Abstract
Zebrafish is a powerful vertebrate model system for studying development, modeling disease, and performing drug screening. Recently a variety of genetic tools have been introduced, including multiple strategies for inducing mutations and generating transgenic lines. However, large-scale screening is limited by traditional genotyping methods, which are time-consuming and labor-intensive. Here we describe a technique to analyze zebrafish genotypes by PCR combined with high-resolution melting analysis (HRMA). This approach is rapid, sensitive, and inexpensive, with lower risk of contamination artifacts. Genotyping by PCR with HRMA can be used for embryos or adult fish, including in high-throughput screening protocols.
Keywords: Basic Protocol, Issue 84, genotyping, high-resolution melting analysis (HRMA), PCR, zebrafish, mutation, transgenes
Introduction
Zebrafish (Danio rerio) is a vertebrate model system widely used for studies of development and disease modeling. Recently, numerous transgenic and mutation technologies have been developed for zebrafish. Rapid transgenesis techniques, usually based on a Tol2 transposon system1, have been combined with improved cloning options for multiple DNA fragment assembly2. Zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) have been used to target loci in both somatic and germline cells in zebrafish3,4. These techniques can efficiently generate genetically modified animals, with high-frequency mutation creation and germ-line transmission3,4.
Despite these advances, traditional genotyping techniques in zebrafish limit the full power of the mutagenesis and transgenesis tools. PCR followed by gel electrophoresis, sometimes combined with restriction enzyme digestion, is widely used to detect genome modification, but is time-consuming and less sensitive to identify small insertions or deletions. TaqMan probe assays have high initial costs and require careful optimization. Sequencing of PCR products can take several days and is not practical for large-scale screening. Restriction fragment length polymorphism (RFLP) analysis can only discriminate SNPs affecting a limited range of restriction enzyme recognition sites.
High-resolution melting analysis (HRMA), a closed-tube post-PCR analysis method, is a recently developed method that is rapid, sensitive, inexpensive, and amenable to screening large numbers of samples. HRMA can be used to detect SNPs, mutations, and transgenes5-7. HRMA is based on thermal denaturation of double-stranded DNAs, and each PCR amplicon has a unique dissociation (melt) characteristic5. Samples can be discriminated due to their different nucleotide composition, GC content, or length, typically in combination with a fluorescent dye that only binds double-stranded DNA8. Thus, HRMA can distinguish different genotypes based on the different melt-curve characteristics. Because HRMA uses low-cost reagents and is a single-step post-PCR process, it can be used for high-throughput strategies. HRMA is nondestructive, so following analysis the PCR amplicons can be used for other applications. HRMA has been applied in many organisms and systems, including cell lines, mice, and humans9-11. Its use has recently been described in zebrafish to detect mutations induced by zinc finger nucleases (ZFNs) and TALENs6,12,13.
In this paper, we describe how to perform PCR-based HRMA in embryonic and adult zebrafish (Figure 1). This protocol is suitable for detecting SNPs, transgenes, and mutations, including single base-pair changes, insertions, or deletions.
Protocol
1. DNA Preparation
Prepare DNA lysis buffer: 50 mM KCl, 10 mM Tris-HCl pH 8.3, 0.3% Tween 20, 0.3% NP40. Add fresh Proteinase K to a final concentration of 1 mg/ml on the day of use12.
- Tissue collection:
- For adult fish fin clip:
- Anesthetize fish: Place the fish in 0.004% MS-222 (tricaine) solution. Wait until gill movement slows.
- Put fish on a stack of 5-10 Kimwipes and cut a small piece of the tail fin, about 2-3 mm, with a sterile razor blade.
- Quickly place the fish in a labeled tank with fresh water for recovery; carefully label both the tank and corresponding tube that will contain the fin clip. Change the water and feed the fish every other day.
- Pick up the fin clip with a sterile pipette tip and transfer it into a tube filled with 100 μl DNA lysis buffer.
- Discard the pipette tip, and discard the razor blade into a sharps container.
- For embryo tail clip:
- Place embryos into 0.004% MS-222 (tricaine) solution. Wait 2 min.
- Cut a piece of tail with forceps under a dissecting microscope.
- Put the tail into a labeled tube filled with 30 μl DNA lysis buffer.
- Quickly put the embryo into a tube containing 100 μl 4% paraformaldehyde.
- Label tubes with embryos and their corresponding tail tubes.
- Store the tubes with embryos at 4 °C overnight for fixation.
- Clean the forceps using Milli-Q water and Kimwipes between each embryo to minimize contamination.
- For whole embryos:
- Place embryos into 0.004% MS-222 (tricaine) solution. Wait 2 min.
- Put 1-5 embryos in a tube filled with 100 μl DNA lysis buffer. Dechorionation is not necessary.
DNA digestion Incubate tubes with tissue (fin or tail clips or embryos) at 55 °C for 4 hr up to overnight12.
Inactivate the Proteinase K Heat tubes to 95 °C for 15 min12. DNA should be used for PCR immediately, or stored at -20 °C for up to 3 months.
2. PCR
Design Primers either manually or by primer design software (e.g. Lightscanner Primer Design Software- Biofire). PCR products for HRMA are usually 50-200 bp; PCR products for SNP detection should be smaller, typically 50-80 bp.
- PCR
- Perform PCR reaction in a 96-well or 384-well plate.
- Use 10 μl total volume: 4 μl LightScanner master mix (0.1 U hot-start Taq-AB polymerase, buffer, 0.2 mM dNTPs, 2 mM magnesium chloride, and 1x fluorescent double-stranded DNA-binding dye); 5 pmol each primer, and 1 μl genomic DNA template extracted from adult tail fin or 3 μl genomic DNA from embryonic tail13.
- Cover samples with 30 μl mineral oil.
- Cover the plate with an optically-transparent adhesive seal.
- Optimize PCR cycling conditions- typical conditions are 95 °C for 5 min and 30 cycles of 10 sec at 95 °C, 25 sec at 60 °C, 30 sec at 72 °C, ending with 95 °C for 30 sec and cooled to 15 °C.
- Store PCR plates at 4 °C or continue directly to HRMA.
- Confirm our PCR product by analysis of its size using agarose gel electrophoresis during initial optimization of PCR; and if indicated, by sequencing of the PCR product.
3. HRMA
- Heat plates
- Place plate in a melt analysis system.
- Open the software (LightScanner Software Call-IT 2.0).
- Heat plates from 60-95 °C.
- Create a new data storage file.
- Analyze HRMA data (Figure 2). Multiple steps of analysis are possible. We show the most commonly used set of data manipulations.
- Subset setting Click Subset tab. Select the wells with samples.
- Normalization
- Select the Normalize tab in the left panel. To eliminate fluorescence variance, manually position the parallel double-line in pre- (initial) and post (final) -melt regions as illustrated (Figure 2C, arrowheads) and normalize premelt and post-melt fluorescence signals of all samples to 1 and 0, respectively14.
- Adjust the positioning of the lines so that the melt region is in the region between the pre- and post-melt lines, but not selected. In general, the Lower Min and Lower Max (and Upper Min and Upper Max) temperature lines should be placed approximately 1 °C apart.
- Choose temperature positions for normalization such that all samples have maximum or full (100%) fluorescence for the premelt region and minimum or no (0%) fluorescence for the post-melt region (as best as possible). Normalization should result in a near-horizontal line at the maximum fluorescence of 1 that extends until the melt point and then a near-horizontal line from the melt point at the minimum fluorescence of 0; there should not be fluorescence levels above 1 or below 0. For example, in Figure 2C, normalize the premelting curves using temperature ranges of 79-80 °C.
- Grouping/Clustering Distinguish genotypes based on their melting temperature (Figure 2C, green box). Select Grouping.
- Select Autogroup from the Standards selection list under the Grouping section.
- Select Normal or High for melting profiles with single transitions or multiple transitions respectively from the Sensitivity selection list under the Grouping section.
- Select Compute Groups under the Grouping section.
- Go to the File menu and click Save to save the results.
Representative Results
The protocol can be performed during a single day or separated in steps over several days (flow diagram of work is shown in Figure 1). DNA extraction is followed by the melt and analysis of PCR amplicons. The temperatures for the melt of the amplicon depend on the size and GC-content, but generally start and end temperatures of 50 ˚C and 95 ˚C are appropriate (Figures 2A and 2B). Once the melt is performed, analysis of the fluorescence melt curves typically requires normalization of the variation of the different sample curves, using pre- and post-melt regions as standards (Figure 2C). This improves comparison of results from different samples in which the variation of fluorescence is related to minor experimental variations. Each pair of temperature lines for normalization should be placed approximately 1 ˚C apart (Figure 2C, arrowheads). Grouping of data results is represented in two different ways: an upper graph that shows melt curve profiles, and a lower graph that shows a subtractive difference plot in comparison to a reference sample (Figure 2D).
HRMA analysis can be used to detect mutations (Figures 3A and 3B) or transgenes (Figure 3C). In Figure 3A, two different mutations in eif2b5 gene are shown (red and blue curves in Figure 3A) by subtracting the normalized fluorescence data of the wild-type sample. It does not matter which genotype is chosen for reference. More subtle or confusing differences in melt-curves can be distinguished by using the subtractive difference plot. In Figure 3B, an example of four different genotypes in a single collection of embryos is shown.
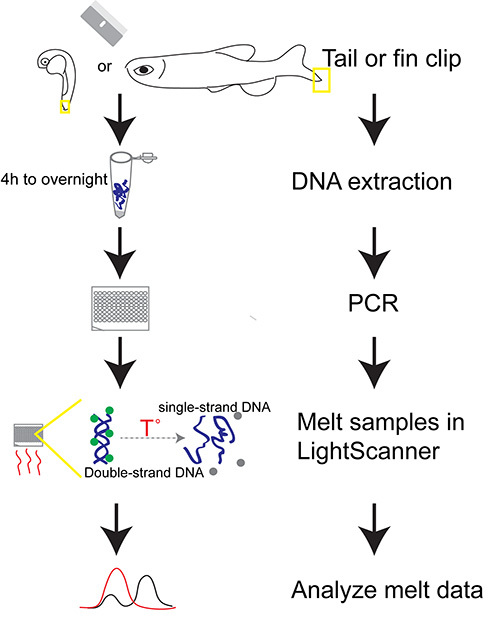 Figure 1. Outline of protocol for PCR-based HRMA of zebrafish genomic DNA. DNA extraction from whole tissue (fin-clip or embryo) is followed by PCR amplification in the presence of a fluorescent double-stranded DNA-binding dye. The PCR amplicon is denatured and fluorescence signal is recorded, followed by melt-curve analysis, to detect the amplicon and/or to differentiate genotypes.
Figure 1. Outline of protocol for PCR-based HRMA of zebrafish genomic DNA. DNA extraction from whole tissue (fin-clip or embryo) is followed by PCR amplification in the presence of a fluorescent double-stranded DNA-binding dye. The PCR amplicon is denatured and fluorescence signal is recorded, followed by melt-curve analysis, to detect the amplicon and/or to differentiate genotypes.
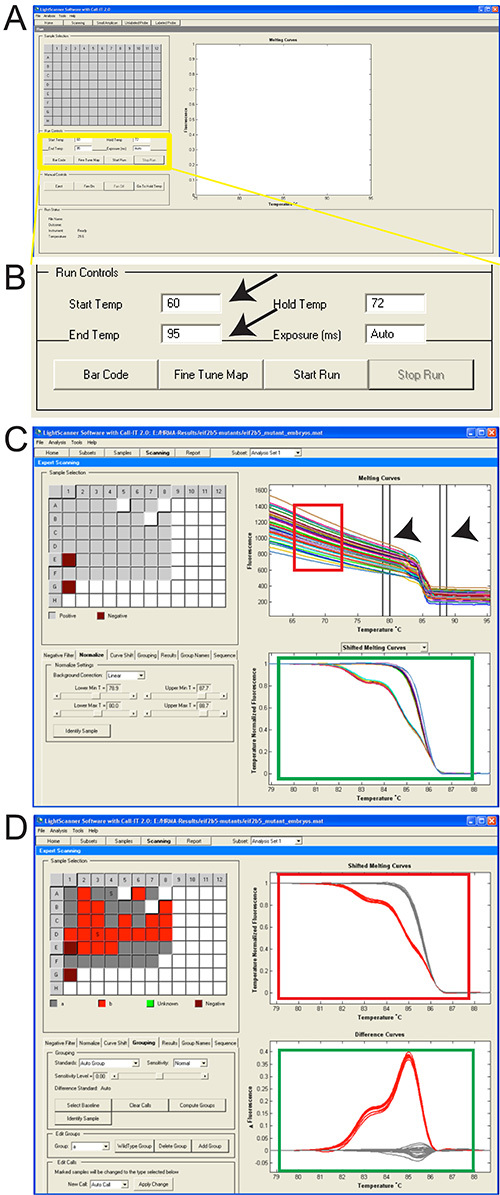 Figure 2. Screen-shots of software to perform HRMA and analyze results.
A. Screen shot of LightScanner software; yellow box is magnified in “B”. B. Magnified view of boxed region in “A”. Setting Start and End Temp are shown (arrows). C. Position the premelt and post-melt parallel lines for normalization (arrowheads). Notice the fluorescence variance in pre- and post-melting regions (red box). Lower graph (green box) shows melt-curves derived from the raw data plots following normalization. D. Upper graph shows melt curve profiles after automatic grouping; different genotypes are illustrated in different colors (red box). Lower graph (green box) shows the fluorescence difference curve. Click here to view larger image.
Figure 2. Screen-shots of software to perform HRMA and analyze results.
A. Screen shot of LightScanner software; yellow box is magnified in “B”. B. Magnified view of boxed region in “A”. Setting Start and End Temp are shown (arrows). C. Position the premelt and post-melt parallel lines for normalization (arrowheads). Notice the fluorescence variance in pre- and post-melting regions (red box). Lower graph (green box) shows melt-curves derived from the raw data plots following normalization. D. Upper graph shows melt curve profiles after automatic grouping; different genotypes are illustrated in different colors (red box). Lower graph (green box) shows the fluorescence difference curve. Click here to view larger image.
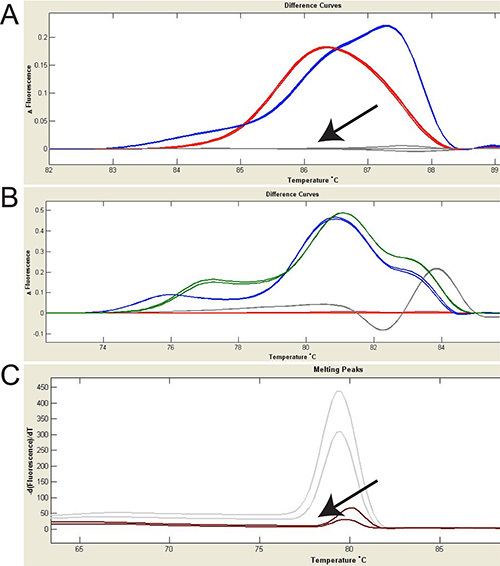 Figure 3. Using HRMA to identify mutations and transgenes. Fluorescence difference curves are shown; change in fluorescence (y-axis) to temperature (x-axis) is shown. A. HRMA was used to detect fish carrying mutations in the eif2b5 gene. Wild type is shown in grey (arrow). Red and blue colors represent two different eif2b5 mutations, confirmed by sequencing of the PCR product. B. Four different foxP2 mutant alleles are identified with HRMA. Red: zc82/+ (8 bp deletion), green: zc83/+ (17 bp deletion), blue: zc82/zc83 (8bp deletion/17bp deletion), grey: zc83/zc83 (17bp deletion homozygotes). C. Gal4 transgenic fish (grey curves) can be distinguished from wild type (brown curve, arrow). Notice that for transgene detection, normalization should not be performed. Click here to view larger image.
Figure 3. Using HRMA to identify mutations and transgenes. Fluorescence difference curves are shown; change in fluorescence (y-axis) to temperature (x-axis) is shown. A. HRMA was used to detect fish carrying mutations in the eif2b5 gene. Wild type is shown in grey (arrow). Red and blue colors represent two different eif2b5 mutations, confirmed by sequencing of the PCR product. B. Four different foxP2 mutant alleles are identified with HRMA. Red: zc82/+ (8 bp deletion), green: zc83/+ (17 bp deletion), blue: zc82/zc83 (8bp deletion/17bp deletion), grey: zc83/zc83 (17bp deletion homozygotes). C. Gal4 transgenic fish (grey curves) can be distinguished from wild type (brown curve, arrow). Notice that for transgene detection, normalization should not be performed. Click here to view larger image.
Discussion
PCR combined with HRMA is a powerful technology for zebrafish genotyping. The advantages of this approach are its speed, robustness, and sensitivity to detect even point mutations. The entire protocol, from fin-clip to melt-curve analysis, can be performed in less than eight hours by a single individual. In addition, the technique is amenable for high-throughput screening; does not require the use of ethidium bromide; and is sealed for all PCR and analysis steps which helps minimize contamination issues.
A critical step for this technique is PCR amplicon and primer design. Typical length for PCR products in HRMA is 50-200 bp. Short amplicons increase sensitivity and are ideal for detecting single nucleotide changes. If melt-curves of closely related PCR product species can not be distinguished easily, a small oligonucleotide complementary to the SNP and blocked at the 3’ end can be included in the PCR reaction (Lunaprobes; probes). The probes are generated by asymmetric PCR using different concentration of the forward and reverse primers, with a 3’ C3 blocker to prevent probe extension in the subsequent HRMA PCR. The probe is then included in the HRMA PCR reaction. HRMA then occurs with the probe, in which the asymmetric probe binds to the PCR product and generates probe-target duplexes with different melt-curves that can be used to distinguish alleles.
Several companies offer melt-curve analysis systems. These include the Biofire Lightscanner, the Roche LightcyclerVR 480 and the Qiagen Rotor-Gene Q. Several fluorescent, DNA-binding dyes are available, including LC Green PLUS and SYT09 EvaGreen. There is some variation in sensitivity in mutation detection based on different fluorescent dyes and melt-curve machines8,15. Nonsaturating dyes, for example, SYBR Green I, are unsuitable for most HRMA applications: at high concentrations, SYBR Green I inhibits the activity of DNA polymerase; and at lower concentrations, SYBR Green I cannot precisely measure melting behavior due to its redistribution from melted regions back to regions of dsDNA16. In general most existing Real Time PCR platforms are capable of preforming HRMA using a software package add-on and a second-generation fluorescent DNA binding dye. While technical details of the software and sequence of experimental steps vary slightly between platforms, the overall scientific concepts are essentially identical.
Our lab and others have demonstrated that HRMA can be used in both embryonic and adult zebrafish to detect transgenes, and mutations induced by ZFNs and TALENs6,12,13. HRMA has been used to discover polymorphisms in zebrafish6,12. Our lab uses HRMA both for initial screening of TALEN-induced mutations as well as for sorting of established lines (TSQ and JLB, unpublished). Other potential applications that could be applied to zebrafish include methylation-sensitive HRMA (ms-HRMA)17, quantification of copy-number variants5; and pathogen detection in vivariums18.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank members of the Blaschke, Grunwald, and Wittwer labs for advice and technical assistance. This work is supported by the PCMC Foundation, NIH R01 MH092256 and DP2 MH100008, and the March of Dimes Foundation research grant #1-FY13-425, to JLB.
References
- Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell. 2004;7(1):133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, et al. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 2007;236(11):3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Sander JD, Cade L, et al. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat. Biotechnol. 2011;29(8):697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B. Heritable gene targeting in zebrafish using customized TALENs. Nat. Biotechnol. 2011;29(8):699–700. doi: 10.1038/nbt.1939. [DOI] [PubMed] [Google Scholar]
- Vossen RHAM, Aten E, Roos A, Den Dunnen JT. High-resolution melting analysis (HRMA): more than just sequence variant screening. Hum. Mutation. 2009;30(6):860–866. doi: 10.1002/humu.21019. [DOI] [PubMed] [Google Scholar]
- Dahlem TJ, Hoshijima K, et al. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 2012;8(8) doi: 10.1371/journal.pgen.1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew M, Pryor R, et al. Genotyping of single-nucleotide polymorphisms by high-resolution melting of small amplicons. Clin. Chem. 2004;50(7):1156–1164. doi: 10.1373/clinchem.2004.032136. [DOI] [PubMed] [Google Scholar]
- Herrmann MG, Durtschi JD, Bromley LK, Wittwer CT, Voelkerding K. V Amplicon DNA melting analysis for mutation scanning and genotyping: cross-platform comparison of instruments and dyes. Clin. Chem. 2006;52(3):494–503. doi: 10.1373/clinchem.2005.063438. [DOI] [PubMed] [Google Scholar]
- Carrillo J, Martínez P, et al. High resolution melting analysis for the identification of novel mutations in DKC1 and TERT genes in patients with dyskeratosis congenita. Blood Cell. Mol. Dis. 2012;49(3-4):140–146. doi: 10.1016/j.bcmd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Thomsen N, Ali RG, Ahmed JN, Arkell RM. High resolution melt analysis (HRMA); a viable alternative to agarose gel electrophoresis for mouse genotyping. PloS one. 2012;7(9) doi: 10.1371/journal.pone.0045252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorkas PA, Poumpouridou N, Agelaki S, Kroupis C, Georgoulias V, Lianidou ES. PIK3CA hotspot mutation scanning by a novel and highly sensitive high-resolution small amplicon melting analysis method. J. Mol. Diagn. 2010;12(5):697–704. doi: 10.2353/jmoldx.2010.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parant JM, George SA, Pryor R, Wittwer CT, Yost HJ. A rapid and efficient method of genotyping zebrafish mutants. Dev. Dyn. 2009;238(12):3168–3174. doi: 10.1002/dvdy.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Hoshijima K, et al. Zebrafish foxP2 zinc finger nuclease mutant has normal axon pathfinding. PloS one. 2012;7(8) doi: 10.1371/journal.pone.0043968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra A, Siji A, Sridhar TS, Phadke K, Vasudevan A. Evaluation of High Resolution Melting analysis as an alternate tool to screen for risk alleles associated with small kidneys in Indian newborns. BMC Nephrol. 2011;12(1) doi: 10.1186/1471-2369-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MG, Durtschi JD, Wittwer CT, Voelkerding K. V Expanded instrument comparison of amplicon DNA melting analysis for mutation scanning and genotyping. Clin. Chem. 2007;53(8):1544–1548. doi: 10.1373/clinchem.2007.088120. [DOI] [PubMed] [Google Scholar]
- Wittwer CT. High-Resolution Genotyping by Amplicon Melting Analysis Using LCGreen. Clin. Chem. 2003;49(6):853–860. doi: 10.1373/49.6.853. [DOI] [PubMed] [Google Scholar]
- Dimitrakopoulos L, Vorkas PA, Georgoulias V, Lianidou ES. A closed-tube methylation-sensitive high resolution melting assay (MS-HRMA) for the semi-quantitative determination of CST6 promoter methylation in clinical samples. BMC Cancer. 2012;12:486–48. doi: 10.1186/1471-2407-12-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng K, Gaydos CA, et al. Comparative analysis of two broad-range PCR assays for pathogen detection in positive-blood-culture bottles: PCR-high-resolution melting analysis versus PCR-mass spectrometry. J. Clin. Microbiol. 2012;50(10):3287–3292. doi: 10.1128/JCM.00677-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


