Abstract
Reliable tools for investigating ovarian cancer initiation and progression are urgently needed. While the use of ovarian cancer cell lines remains a valuable tool for understanding ovarian cancer, their use has many limitations. These include the lack of heterogeneity and the plethora of genetic alterations associated with extended in vitro passaging. Here we describe a method that allows for rapid establishment of primary ovarian cancer cells form solid clinical specimens collected at the time of surgery. The method consists of subjecting clinical specimens to enzymatic digestion for 30 min. The isolated cell suspension is allowed to grow and can be used for downstream application including drug screening. The advantage of primary ovarian cancer cell lines over established ovarian cancer cell lines is that they are representative of the original specific clinical specimens they are derived from and can be derived from different sites whether primary or metastatic ovarian cancer.
Keywords: Medicine, Issue 84, Neoplasms, Ovarian Cancer, Primary cell lines, Clinical Specimens, Downstream Applications, Targeted Therapies, Epithelial Cultures
Introduction
Despite its relatively low incidence, ovarian cancer is the deadliest of the gynecological diseases and the fifth leading cause of cancer deaths among women1,2. This is mainly due to the lack of reliable tools and models that faithfully recapitulate the initiation and progression of the disease3. Most of our knowledge today about ovarian cancer has been possible through the use of immortalized ovarian surface epithelial cells (IOSEs), ovarian cancer cell lines and primary ovarian cancer cells recovered from ascitic fluid4-7. Unfortunately, their use has several limitations including a number of genetic and phenotypic changes associated with immortalization process or in vitro passages and the heterogeneity of the population resulting from ascitic fluid preparation.
Therefore, primary ovarian cancer cells derived from identifiable and specific solid specimens of ovarian cancer represent a unique tool for studying ovarian cancer progression.
The main difficulties involved in the obtaining these malignant cells are due to an overgrowth of stromal cells or fibroblasts along with loss of viability and premature lack of proliferative capacity in the culture of these EOC cells. Several methods to create single-cell suspensions from solid tumors currently exist, through mechanical means or enzymatic dissociation, however certain techniques yield a greater amount of the preferred outcome8. Here, we show that enzymatic digestion with dispase II results in an effective recovery of viable, fibroblast-free EOC cells. The so-obtained EOC cultures are highly susceptible to genetic manipulation and are also useful in drug screening tests, indicating that these EOC cultures are suitable for many downstream applications.
Protocol
Ethics Statement Solid specimens of ovarian cancer were obtained from the University of Minnesota Tissue Procurement Facility (TPF) after Institutional Review Board Committee: Human Subject Board (IRB) approval.
1. Reagent Setup
Prepare Complete DMEM medium by supplementing DMEM with 10% FBS, and 100 units penicillin-streptomycin. Store the medium at 4 °C and warm to 37 °C prior to use.
Aliquot dispase II into separate volumes of 5-10 ml to avoid multiple freeze thaws and store at -20 °C in a nonfrost-free freezer.
2. Tissue Collection
Collect solid specimens of ovarian cancer (~5 g in size) at the time of surgery from areas macroscopically identified as cancer by a pathologist. Clinical specimens are de-identified and registered according to institutional protocols.
Place specimens in a sterile, screw lid, polypropylene specimens container filled with 30 ml of ice-cold PBS. Transport the specimens from the surgical pathology laboratory to the research laboratory for processing on ice, and within 30 min from specimen collection (Figure 1).
3. Tissue Processing
Working under sterile conditions, transfer the samples onto a Petri dish (60 mm x 60 mm) containing 10 ml of fresh, ice-cold PBS and using a sterile razor blade, further cut into the smallest pieces possible (2 mm or less) (Figures 2A and 2B).
Transfer the minced tissues into a 15 ml conic tube containing 10 ml of prewarmed (37 °C for 30 min) dispase II (2.4 U/ml) in DMEM and incubate at 5% CO2 and 37 °C for 30 min. To ensure optimal digestion of the specimens, manually agitate the cell slurry every 5 min.
After 30 min incubation, transfer (using a 10 ml serological pipette) the cell slurry onto a cell strainer (70 μm mesh) placed on top of a 50 ml conical tube and apply a gentle pressure against the mesh using a syringe plunger. Discard any undissociated tissue (remaining on the top of the mesh) and collect the obtained cell suspension in the 50 ml sterile conical tube. Centrifuge at 320 x g for 7 min at 4 °C (Figure 3).
Discard the supernatant and resuspend the cell pellet in 10 ml of DMEM containing 10% FBS.
Incubate the cell suspension in a Petri dish at 5% CO2 and 37 °C (Figure 4).
Change the medium after 24 hr from the initial plating. This allows for removal of cellular debris and the majority of the erythrocytes present in culture.
Change the medium every three days for the following two weeks, after which the cultures of primary EOC are ready for downstream applications.
Representative Results
Fresh clinical specimens of ovarian cancer are collected after surgery (Figure 1) and cut in small pieces (Figures 2A and 2B) constituting the cell slurry. This allows for optimal exposure of the specimens to the enzymatic treatment. Cell slurry is exposed to enzymatic digestion and incubated at 37 °C for 30 min. During the incubation time the slurry becomes increasingly cloudy (especially after each agitation) and this is a sign of tissue disaggregation. At the end of the incubation time, the slurry is transferred onto a cell strainer to separate EOC cells from any undissociated tissue (Figure 3). The recovered cell suspension is placed at 5% CO2 and 37 °C (Figure 4). At this stage the morphology of the cell cultures reveals the presence of both EOC cells as a single cell suspension and in small clumps. The culture at this point also reveals the presence of erythrocytes and small debris as shown in Figure 5. Importantly while the EOC will slowly (over a period of 1-3 days) adhere to the plastic, erythrocytes and cell debris will not and they will eventually and progressively "disappear from the culture". By day 3 from initial plating, the EOC cultures show increasing adherent EOC cell clusters and progressively less erythrocyte and debris as shown in Figure 6. By day 6-7, EOC cells form swirl-like shapes (arrow) and start to spread to the plastic to form larger multicellular aggregates leading to the typical cobblestone morphology of EOC cells as shown in Figure 7. By day 14, EOC cells typically become confluent (Figure 8) and are now ready for downstream testing and applications.
 Figure 1. Collection of clinical specimens. Solid specimens of ovarian cancer are collected at the time of surgery and placed in a sterile, screw lid, polypropylene specimen container filled with 30 ml of ice-cold PBS.
Figure 1. Collection of clinical specimens. Solid specimens of ovarian cancer are collected at the time of surgery and placed in a sterile, screw lid, polypropylene specimen container filled with 30 ml of ice-cold PBS.
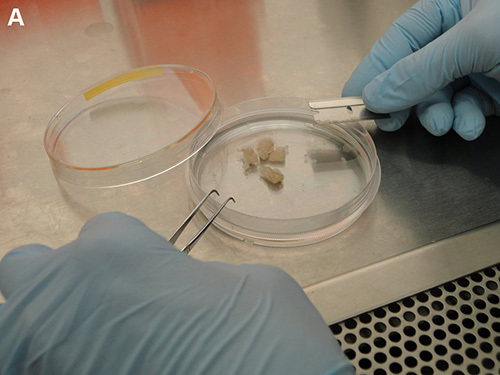
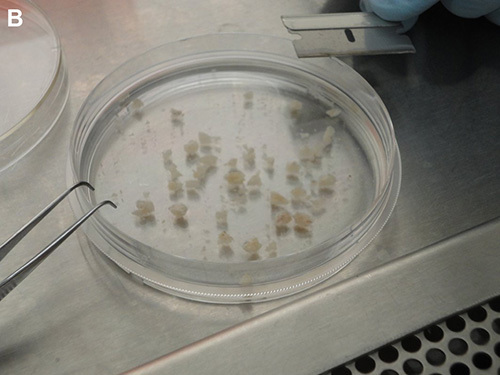 Figure 2. Processing of clinical specimens. A) Solid specimen transferred onto a Petri dish containing 10 ml of fresh, ice-cold 1x PBS. B) Clinical specimens further cut into pieces about 2 mm in size.
Figure 2. Processing of clinical specimens. A) Solid specimen transferred onto a Petri dish containing 10 ml of fresh, ice-cold 1x PBS. B) Clinical specimens further cut into pieces about 2 mm in size.
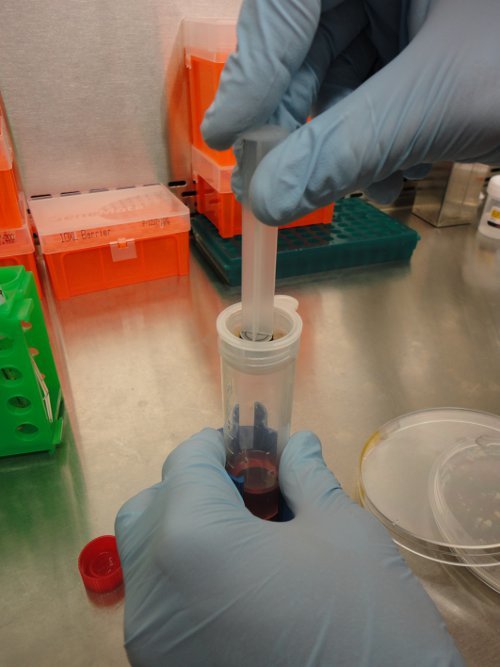 Figure 3. Separation of EOC cells from undissociated tissues. Following enzymatic digestion, clinical specimens are transferred onto a cell strainer and a gentle pressure is applied on the digested clinical specimens by using a syringe plunger. This allow for mechanical detachment of EOC from connective tissues and recovery of EOC cells.
Figure 3. Separation of EOC cells from undissociated tissues. Following enzymatic digestion, clinical specimens are transferred onto a cell strainer and a gentle pressure is applied on the digested clinical specimens by using a syringe plunger. This allow for mechanical detachment of EOC from connective tissues and recovery of EOC cells.
 Figure 4. Plating of resulting EOC cells suspension. After removing any undissociated tissues, the cell suspension is centrifuged and resuspended in 10 ml of DMEM containing 10% FBS and incubated in a Petri dish at 5% CO2 and 37 °C.
Figure 4. Plating of resulting EOC cells suspension. After removing any undissociated tissues, the cell suspension is centrifuged and resuspended in 10 ml of DMEM containing 10% FBS and incubated in a Petri dish at 5% CO2 and 37 °C.
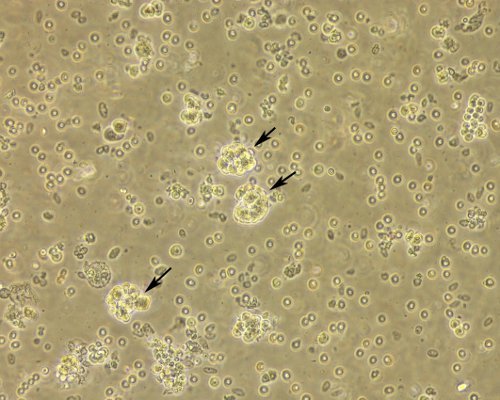 Figure 5. Morphology of EOC cell cultures immediately after processing. Cell suspension immediately after plating shows EOC as a single cell suspensions and in clumps (both indicated by the arrows), erythrocytes and cell debris still abound at this stage. Original magnification, 20X.
Figure 5. Morphology of EOC cell cultures immediately after processing. Cell suspension immediately after plating shows EOC as a single cell suspensions and in clumps (both indicated by the arrows), erythrocytes and cell debris still abound at this stage. Original magnification, 20X.
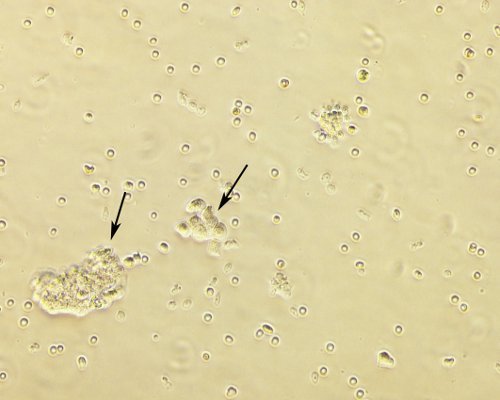 Figure 6. Morphology of EOC cell cultures at day 3 after plating. EOC cell culture at day 3 after plating shows semi-adherent EOC cell cluster (indicated by the arrow) and less erythrocyte contamination. Original magnification, 20X.
Figure 6. Morphology of EOC cell cultures at day 3 after plating. EOC cell culture at day 3 after plating shows semi-adherent EOC cell cluster (indicated by the arrow) and less erythrocyte contamination. Original magnification, 20X.
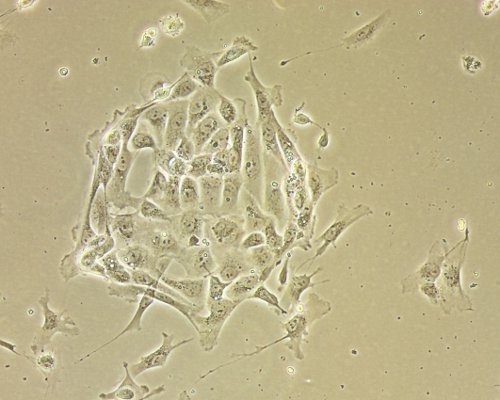 Figure 7. Established EOC cultures after a week from plating. EOC cell culture one week after initial plating shows swirl-like clusters of cells spreading on the tissue culture plastic. Original magnification, 20X.
Figure 7. Established EOC cultures after a week from plating. EOC cell culture one week after initial plating shows swirl-like clusters of cells spreading on the tissue culture plastic. Original magnification, 20X.
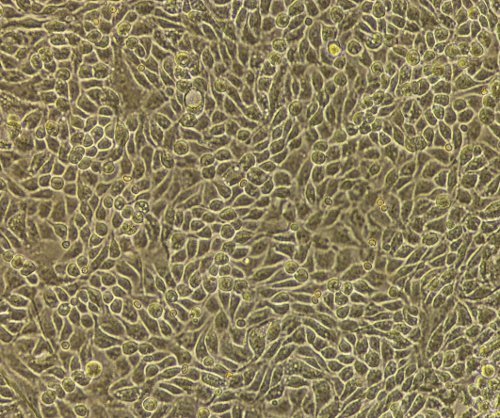 Figure 8. Confluent EOC cultures. Confluent monolayer of EOC cells showing the typical epithelial cobblestone morphology after 14 days in culture.
Figure 8. Confluent EOC cultures. Confluent monolayer of EOC cells showing the typical epithelial cobblestone morphology after 14 days in culture.
Discussion
A better understanding of ovarian cancer etiology and development is crucial to improving the outcome of women affected by this devastating disease. In this context, the use of established and "commercially available" ovarian cancer cell line has undoubtedly been tremendously useful. However, today we know that cancer cell lines are not representative of the human tumor they originated from in many aspects including the lack of heterogeneity9,10.
Here, we report a rapid and reliable method for isolation and culturing of primary ovarian cancer cells directly from clinical specimens of ovarian cancer within 30 min of surgical specimen isolation.
Because the primary cells are isolated from a solid specimen versus ascites fluid, this has the advantage of isolating cells from early stages of the disease prior to the time when ascites fluid formation occurs. This technique also has the advantage of isolating primary ovarian cancer cells from a specific site. The success in obtaining viable primary ovarian cancer cells using this protocol is largely dependent on how quickly the sample can be processed following surgery. For best results, the specimens should be received within 30 min from surgery. If this is not possible, the specimen should be kept at 4 °C (in PBS) overnight and processed the following day. In our experience, processing of samples following overnight storage decreases the yield. Exposure to dispase II for longer than 30 min also reduces cell viability and should not be performed. An alternative to dispase II treatment can be treatment with collagenase or hyaluronidase. However, this results in decreased yield8. Because of the great variability in clinical specimens, some may have larger red blood cell contamination as compared to others. If that is the case, we suggest "washing" the minced tissues (step 3.1) with PBS prior exposing them to enzymatic digestion. This will reduce the number of red blood cells in the preparation and ultimately facilitate adhesion of the primary epithelial cells.
Because the clinical specimens are not sterile (they lose sterility as soon as they arrive at the surgical pathology laboratory), it is critical to perform all of the steps under strictly sterile conditions. While contamination of the culture is possible, typically it occurs in less than 10% of the cases. The above protocol may be used with any consistency of clinical specimen.
The major limitation of this protocol is that the number of cells recovered from each samples is highly dependent on the size of the specimens provided. A typical specimen's size is about 3 cm x 3 cm. Another limitation is represented by the fact that while primary ovarian cancer cells grow exponentially for up to 10 passages in vitro (therefore providing the opportunity to make viable stocks) they ultimately senesce and die, thus limiting the availability of cells from a single donor. While we do not perform any separation of tumor cells from fibroblasts, we hypothesize that the relatively high (10%) concentration of serum throughout the process may give ovarian cancer cells a survival advantage over fibroblasts. It is also likely that cells that are less prone to separation from the stromal compartment (fibroblasts) may not disaggregate and will remain on the filter during separation.
Cells obtained with this protocol are highly suitable for future applications including investigations aimed to understand the processes leading to ovarian cancer initiation and development. Future applications also include using these primary cells for in vitro and in vivo testing of novel chemotherapy agents for ovarian cancer as well as biomarkers for early detection.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We would like to thank the staff of the Tissue Procurement Facility of the University of Minnesota for assistance with patient tissue samples collection. This work was supported by the Department of Defense Ovarian Cancer Research Program (OCRP) OC093424 to MB, by the Randy Shaver Cancer Research and Community Fund to MB, by the Minnesota Ovarian Cancer Alliance to MB and by the Gynecological Oncology Departmental fund to MB. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Kurman RJ, Visvanathan K, Roden R, Wu TC, Ie M Shih. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am. J. Obstet. Gynecol. 2008;198(4):351–356. doi: 10.1016/j.ajog.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E, Kurman RJ, Shih IM. Ovarian Cancer Is an Imported Disease: Fact or Fiction. Curr. Obstet. Gynecol. Rep. 2012;1(1):1–9. doi: 10.1007/s13669-011-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarojini S, et al. Early detection biomarkers for ovarian. J. Oncol. 2012. [DOI] [PMC free article] [PubMed]
- Shepherd TG, Theriault BL, Campbell EJ. Nachtigal M.W. Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nat. Protoc. 2006;1(6):2643–2649. doi: 10.1038/nprot.2006.328. [DOI] [PubMed] [Google Scholar]
- Tsao SW, et al. Characterization of human ovarian surface epithelial cells immortalized by human papilloma viral oncogenes (HPV-E6E7 ORFs) Exp. Cell. Res. 1995;218(2):499–507. doi: 10.1006/excr.1995.1184. [DOI] [PubMed] [Google Scholar]
- Auersperg N, Maines-Bandiera SL, Dyck HG, Kruk PA. Characterization of cultured human ovarian surface epithelial cells: phenotypic plasticity and premalignant changes. Lab. Invest. 1994;71(4):510–518. [PubMed] [Google Scholar]
- Brewer M, et al. In vitro model of normal, immortalized ovarian surface epithelial and ovarian cancer cells for chemoprevention of ovarian cancer. Gynecol. Oncol. 2005;98(2):182–192. doi: 10.1016/j.ygyno.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Sueblinvong T, et al. Establishment, characterization and downstream application of primary ovarian cancer cells derived from solid tumors. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell S. In vivo-in vitro tumour cell lines: characteristics and limitations as models for human cancer. Br. J. Cancer Suppl. 1980;41(4):118–122. [PMC free article] [PubMed] [Google Scholar]
- Wong C, Chen S. The development, application and limitations of breast cancer cell lines to study tamoxifen and aromatase inhibitor resistance. J. Steroid. Biochem. Mol. Biol. 2012;131(3-5):83–92. doi: 10.1016/j.jsbmb.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


