Bechara 2009). Our findings were consistent with the general conception that alcohol-related stimuli trigger an urge mediated through the insula, which in turn may facilitate the implicit/automatic processing of habitual behaviors and weaken regulatory processing of self-controlled behaviors (Naqvi & Bechara 2009). This study provides preliminary support that these neural mechanisms can be elicited by an alcohol IAT, thus providing an additional step toward increasing our understanding of associative habit processes and their regulatory influence over addictive behaviors.
Behaviorally, we found a significant difference between the heavy and light drinkers during task performance in the scanner with heavier drinkers having stronger positive implicit associations toward alcohol, assessed through rate of processing/reaction time. This was expected based on behavioral studies suggesting that (1) the IAT taps into spontaneous or automatic activation of associations as well as controlled processes during compatible and incompatible trial performance; and (2) the IAT is related to level of drinking behavior.
Neural activity generated by the different task conditions during fMRI were consistent with the detection of potential habit learning, reflecting neural response to more or less effortful processing of concepts resulting from repetitive experience with a reinforcing behavior. With repetitive alcohol use, associations in memory are strengthened and can overwhelm control processes. Patterns of associations can then signal and drive behavior without the necessary involvement of deliberative or control processes (cf., White 1996; Stacy et al. 2004; Wiers et al. 2007; Stacy & Wiers 2010). Behavior then becomes increasingly under cue control and less under voluntary control. In the brain, we expect to observe a ‘transition from prefrontal cortical control to subcortical striatal control and within the striatum from ventral to dorsal domains of the striatum’ (Vollstädt-Klein et al. 2010, p. 1741) as habitual behaviors develop (Schneider & Chein 2003; Everitt & Robbins 2005; Robbins, Ersche & Everitt 2008; cf. Yin & Knowlton 2006). Our imaging data revealed within- and between-group differences during compatible and incompatible trials in both controlled and automatic associative systems, described later.
Between-group neural response observed for full-factor analysis
The imaging data showed an interaction effect between groups and task conditions and processing of stimuli in some key hypothesized regions. Findings revealed a difference among the heavy and light drinkers in regions of the brain implicated in habit-based (associative) learning (see Robbins et al. 2008). The heavy drinking group showed significantly greater activity in the left putamen and right insula during compatible association trials. It was expected that individuals with repetitive experiences with alcohol use would be able to perform trials comprised of concepts highly associated with alcohol fairly easily, requiring little need for engagement of more deliberative or control processes. Alternatively, the light drinking group with significantly less alcohol use experience showed no significant differences in activity in the putamen and insula during compatible trials. Overall, little activity in the putamen during task performance was observed in the light drinkers.
In contrast, light drinkers showed significantly greater activity in the left OFC relative to our heavier drinkers during both compatible and incompatible association trials. This finding was somewhat consistent with our prediction. That is, we had predicted that lighter drinkers would show more activity in regions requiring more reflective/effortful processing of information during incompatible trials relative to heavy drinkers, as a result of perhaps poorer controlled processing among the heavier drinkers, but we had not predicted similar findings for the compatible trials. However, this finding is generally consistent with other imaging studies observing diminished control ability and abnormal/reduced frontal activity in humans with substance use problems as a result of learned responses to drug cues (see Volkow & Fowler 2000; Dom et al. 2005; Everitt et al. 2008; Koob & Volkow 2010).
The full-factor analysis also revealed significant bilateral activity across both groups during incompatible trials in the DLPFC, suggesting that both groups of drinkers exhibited some deliberative processes during categorization on these trials. This result is not surprising given these trials are more difficult to perform, requiring more effortful processing of information and controlled attention (see Kane & Engle 2002). Between groups, the lighter drinkers showed a larger effect than heavy drinkers and this effect was also observed in the within-group comparison (incompatible > compatible contrast) among light drinkers.
Within-group neural response on compatible and incompatible trials
Significant within-group differences were also observed when we compared compatible with incompatible trials. Heavy drinkers revealed significant striatal-amygdala activity, reflecting implicit/associative learning dependent on a neural system that has been described as the ‘impulsive’ system (e.g. Bechara 2005; Bechara, Noel & Crone 2006). The response for heavy drinkers on the trials of automatic associations for positive outcomes of alcohol use is suggestive of the development of some habit-based associative memories attributed to repetitions of reinforced behavior and reflected by increased activity in the putamen (considered to contribute to habit-like responding) and amygdala. Various associative memories involving behavior depend in large part on regions of the striatum (Eldridge, Masterman & Knowlton 2002; Yin & Knowlton 2006). The dorsal striatum has been implicated in habit learning in animal studies (e.g. Barnes et al. 2005; Atallah et al. 2007; Corbit, Nie & Janak 2012) and studies with humans (e.g. Knowlton et al. 1996; Tricomi, Balleine & O'Doherty 2009; Vollstädt-Klein 2010). The amygdala has been implicated in the processing of reinforcers and relevance of stimuli (White & McDonald 2002), as well as stimulus-reward associations (Yin & Knowlton 2006).
Further, the heavy drinkers showed greater insula activity during compatible trials relative to incompatible trials and relative to light drinkers. The insula has been linked to habit neural circuitry, including cued induced urges through interoceptive information processing and emotional memories of drug effects (Naqvi & Bechara 2009; Tang et al. 2012). Self-reported alcohol urges were not correlated with neural response in the insula in this study, but this is not surprising because assessment of urges occurred after task performance and a short time delay outside the scanner. It is possible that during task performance exposure to positive alcohol-related associations elicited automatic activation of emotional memories or representations of alcohol experiences, without conscious awareness, reflected in insula activation. It is feasible that such urges occurred in both heavy and light drinkers (because light drinkers might also experience an urge to drink). Insula activation has been observed in other appetitive behaviors, including food and smoking cue reactivity studies, also with no significant correlation to self-reported urges/craving (for meta analysis, see Tang et al. 2012).
Interestingly, contrary to our initial prediction, we observed no significant activity in heavy or light drinkers in the ventral striatum, which has traditionally been implicated in motivation and reward. Among the heavy drinkers, this finding is consistent with some contemporary theories that argue that what originates as motivational (supported by the nucleus accumbens) essentially transfers control to the dorsal striatum as habit-based after repetitive reward-based learning, and without need for involvement of the accumbens (e.g. see Porrino et al. 2004; Everitt & Robbins 2005; Robbins et al. 2008; cf. Yin & Knowlton 2006).
Limitations
More research and larger samples are needed to parse various interacting cortical and subcortical regions involved in appetitive behaviors and task-related findings. Additionally, future research might want to compare alcohol dependent individuals, or abstinent alcoholics, and light drinkers given the IAT correlates with level of drinking, which may further help differentiate the roles of the various neural regions and processes involved in habitual behaviors. Further, one might argue that participants in this study are too young to be considered habitual drinkers. However, heavy drinking could result in a lifetime of alcohol abuse given some subcortical regions are fully developed that support habit learning. In addition, most of the heavy drinkers in the study self-reported drinking problems on the AUDIT with scores suggestive of hazardous, habitual use, and many heavy drinking emerging adults begin drinking during adolescence (e.g. Hill et al. 2000). Also, it is not possible to rule out that our findings may be diluted by learning over the course of the task, or that differences in brain activity found may not be specific to the alcohol-related IAT. Additionally, although the groups in the study were not significantly different with respect to gender, they were not equal in number. Based on findings from our behavioral research, we did not expect gender differences on indirect tests of associations (e.g. Ames et al. 2007). Future imaging studies involving alcohol and other drug-related IATs might evaluate potential gender differences on this task.
Finally, although we assessed past 3-year drinking behavior, we did not assess onset or duration of drinking experiences, which could affect alcohol habit development. Nevertheless, this was a first step in evaluating an alcohol IAT in the scanner in emerging adults, some of who are engaging in hazardous drinking behaviors.
Some of our findings differed from previous IAT studies, perhaps because of the nature of the population and the type of behavior studied. More research is needed to further understand the neural mechanisms underlying condition effects of the IAT and to tease out processes involving associative/habit learning as a result of appetitive behaviors (i.e. dopamine-dependent behaviors) and implicit attitudes toward objects and/or beliefs.
Summary
In sum, the findings from this study contribute insight into the neural substrates of alcohol-related associative memory processes and executive control regions engaged during compatible and incompatible IAT conditions. Our imaging findings showed a difference among heavy and light drinkers in regions of the brain implicated in habit (associative) learning. The observed pattern of neural activity within groups across conditions is consistent with what might be expected in the transition from processes that are predominantly reflective, effortful or control-related to more automatic processing with learning or experience (e.g. Schneider & Chein 2003; Chein & Schneider 2005; also see Vollstädt-Klein et al. 2010). Essentially, performance of well-learned behaviors in response to strong associations does not require as much effort or strong involvement of neural regions implicated in control processes. This work extends findings derived through behavioral research, demonstrating the consistent predictive effects of associative memory processes in alcohol use and has implications for intervention research. Because implicit associative memory processes perpetuating hazardous behavior may override control processes, linking neural and behavioral findings to intervention research is key for future research in this area.
Supplementary Material
Figure S1 Significant activity for heavy drinkers during compatible—incompatible trials
Figure S2 Significant activity for light drinkers during incompatible—compatible trials
Figure S3 Group effects in compatible association trials
Figure S4 Group effects in incompatible association trials
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse (DA024772, DA023368, DA024659) and the National Institute on Alcohol Abuse and Alcoholism (AA017996) and the National Cancer Institute (CA152062). We thank Marcia McGuire for her support on this project.
Footnotes
Supporting Information: Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
References
- Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcohol Clin Exp Res. 1997;21:613–619. [PubMed] [Google Scholar]
- Ames SL, Franken IHA, Coronges K. Implicit cognition and drugs of abuse. In: Wiers RW, Stacy AW, editors. Handbook of Implicit Cognition and Addiction. Thousand Oaks, CA: SAGE Publications; 2006. pp. 363–378. [Google Scholar]
- Ames SL, Grenard JL, Thush C, Sussman S, Wiers RW, Stacy AW. Comparison of indirect assessments of association as predictors of marijuana use among at-risk adolescents. Exp Clin Psychopharmacol. 2007;15:204–218. doi: 10.1037/1064-1297.15.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Atallah HE, Lopez-Paniagua D, Rudy JW, O'Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat Neurosci. 2007;10:126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders J, Monteiro MG. AUDIT. The Alcohol Use Disorders Identification Test. Guidelines for use in primary health care. 2nd. Geneva, Switzerland: 2001. ••. [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Noel X, Crone EA. Loss of willpower: abnormal neural mechanisms of impulse control and decision making in addiction. In: Wiers RW, Stacy AW, editors. Handbook of Implicit Cognition and Addiction. Thousand Oaks, CA: Sage Publications, Inc.; 2006. pp. 215–233. [Google Scholar]
- Beer JS, Stallen M, Lombardo MW, Gonsalkorale K, Cunningham WA, Sherman JW. The quadruple process model approach to examining the neural underpinnings of prejudice. Neuroimage. 2008;43:775–783. doi: 10.1016/j.neuroimage.2008.08.033. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Reward learning: reinforcement, incentives, and expectations. In: Medin DL, editor. The Psychology of Learning and Motivation: Advances in Research and Theory. Vol. 40. San Diego, CA: Academic Press; 2001. pp. 223–278. [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Sriram N, Soon CS, Lee KM. Dorsolateral prefrontal cortex and the implicit association of concepts and attributes. Neuroreport. 2000;11:135–140. doi: 10.1097/00001756-200001170-00027. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta analytic evidence of a domain-general control network for learning. Brain Res Cogn Brain Res. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry. 2012;72:389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Houwer J, Crombez G, Koster EHW, De Beul N. Implicit alcohol-related cognitions in a clinical sample of heavy drinkers. J Behav Ther Exp Psychiatry. 2004;35:275–286. doi: 10.1016/j.jbtep.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. Br J Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Masterman D, Knowlton BJ. Intact implicit habit learning in Alzheimer's disease. Behav Neurosci. 2002;116:722–726. [PubMed] [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Curr Dir Psychol Sci. 2002;11:19–23. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory and general fluid intelligence: a latent variable approach. J Exp Psychol Gen. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Cognitive bias and drug craving in recreational cannabis users. Drug Alcohol Depend. 2004;74:105–111. doi: 10.1016/j.drugalcdep.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Fleming MF, Barry KL, MacDonald R. The alcohol use disorders identification test (AUDIT) in a college sample. Int J Addict. 1991;26:1173–1185. doi: 10.3109/10826089109062153. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline JB. To smooth or not to smooth?: Bias and efficiency in fMRI time-series analysis. Neuroimage. 2000;12:196–208. doi: 10.1006/nimg.2000.0609. [DOI] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM, Ungerleider LG, et al. Dynamic mapping of human cortical development during childhood and adolescence. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol. 1998;74:1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the Implicit Association Test: I. An improved scoring algorithm. J Pers Soc Psychol. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Poehlman TA, Uhlmann EL, Banaji MR. Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. J Pers Soc Psychol. 2009;97:17–41. doi: 10.1037/a0015575. [DOI] [PubMed] [Google Scholar]
- Hill KG, White HR, Chung IJ, Hawkins JD, Catalano RF. Early adult outcomes of adolescent binge drinking: person- and variable-centered analyses of binge drinking trajectories. Alcohol Clin Exp Res. 2000;24:892–901. [PMC free article] [PubMed] [Google Scholar]
- Hopfield JJ, Tank DW. Computing with neural circuits: a model. Science. 1986;233:625–633. doi: 10.1126/science.3755256. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW. Are drinkers implicitly positive about drinking alcohol? Personalizing the alcohol-IAT to reduce negative extrapersonal contamination. Alcohol Alcohol. 2007;42:301–307. doi: 10.1093/alcalc/agm015. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW. Implicitly positive about alcohol? Implicit positive associations predict drinking behavior. Addict Behav. 2008;33:979–986. doi: 10.1016/j.addbeh.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Jajodia A, Earleywine M. Measuring alcohol expectancies with the Implicit Association Test. Psychol Addict Behav. 2003;17:126–133. doi: 10.1037/0893-164x.17.2.126. [DOI] [PubMed] [Google Scholar]
- Kahneman D. A perspective on judgment and choice: mapping bounded rationality. Am Psychol. 2003;58:697–720. doi: 10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Hum Brain Mapp. 2007;28:915–930. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KM, Wood JN, Spampinato MV, Grafman J. Politics on the brain: an fMRI investigation. Soc Neurosci. 2006;1:25–40. doi: 10.1080/17470910600670603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Nakic M, Wheatley T, Richell R, Martin A, Blair RJ. The neural basis of implicit moral attitude—an IAT study using event-related fMRI. Neuroimage. 2006;30:1449–1457. doi: 10.1016/j.neuroimage.2005.11.005. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Thompsen DM. Implicit and explicit measures of alcohol and smoking cognitions. Psychol Addict Behav. 2006;20:436–444. doi: 10.1037/0893-164X.20.4.436. [DOI] [PubMed] [Google Scholar]
- McCusker CG. Cognitive biases and addiction: an evolution in theory and method. Addiction. 2001;96:47–56. doi: 10.1046/j.1360-0443.2001.961474.x. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi H. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) College Drinking. NIAAA College Fact Sheet. 2012 Apr; Available at: http://pubs.NIAAA.NIH.gov. Accessed ••.
- Nosek BA, Greenwald AG, Banaji MR. Understanding and using the Implicit Association Test: II. Method variables and construct validity. Pers Soc Psychol Bull. 2005;31:166–180. doi: 10.1177/0146167204271418. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queller S, Smith ER. Subtyping versus bookkeeping in stereotype learning and change: connectionist simulations and empirical findings. J Pers Soc Psychol. 2002;82:300–313. [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AK, et al. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Reich R, Below M, Goldman MS. Explicit and implicit measures of expectancy and related alcohol cognitions: a meta-analytic comparison. Psychol Addict Behav. 2010;24:13–25. doi: 10.1037/a0016556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Mechanisms of action of addictive stimuli. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rooke SE, Hine DW, Thorsteinsson EB. Implicit cognition and substance use: a meta-analysis. Addict Behav. 2008;33:1314–1328. doi: 10.1016/j.addbeh.2008.06.009. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS® 9.3. Cary, NC: SAS Institute Inc.; 2011. [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Puente JR, Grant M. Development of the Alcohol Use Disorders Screening Test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption. II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schneider W, Chein JM. Controlled & automatic processing: behavior, theory, and biological mechanisms. Cogn Sci. 2003;27:525–559. [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and the college drinker: biological basis of propensity to use and misuse alcohol. J Stud Alcohol. 2002;14(Suppl):71–81. doi: 10.15288/jsas.2002.s14.71. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stacy AW. Memory association and ambiguous cues in models of alcohol and marijuana use. Exp Clin Psychopharmacol. 1995;3:183–194. [Google Scholar]
- Stacy AW. Memory activation and expectancy as prospective predictors of alcohol and marijuana use. J Abnorm Psychol. 1997;106:61–73. doi: 10.1037//0021-843x.106.1.61. [DOI] [PubMed] [Google Scholar]
- Stacy AW, Ames SL, Knowlton B. Neurologically plausible distinctions in cognition relevant to drug abuse etiology and prevention. Subst Use Misuse. 2004;39:1571–1623. doi: 10.1081/ja-200033204. [DOI] [PubMed] [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annu Rev Clin Psychol. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional FMRI studies. Physiol Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Thush C, Wiers RW, Ames SL, Grenard JL, Sussman S, Stacy AW. Apples and oranges? Comparing implicit measures of alcohol-related cognition predicting alcohol use in at-risk adolescents. Psychol Addict Behav. 2008;23:146–151. doi: 10.1037/0893-164X.21.4.587. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, O'Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, Heitz RP, Schrock JC, Engle RW. An automated version of the operation span task. Behav Res Methods Instrum Comput. 2005;37:498–505. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]
- Vetulani J. Drug addiction. Part II. Neurobiology of addiction. Pol J Pharmacol. 2001;53:303–317. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized b a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Schacter DL. Interactions between forms of memory: when priming hinders new episodic learning. J Cogn Neurosci. 2000;12:S52–S60. doi: 10.1162/089892900564064. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang Z, Aguirre GK, Detre JA. To smooth or not to smooth? ROC analysis of perfusion fMRI data. Magn Reson Imaging. 2005;23:75–81. doi: 10.1016/j.mri.2004.11.009. [DOI] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–949. [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Bartholow BD, van den Wildenberg E, Thush C, Engels R, Sher K, Grenard J, Ames S, Stacy AW. Automatic and controlled processes and the development of addictive behavior in adolescents: a review and a model. Pharmacol Biochem Behav. 2007a;86:263–283. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Houben K, de Kraker J. Implicit cocaine associations in active cocaine users and controls. Addict Behav. 2007b;32:1284–1289. doi: 10.1016/j.addbeh.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Stacy AW. Handbook of Implicit Cognition and Addiction. Thousand Oaks, CA: SAGE Publications; 2006. [Google Scholar]
- Wiers RW, Van de Luitgaarden J, Van den Wildenberg E, Smulders FTY. Challenging implicit and explicit alcohol-related cognitions in young heavy drinkers. Addiction. 2005;100:806–819. doi: 10.1111/j.1360-0443.2005.01064.x. [DOI] [PubMed] [Google Scholar]
- Wiers RW, van Woerden N, Smulders FTY, de Jong PJ. Implicit and explicit alcohol-related cognitions in heavy and light drinkers. J Abnorm Psychol. 2002;111:648–658. doi: 10.1037/0021-843X.111.4.648. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Significant activity for heavy drinkers during compatible—incompatible trials
Figure S2 Significant activity for light drinkers during incompatible—compatible trials
Figure S3 Group effects in compatible association trials
Figure S4 Group effects in incompatible association trials


