Abstract
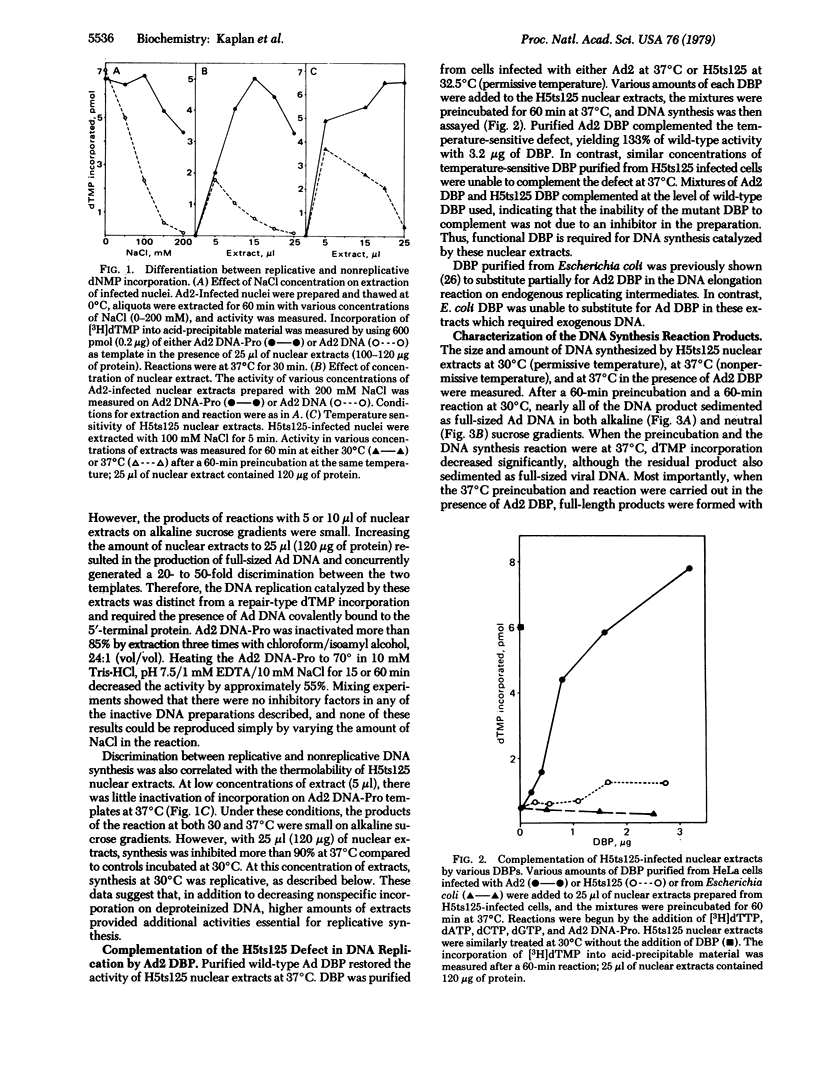
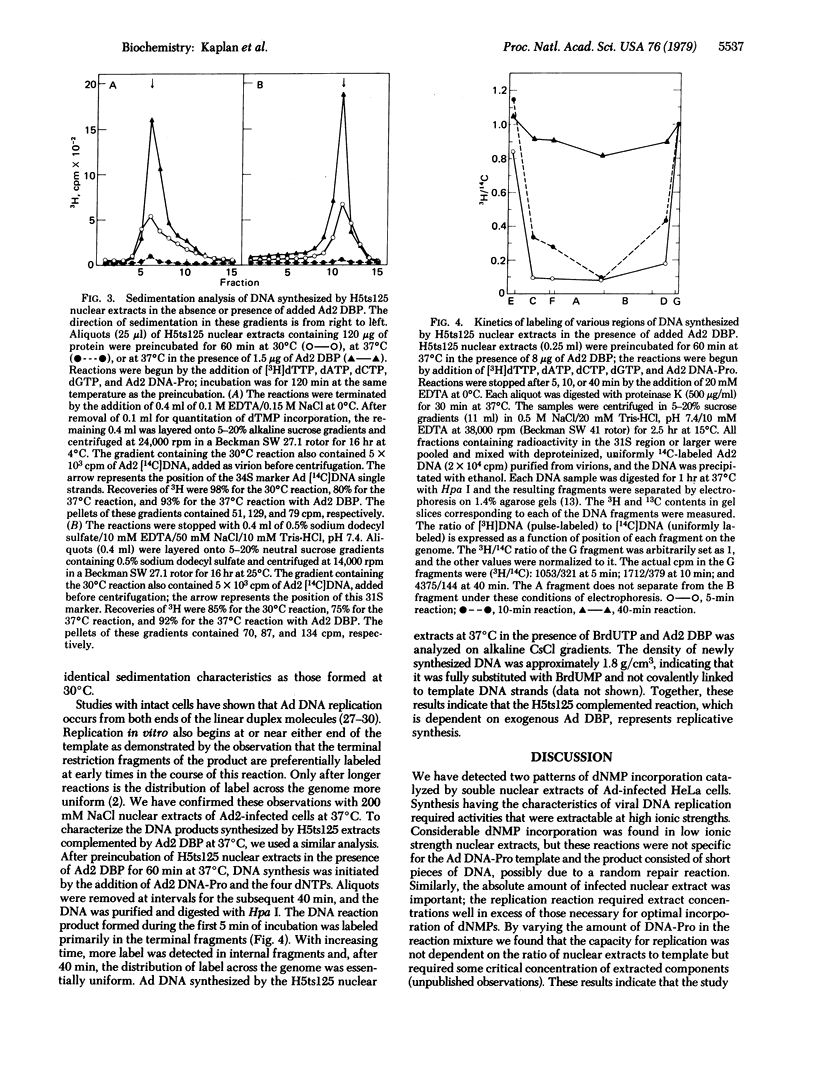
Soluble extracts of adenovirus-infected HeLa cell nuclei support DNA replication on exogenous adenovirus DNA templates. Conditions of synthesis using both wild-type and temperature-sensitive extracts have been defined. Nuclear extracts prepared from cells permissively infected with the adenovirus mutant H5ts125 expressed the temperature-sensitive phenotype and could be inactivated at 37 degrees C in vitro. These extracts were completely complemented by the addition of wild-type adenovirus DNA binding protein but not by H5ts125 DNA binding protein. Enhancement by binding protein in the mutant extracts represents replication, as demonstrated by the production of full-sized products and orderly chain elongation originating, as in vivo, at both ends of the linear DNA. Replicative synthesis required the 5'-terminal protein bound covalently to template DNA and could be inhibited by denaturation of this 55,000-dalton protein. Various inhibitors of eukaryotic DNA polymerases, such as aphidicolin and 2',3'-dideoxythymidine triphosphate, inhibited replication of exogenous adenovirus templates in this system as they do in previously reported systems that only elongate endogenous replicating intermediates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud M. M., Horwitz M. S. The DNA polymerases associated with the adenovirus type 2 replication complex: effect of 2'-3'-dideoxythymidine-5'-triphosphate on viral DNA synthesis. Nucleic Acids Res. 1979 Mar;6(3):1025–1039. doi: 10.1093/nar/6.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kronberg A. Enzymatic replication of phiX174 duplex circles: continuous synthesis. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):295–302. doi: 10.1101/sqb.1979.043.01.036. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Berget S. M., Sharp P. A. Characterization of single-stranded viral DNA sequences present during replication of adenovirus types 2 and 5. Cell. 1976 Dec;9(4 Pt 1):559–571. doi: 10.1016/0092-8674(76)90038-6. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Young C. S. Genetics of adenoviruses. Adv Cancer Res. 1976;23:91–130. doi: 10.1016/s0065-230x(08)60544-8. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Bidirectional replication of adenovirus type 2 DNA. J Virol. 1976 Apr;18(1):307–315. doi: 10.1128/jvi.18.1.307-315.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S. Intermediates in the synthesis of type 2 adenovirus deoxyribonucleic acid. J Virol. 1971 Nov;8(5):675–683. doi: 10.1128/jvi.8.5.675-683.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Kaplan L. M., Abboud M., Maritato J., Chow L. T., Broker T. R. Adenovirus DNA replication in soluble extracts of infected cell nuclei. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):769–780. doi: 10.1101/sqb.1979.043.01.084. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Temperature-sensitive replication of H5ts125 adenovirus DNA in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4291–4295. doi: 10.1073/pnas.75.9.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Kaplan L. M., Kelinman R. E., Horwitz M. S. Replication of adenovirus type 2 DNA in vitro. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4425–4429. doi: 10.1073/pnas.74.10.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedinger C., Gissinger F., Chambon P. Animal DNA-dependent RNA polymerases. Molecular structures and immunological properties of calf-thymus enzyme AI and of calf-thymus and rat-liver enzymes B. Eur J Biochem. 1974 May 15;44(2):421–436. doi: 10.1111/j.1432-1033.1974.tb03500.x. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Burke R. L., Hibner U., Barry J., Alberts B. Probing DNA replication mechanisms with the T4 bacteriophage in vitro system. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):469–487. doi: 10.1101/sqb.1979.043.01.053. [DOI] [PubMed] [Google Scholar]
- Longiaru M., Ikeda J. E., Jarkovsky Z., Horwitz S. B., Horwitz M. S. The effect of aphidicolin on adenovirus DNA synthesis. Nucleic Acids Res. 1979 Jul 25;6(10):3369–3386. doi: 10.1093/nar/6.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Richardson C. C., Romano L. J., Kolodner R., LeClerc J. E., Tamanoi F., Engler M. J., Dean F. B., Richardson D. S. Replication of bacteriophage T7 DNA by purified proteins. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):427–440. doi: 10.1101/sqb.1979.043.01.049. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Sumida-Yasumoto C., Ikeda J. E., Benz E., Marians K. J., Vicuna R., Sugrue S., Zipursky S. L., Hurwitz J. Replication of phiX174 DNA: in vitro synthesis of phiX RFI DNA and circular, single-stranded DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):311–329. doi: 10.1101/sqb.1979.043.01.038. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Kuijk M. G. Studies on the mechanism of replication of adenovirus DNA. V. The location of termini of replication. Virology. 1977 Mar;77(1):149–157. doi: 10.1016/0042-6822(77)90414-7. [DOI] [PubMed] [Google Scholar]
- Van Der Vliet P. C., Levine A. J., Ensinger M. J., Ginsberg H. S. Thermolabile DNA binding proteins from cells infected with a temperature-sensitive mutant of adenovrius defective in viral DNA synthesis. J Virol. 1975 Feb;15(2):348–354. doi: 10.1128/jvi.15.2.348-354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Vliet P. C., Zandberg J., Jansz H. S. Evidence for a function of the adenovirus DNA-binding protein in initiation in DNA synthesis as well as in elongation of nascent DNA chains. Virology. 1977 Jul 1;80(1):98–110. doi: 10.1016/0042-6822(77)90383-x. [DOI] [PubMed] [Google Scholar]
- Vliet P. C., Sussenbach J. S. An adenovirus type 5 gene function required for initiation of viral DNA replication. Virology. 1975 Oct;67(2):415–426. doi: 10.1016/0042-6822(75)90443-2. [DOI] [PubMed] [Google Scholar]
- Weingärtner B., Winnacker E. L., Tolun A., Pettersson U. Two complementary strand-specific termination sites for adenovirus DNA replication. Cell. 1976 Oct;9(2):259–268. doi: 10.1016/0092-8674(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Wist E., Prydz H. The effect of aphidicolin on DNA synthesis in isolated HeLa cell nuclei. Nucleic Acids Res. 1979 Apr;6(4):1583–1590. doi: 10.1093/nar/6.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. Nucleotide sequence analysis of DNA. I. Partial sequence of the cohesive ends of bacteriophage lambda and 186 DNA. J Mol Biol. 1970 Aug;51(3):501–521. doi: 10.1016/0022-2836(70)90004-5. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Kwant M. M. Role of DNA polymerase gamma in adenovirus DNA replication. Nature. 1978 Nov 30;276(5687):532–534. doi: 10.1038/276532a0. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]


