Abstract
Monitoring minimal residual disease is an important way to identify patients with acute myeloid leukemia at high risk of relapse. In this study we investigated the prognostic potential of minimal residual disease monitoring by quantitative real-time polymerase chain reaction analysis of NPM1 mutations in patients treated in the AMLCG 1999, 2004 and 2008 trials. Minimal residual disease was monitored - in aplasia, after induction therapy, after consolidation therapy, and during follow-up - in 588 samples from 158 patients positive for NPM1 mutations A, B and D (with a sensitivity of 10−6). One hundred and twenty-seven patients (80.4%) achieved complete remission after induction therapy and, of these, 56 patients (44.1%) relapsed. At each checkpoint, minimal residual disease cut-offs were calculated. After induction therapy a cut-off NPM1 mutation ratio of 0.01 was associated with a high hazard ratio of 4.26 and the highest sensitivity of 76% for the prediction of relapse. This was reflected in a cumulative incidence of relapse after 2 years of 77.8% for patients with ratios above the cut-off versus 26.4% for those with ratios below the cut-off. In the favorable subgroup according to European LeukemiaNet, the cut-off after induction therapy also separated the cohort into two prognostic groups with a cumulative incidence of relapse of 76% versus 6% after 2 years. Our data demonstrate that in addition to pre-therapeutic factors, the course of minimal residual disease in an individual is an important prognostic factor and could be included in clinical trials for the guidance of post-remission therapy. The trials from which data were obtained were registered at www.clinicaltrials.gov (#NCT01382147, #NCT00266136) and at the European Leukemia Trial Registry (#LN_AMLINT2004_230).
Introduction
Molecular analyses have led to an improvement of the prognostic evaluation of patients with acute myeloid leukemia (AML). The European LeukemiaNet (ELN) has published a classification that identifies prognostic subgroups based on the patients’ cytogenetic and molecular genetic characteristics. At present, therapy decisions are guided by pre-therapeutic risk assessments.1 Nevertheless, there are still patients who suffer from relapse despite belonging to the favorable risk group2,3 and more individualized therapy regimens are needed to prevent relapses.
Monitoring minimal residual disease (MRD) could become an important way to identify patients at high risk of relapse.4 In patients with acute lymphoblastic leukemia or chronic myeloid leukemia, the MRD status routinely guides therapy decisions at different check points.5,6 Patients with acute lymphoblastic leukemia in complete remission after treatment within the GMALL protocol and with a MRD level of >10−4 at week 16 should undergo hematopoietic allogeneic stem cell transplantation (HSCT). Pre-emptive arsenic trioxide therapy in patients with acute promyelocytic leukemia with t(15;17)(q22;q12) PML-RARA, initiated on the basis of increasing MRD levels, was seen to prevent relapses in the MRC 15 trial.7
AML has a wide spectrum of molecular markers.1 Some of these markers can be assessed by quantitative real-time polymerase chain reaction (RT-PCR), e.g. leukemia-specific fusion transcripts, mutated genes, or aberrantly expressed genes. These molecular markers can be monitored with higher sensitivity by RT-PCR than by other methods such as immunophenotyping.4,8
MRD assessments after induction and consolidation therapy showed a significant impact on relapse-free and overall survival and serial MRD assessments throughout the further follow-up could predict impending relapse.9–12 One of the established MRD markers in AML is the mutated Nucleophosmin 1 gene (NPM1). In approximately 30% of all AML patients NPM1 is mutated with the main point mutations A, B, and D which occur in 95% of all NPM1-mutated patients.13,14 Depending on an additional fms-like tyrosine kinase receptor 3 internal tandem duplication (FLT3-ITD) the general prognosis of patients with NPM1 mutations is favorable.1,15 NPM1 mutations are reliable markers for monitoring MRD after conventional chemotherapy, allogeneic HSCT, or during the subsequent follow-up.10,11,16,17 There has been some discussion on the stability of this MRD marker in relapse, but NPM1 mutations could be detected in at least 91% of all relapsed patients.10,18
In this study, we analyzed MRD monitoring by RT-PCR in 158 adult patients with NPM1 A, B and D mutations treated with high-dose cytarabine induction therapies within the AMLCG 1999, 2004 and 2008 trials. We established clinically useful cut-offs at different time points and showed the impact of MRD levels, in terms of NPM1 mutation ratios, on clinical outcome.
Methods
Patients
Since 2005 all AML patients have been routinely retrospectively and prospectively screened for NPM1 mutations by melting curve-based LightCycler assay19 at the Laboratory for Leukemia Diagnostics of the Department of Internal Medicine III, University of Munich, Grosshadern. Patients screened positive for NPM1 mutations (NPM1mut) and with at least one RT-PCR result after the initial diagnosis were included in this retrospective study. All patients were enrolled into one of the following AMLCG trials: AMLCG 1999 (n=91),20 AMLCG 2004 (n=29),21 and AMLCG 2008 (n=38, NCT01382147) and gave written informed consent to treatment and genetic analyses according to the Declaration of Helsinki. All trials were approved by the relevant institutional review boards and ethics committees of the participating institutions.
Treatment, sampling and minimal residual disease monitoring
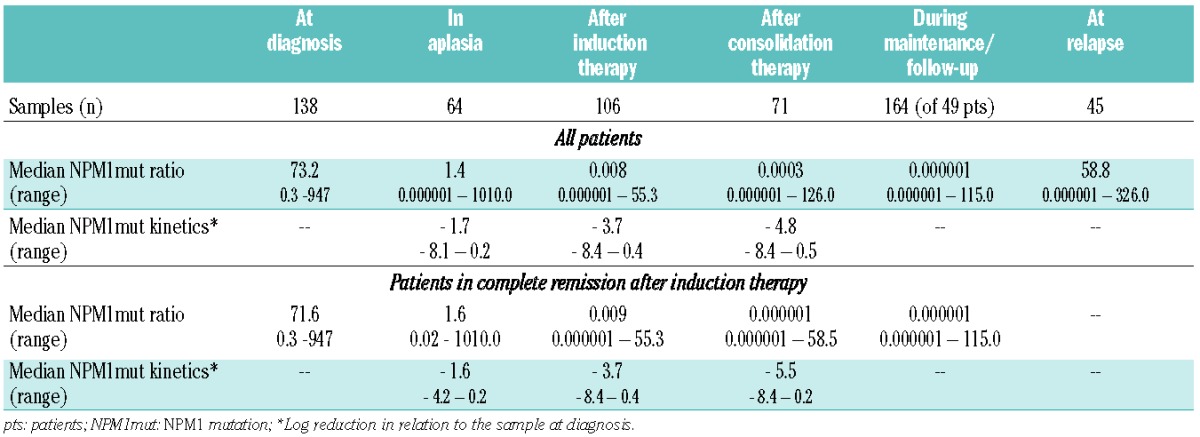
Younger patients, i.e. those under the age of 60, received either sequential high-dose cytarabine with mitoxantrone (sHAM21), or double induction with standard-dose cytarabine, daunorubicin and thioguanine followed by high-dose cytarabine with mitoxantrone (TAD-HAM), or two courses of high-dose cytarabine with mitoxantrone (HAM-HAM20). In older patients, over the age of 60, induction therapy consisted of either reduced dose sHAM21 or one to two courses of intermediate-dose cytarabine with mitoxantrone (HAM-HAM20,21). Post-induction therapy consisted of TAD consolidation (with subsequent maintenance therapy according to the AMLCG standard20,22) and/or HSCT. MRD was monitorined by RT-PCR in 588 bone marrow samples from 158 patients. It was recommended that bone marrow was collected and MRD analyzed at diagnosis, during aplasia within induction therapy, after induction therapy, after consolidation therapy, and every 3 months during the maintenance therapy or after completion of the therapy (Table 1, Online Supplementary Figure S1).
Table 1.
Sampling and median NPM1mut ratios of 158 patients at different MRD checkpoints.
Quantitative assessment of NPM1 mutations A, B, and D
Sample preparation and the conditions of the RT-PCR assay of NPM1mut A were as described by Papadaki et al.18 The primers and probes used for NPM1mut B and D were those published by Gorello et al.,16 while the RT-PCR assay conditions and analyses for these mutations were analogous to those described by Papadaki et al. with a maximum sensitivity of 10−6 in a serial dilution of a NPM1mut-negative cell line. MRD levels of the samples were expressed as a ratio of the NPM1mut normalized to the housekeeping gene ABL1 and divided by the NPM1mut/ABL1 ratio of an internal calibrator (the OCI/AML3 cell line).
Clinical endpoints and statistical analyses
The definitions of the clinical endpoints (relapse-free survival and overall survival) and remission criteria follow the International Working Group guidelines.23 Survival data were censored at the time of HSCT for those patients in whom HSCT was performed in first complete remission. Cut-off values in aplasia, after induction therapy and after consolidation therapy of the absolute NPM1mut ratios and of their kinetics (log difference to NPM1mut ratio at diagnosis) were determined by Cox proportional hazards regression models with respect to the highest hazard ratios and the lowest P values. For the prediction of relapse within an observation period of 100 days during the follow-up, we considered the absolute values before relapse for the patients who did replapse or the peak value of measurements for patients who did not relapse during the follow-up. With the help of receiver-operating characteristics (ROC) curve analysis we selected a cut-off for the prediction of relapse within 100 days in the follow-up period. The Kaplan-Meier estimator and log-rank test were used to calculate survival data. The cumulative incidence of relapse was calculated according to Gray.24
All analyses were performed using the SPSS 21 Windows software package (IBM, Armonk, NY, USA), or the software environment package R, version 3.0.1 (see also Online Supplementary Material).
Results
Patients’ characteristics
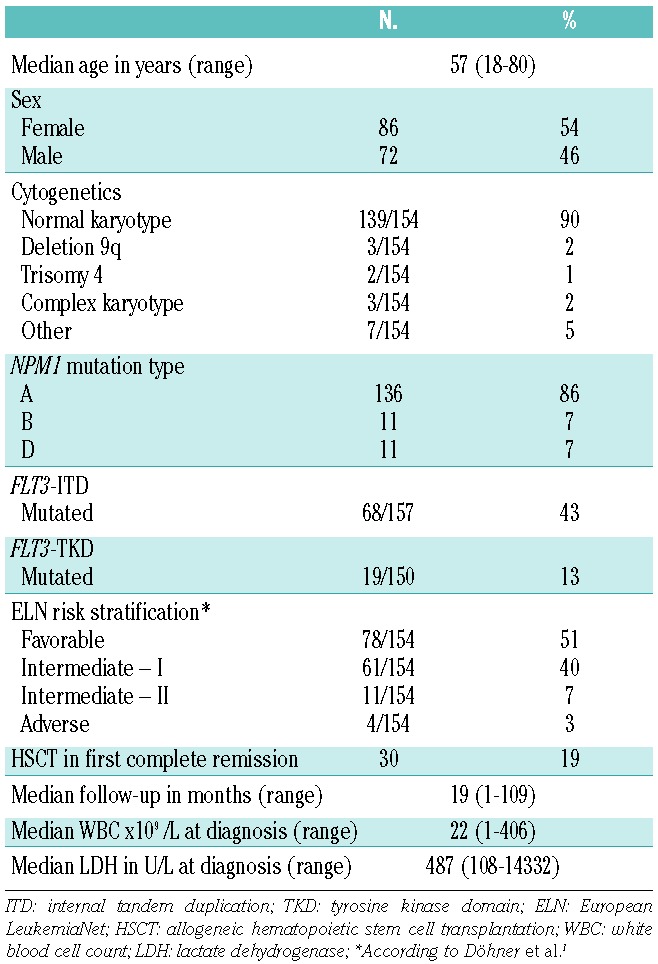
The whole study population consisted of 158 AML patients (median age 57 years, range 18 – 80) with NPM1mut A, B or D. Of these 158 patients, 127 patients achieved a complete remission (80.4%) and 16 an incomplete complete remission (10.1%) after induction therapy. Eight patients (5.1%) had refractory disease, two patients (1.3%) died during induction therapy, and in five patients (3.2%) no remission status was available. HSCT was performed in first complete remission in 30 patients (19%). After a median follow-up of 18.6 months (range, 0.8 – 108.9 months), 56 patients in complete remission had a relapse (44.1%) and 48 patients (30.4%) died. Further characteristics are summarized in Table 2.
Table 2.
Demographics and clinical characteristics of the 158 patients.
NPM1 mutation ratios at different time points
NPM1 mutation ratios at diagnosis
NPM1mut ratios were determined by RT-PCR at diagnosis in 138 bone marrow samples. NPM1mut ratios (median ratio 73.2; range, 0.3 – 947.9, Online Supplementary Figure S2) did not affect the cumulative incidence of relapse (P=0.59), overall survival (P=0.69), or complete remission rate after induction therapy (P=0.78). NPM1mut ratios at diagnosis showed no correlation or association with FLT3-ITD mutation status (P=0.54), ELN risk stratification1 (P= 0.86), blast count (P=0.82), white blood cell count (P=0.76), or lactate dehydrogenase level (P= 0.64).
NPM1 mutation ratios at the time of aplasia during induction therapy
Bone marrow samples from 64 patients in aplasia (day 16 – 18 after the initiation of induction therapy), regardless of the response to induction therapy, were available for analysis. The median bone marrow blast count of all patients during aplasia was 0% (range, 0 – 96%). Patients with refractory disease after induction therapy had a higher blast count in aplasia than patients in complete remission after induction therapy (median 7.5% versus 0%, P=0.03). Bone marrow blast count did not show any impact on cumulative incidence of relapse or overall survival in complete remission patients after induction therapy. Interestingly, neither the absolute NPM1mut ratios nor the NPM1mut kinetics were significantly different between patients who achieved complete remission and patients who did not.
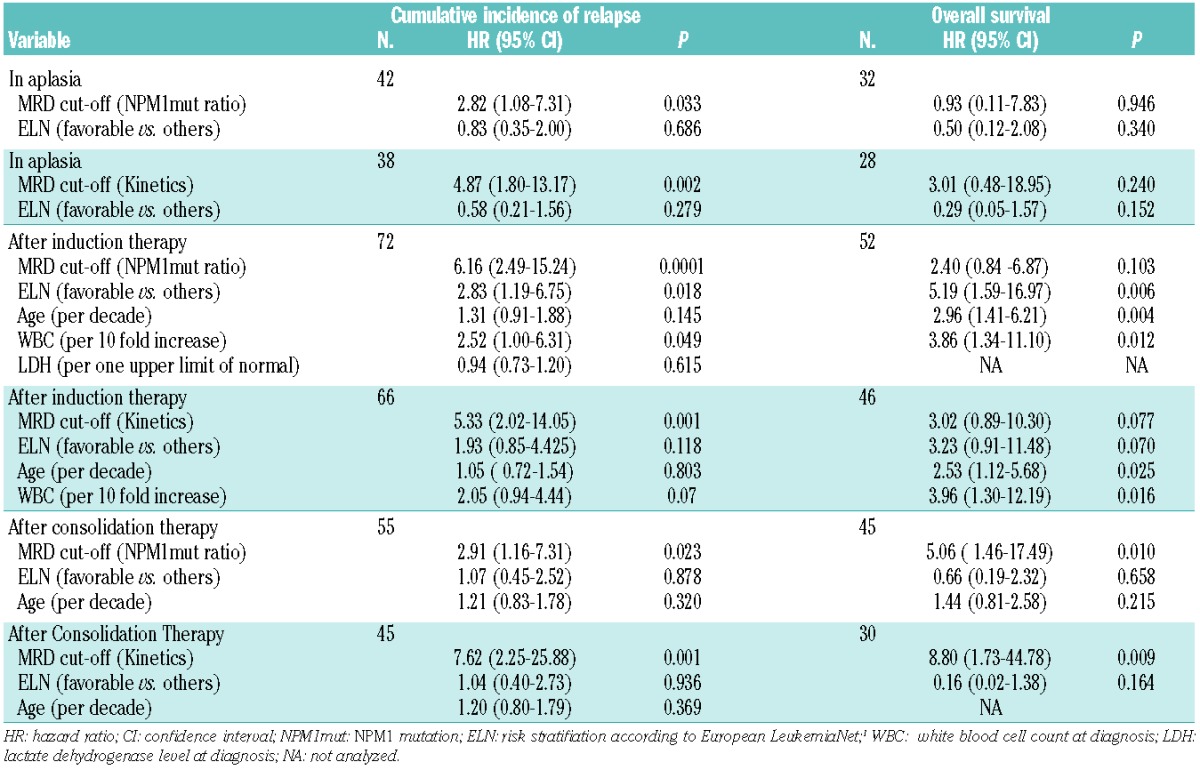
For subsequent analyses only patients who achieved complete remission after induction therapy were analyzed (n=49). At this early stage of treatment all patients still showed detectable RT-PCR signals (Figure 1, Table 1). Patients who stayed in remission showed a trend to having lower NPM1mut ratios and greater NPM1mut log reduction kinetics than those in patients who had a relapse (median NPM1mut ratio 1.00 versus 2.4, P=0.077, and median NPM1mut ratio reduction of −1.6 log versus −1.4 log, P=0.074; Figure 1). To determine the prognostic value of MRD monitoring in complete remission patients, we analyzed Cox regression models. The NPM1mut kinetics but not the absolute levels were significant prognostic indicators for the occurrence of relapse. Clinical cut-offs were determined by Cox proportional hazards regression. A cut-off ratio of 10 for the absolute NPM1mut ratios and a cut-off of -1 log for the kinetics resulted in sensitivities of 29% and 39%, respectively, and specificities of 96% (Table 3). Both cut-offs showed a significant prognostic impact on remission duration [hazard ratio (HR) 2.81, P=0.034, and HR 4.55, P=0.002, respectively] and on cumulative incidence of relapse (Online Supplementary Figure 3A,B). Overall survival was not significantly influenced by MRD levels at this checkpoint (Table 4, Online Supplementary Figure 3C,D). In addition, multivariate analyses revealed that MRD cut-offs at aplasia were significant independent predictors for relapse if combined with the ELN risk stratification (Table 4).
Figure 1.
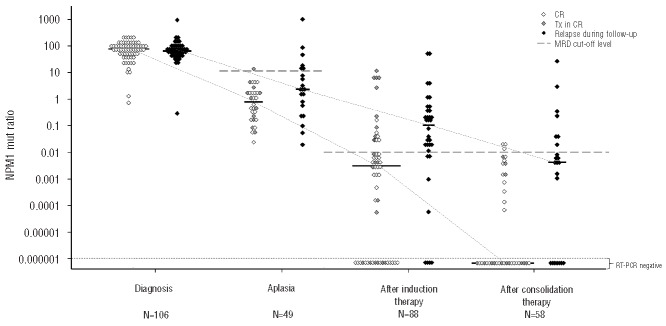
NPM1mut ratios at different checkpoints in patients who achieved complete remission. Black symbols indicate NPM1mut ratios at different checkpoints of patients who relapsed during follow-up, white symbols indicate NPM1mut ratios at different checkpoints of patients with ongoing remission. Gray symbols indicate NPM1mut ratios of patients who subsequently underwent allogeneic stem cell transplantation in first complete remission. Bars indicate median NPM1mut ratios at the specific checkpoint. NPM1mut: NPM1 mutation; RT - PCR: quantitative real-time polymerase chain reaction; CR: NPM1 mutation ratios of patients with ongoing complete remission; Tx in CR: NPM1 mutation ratios of patients who subsequently underwent allogeneic stem cell transplantation in first complete remission.
Table 3.
Results of relapse analyses at different MRD checkpoints.
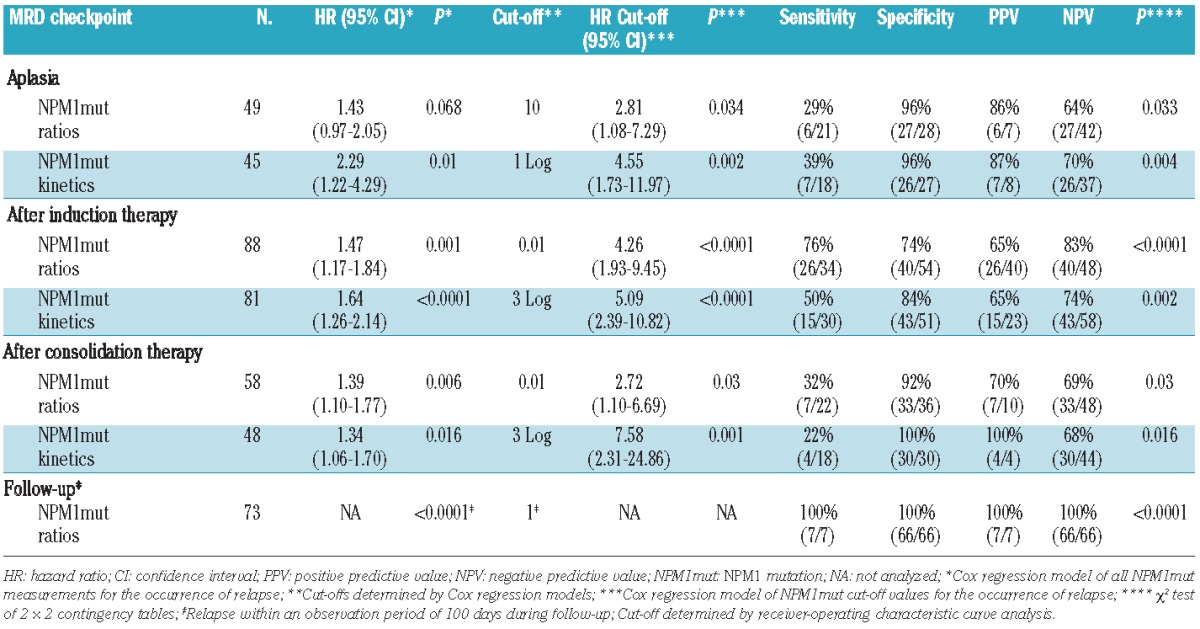
Table 4.
Multivariate analyses.
NPM1 mutation ratios after induction therapy
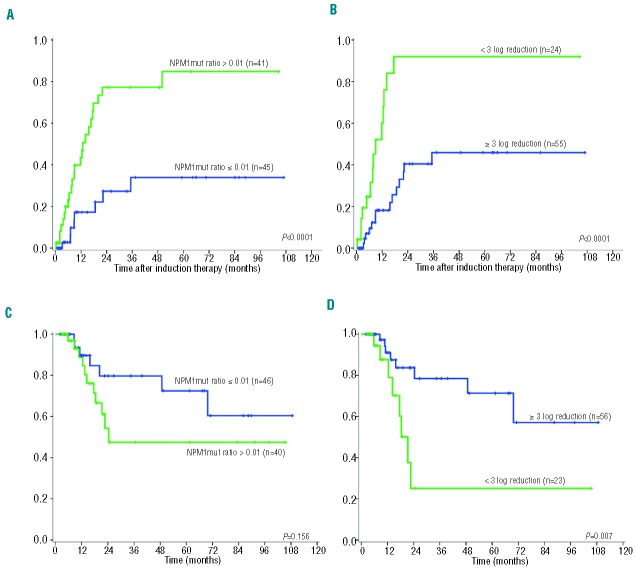
Bone marrow samples from 91 patients in complete remission after induction therapy were available for analysis. Absolute NPM1mut ratios and their kinetics (Table 1) after induction therapy had a significant prognostic impact on the occurrence of relapse (Table 3). Patients with ongoing remission had significantly lower NPM1mut ratios and greater log reductions than patients with relapse during follow-up (median NPM1mut ratio of 0.004 versus 0.11, P=0.001 and median log reduction of −4.4 versus −3.0 log, P=0.001; Figure 1). We determined a cut-off ratio of 0.01 for absolute NPM1mut ratios and a log reduction of −3 log. With these cut-offs we calculated sensitivities of 76% and 50% and specificities of 74% and 81% for the identification of patients at high risk of relapse (Table 3). Both cut-off levels identified patients at risk of relapse (HR 4.26, P<0.0001 for absolute levels, and HR 5.09, P<0.0001 for kinetics, Table 3). The cumulative incidence of relapse after 2 years was 77.8% for MRD cut-off-positive patients and thus significantly higher than that for MRD cut-off-negative patients (26.4%, P<0.0001; Figure 2A,B). This ability to predict relapse persisted in the multivariate model if added to established risk stratification factors: age, ELN risk stratification, white blood cell count, and lactate dehydrogenase level (Table 4). Our cut-off level for absolute NPM1mut ratios showed a trend to affect overall survival (Figure 2C, Table 4) and the cut-off level for kinetics separated two prognostic groups in the Kaplan-Meier plot with statistically significantly different overall survival, although the difference did not reach statistical significance in the multivariate model (Figure 2D, Table 4).
Figure 2.
Cumulative incidence of relapse (A and B) and overall survival (C and D) after induction therapy according to MRD status of NPM1mut ratios and NPM1mut kinetics. (A) and (C) NPM1mut ratios after induction therapy with a NPM1mut cut-off ratio of 0.01; (B) and (D) NPM1mut kinetics after induction therapy with a cut-off of -3 log. NPM1mut: NPM1 mutation.
NPM1 mutation ratios after consolidation therapy
After consolidation therapy 58 patients in complete remission were available for analysis of survival data. The absolute NPM1mut ratios and their kinetics (Table 1) had a significant impact on relapse detection (HR of absolute levels 1.39, P=0.006, and HR of kinetics 1.34, P=0.016, Table 3). In accordance, patients with ongoing remission had significantly lower NPM1mut ratios and greater log reductions than patients with relapse during follow-up (median NPM1mut ratio of 0.00001 versus 0.005, P=0.004 and median log reduction of −6.99 versus −4.39 log, P=0.027, respectively; Figure 1).
Cut-off levels of NPM1mut ratio of 0.01 and of −3 log were established and displayed low sensitivities of 32% and 22% but high specificities of 92% and 100% (Table 3). The cut-off levels separated the cohort into two prognostic groups (Online Supplementary Figure 4A,B) with hazard ratios of 2.72 and 7.58. In the multivariate model with age and ELN risk stratification, the NPM1mut ratios and kinetics exceeding the established cut-off levels were the only prognostic variables for cumulative incidence of relapse and overall survival (Table 4).
NPM1 mutation ratios during follow-up
During the follow-up period, MRD was monitored in 191 bone marrow samples (including the samples after consolidation therapy) from 81 patients in complete remission. In accordance with previously published data on relapse kinetics25 and as a result of individual different MRD monitoring intervals, an evaluation period of 100 days after sampling was chosen for a prediction analysis of relapse. Seventy-three patients in complete remission were available for this prediction analysis. Using ROC analysis a cut-off NPM1mut ratio of 1 was assessed. With this cut-off all patients with an upcoming relapse (sensitivity of 100%) within the next 100 days could be detected prior to relapse with a median time to relapse of 58 days (range, 20 – 98 days). None of the patients with values below this cut-off (specificity of 100%) relapsed in the 100-day observation period (P<0.0001, Table 3).
European LeukemiaNet favorable risk group
Seventy-eight patients (51%) with normal karyotype and no FLT3-ITD were classified as being in the ELN favorable risk group.1 Of these, 80.8% (63 patients) achieved complete remission after induction therapy and 11.5% achieved an incomplete complete remission. Within this group with a favorable prognosis, 45% of the patients had a relapse during the follow-up. In patients in complete remission, there were no differences in relapse rate, remission duration, and overall survival between the prognostically favorable and intermediate I (with FLT3-ITD) groups.
All estimated cut-offs for the whole cohort were confirmed in the ELN favorable subgroup (data not shown). The absolute level of NPM1mut after induction therapy seemed to be the most clinically relevant parameter. At this checkpoint, NPM1mut ratios above the cut-off levels defined patients with an increased risk of relapse as compared to patients with MRD levels below this cut-off (HR 8.59, P=0.005). In accordance with this, the cumulative incidence of relapse was significantly lower in the MRD cut-off negative group (Online Supplementary Figure S6).
Stability of NPM1 mutations at relapse
MRD could be assessed at relapse in 45 patients with a median NPM1mut ratio of 58.8 (range, 0.000001 – 326.0). The NPM1mut ratios at relapse did not differ from those at diagnosis (P=0.25). In three out of the 45 patients (6.7%) no NPM1mut signal could be detected by RT-PCR. One of these patients also lost FLT3-ITD at relapse after 37.6 months and gained a chromosomal aberration t(1;7). In this patient, a DNMT3A mutation present at diagnosis was also detected in the relapse sample. The second patient relapsed after HSCT and lost the initial leukemia-associated immunophenotype at relapse. The third patient relapsed after 5.6 months and gained a JAK2 mutation at relapse (Online Supplementary Table S2).
Impact of consolidation therapy on the course of minimal residual disease
We analyzed the impact of consolidation therapy on the course of MRD in individual patients in complete remission after induction and consolidation therapy. Forty-four patients had paired MRD samples at both checkpoints before and after consolidation therapy (Figure 3).
Figure 3.
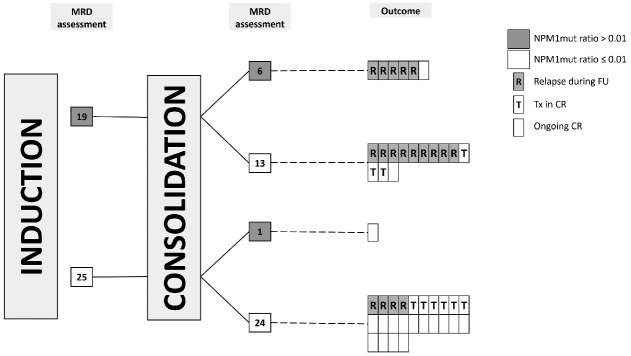
MRD assessment after induction and consolidation therapy in patients with paired samples. In total, 18 patients relapsed after consolidation therapy with a median time to relapse of 9.9 months. Nine patients were transplanted in first complete remission with a median time to allogeneic stem cell transplantation of 1.2 months and 17 patients had an ongoing remission with a median follow-up of 31.1 months after consolidation therapy. NPM1mut: NPM1 mutation; FU: follow-up period; CR: first complete remission; Tx: allogeneic stem cell transplantation in first complete remission.
Nineteen patients showed MRD levels above the estimated cut-off after induction therapy. Six out of these 19 patients stayed MRD-positive (according to the estimated NPM1mut ratio cut-off) after the end of consolidation therapy and five patients relapsed after a median of 4 months (range, 1 – 11 months). The remaining double MRD-positive patient had weakly positive MRD levels at both checkpoints (0.07 and 0.03, respectively) and became RT-PCR negative throughout the maintenance therapy. In 13 patients MRD levels decreased below the estimated cut-off after consolidation therapy. Nevertheless, nine of these patients relapsed, while four remained in complete remission. Three of these four patients underwent HSCT in first complete remission.
Twenty-five patients were MRD cut-off negative after induction therapy and 24 patients stayed MRD cut-off negative after consolidation therapy. Six patients were transplanted in first complete remission and in 14 double MRD cut-off negative patients no relapse occurred after a median follow-up of 26 months (range, 1–105 months). Four double MRD cut-off-negative patients relapsed after a median of 15 months (range, 5–32 months). In one of these patients, MRD sampling 90 days prior to relapse was possible and resulted in a NPM1mut ratio of 8.6, clearly above the follow-up cut-off ratio of 1.
Only one patient was initially MRD cut-off-negative after the induction therapy and showed weakly positive MRD levels after the consolidation therapy (NPM1mut ratio 0.02). The further follow-up sample was RT-PCR-negative and the patient is still in remission after 21 months.
In this analysis of paired samples, MRD status after consolidation therapy in comparison to the MRD status after induction therapy has changed in 14 patients (32%), and in only four patients (29%) did this change in MRD indicate the correct outcome.
Discussion
In this study, we assessed MRD at different checkpoints throughout conventional chemotherapy in 158 AML patients with NPM1 mutations. All patients received intensive, high-dose cytarabine induction therapy within one of three AMLCG trials. Major goals of this study were: (i) to identify relevant assessment checkpoints for MRD and (ii) to establish cut-off levels for the prediction of relapse.
The NPM1mut ratio at diagnosis did not show any impact on survival, or on relapse occurrence. After induction therapy seemed to be the most appropriate checkpoint to assess MRD levels in order to identify patients in complete remission at high risk of relapse. The cut-off values of the NPM1mut ratios and the kinetics of these ratios after induction therapy had high hazard ratios in multivariable analysis and yielded high sensitivity and specificity values. Interestingly, the same cut-off levels after consolidation therapy had less impact on cumulative incidence of relapse than the MRD cut-off after induction therapy. In addition, consolidation therapy showed no clear MRD reduction in MRD cut-off-positive patients after induction therapy. Hence, in this biological subgroup with its slow relapse kinetics,25 a quick and deeper molecular response after induction therapy seems to be more prognostically relevant than persistent MRD positivity after consolidation therapy. Thus, we conclude that MRD levels after induction therapy should be used to identify patients at high risk of relapse.
Krönke et al.,10 and Shayegi et al.11 analyzed clinically relevant MRD checkpoints in NPM1-mutated patients. They, too, identified MRD assessment after induction therapy, or after the achievement of complete remission, as one of the most important MRD checkpoints. They demonstrated that positive MRD levels at this checkpoint identify patients at high risk of relapse and shorter overall suvival. In contrast to Krönke et al. and in accordance with Shayegi et al. we found that a low level of MRD (cut-off ratio of 0.01) after induction therapy is superior to RT-PCR negativity for identifying patients at high risk of relapse (see Online Supplementary Material). During the follow-up period, our estimated cut-off ratio of 1 identified all relapses within the next 100 days after sampling and all patients with lower NPM1mut ratios stayed in remission for the next 100 days. While our analysis confirmed the significant impact of MRD positivity on cumulative incidence of relapse, we observed only a trend for overall survival (Figure 2). This might be the consequence of a smaller cohort of patients, study design, the censored survival data at HSCT, or a short follow-up after relapse.
The monitoring and interpretation of NPM1mut MRD assessments are still restricted to centralized laboratories. As a result of different RT-PCR assays, conditions, treatment protocols and clinical situations, it is very difficult to compare the estimated cut-offs at the different MRD checkpoints. Shayegi et al. established clinical cut-offs by ROC analyses and Cox regression models. After the achievement of complete remission they estimated a cutoff level of 1% and after HSCT a higher cut-off level of 10%.11 We focused our MRD analyses on patients after conventional chemotherapy and identified an NPM1mut ratio of 0.01 as the best cut-off value after induction therapy and a cut-off ratio of 1 during the follow-up period. Only the MRD log reduction can be compared throughout the different MRD studies. Consistent with our data, Schnittger et al.17 also found that a 3-log reduction identifed patients at high risk of relapse. Joint efforts such as the standardization of Wilms’ tumor 1 (WT1) gene expression9 or BCR-ABL5 are needed. Until there is harmonization of NPM1mut RT-PCR assays, enabling subsequent interlaboratory comparison, centralized MRD assessment and interpretation at one laboratory of each study group is obligatory.
The ELN has published a risk stratification considering cytogenetics and molecular genetic analyses at diagnosis.1,3 The ELN favorable risk group of our study cohort is the most clinically interesting subgroup. In contrast to the intermediate I group (with an additional FLT3-ITD) HSCT is usually not recommended in these patients.26 However, in our cohort of patients, 45% of the NPM1mut patients without FLT3-ITD relapsed and we found no significant differences in relapse rate, remission duration, and overall survival between the ELN favorable and intermediate I groups. This might be because of the heterogeneous age of patients within the AMLCG trials, which had no age restrictions. Depending on the quantity of FLT3-ITD mRNA,27 FLT3-ITD might have a prognostic impact in NPM1mut AML and require a more individualized treatment. In other hematologic malignancies, such as acute lymphoblastic leukemia and chronic myeloid leukemia, MRD-guided therapies are already established. Pre-emptive therapy based on MRD monitoring in patients with acute promyelocytic leukemia improved the outcome of patients in the MRC AML15 trial, in a historical comparison to previous trials.7 However, only a few prospective studies with MRD-guided therapies are available in non-acute promyelocytic forms of AML.28–30
Our results and those of others demonstrate the feasibility and reliability of serial NPM1mut MRD monitoring to identify patients at high risk of relapse.10,11,19 Since HSCT in NPM1mut patients does not impair the relapse-free survival26 and, as shown in our analyses, MRD cutoff-positive patients are at high risk of relapse, these patients should be considered for more intensified post-remission therapy, to improve the relapse rate and overall survival, and should undergo HSCT, if eligible.
Instability of MRD markers is a potential problem of MRD-guided therapy and FLT3-ITD can be unstable during follow-up.31,32 The stability of NPM1mut has been debated. In the relapse samples analyzed by Schnittger et al.17 all relapsed patients showed the initial NPM1mut at relapse. This is in contrast to our results and those of Krönke et al.,10 with mutated NPM1 being undetectable at relapse in 6.7% and 9% of patients, respectively. In our analyses, one of the three relapsed patients with undetectable NPM1mut at relapse did relapse after more than 2 years with different chromosomal aberrations and a different leukemia-associated immunophenotype, but with a stable DNMT3A mutation.33 The other two patients relapsed within 6 months after achieving complete remission. One patient also showed the initial leukemia-associated immunophenotype with an additional JAK2 mutation at relapse (Online Supplementary Table S2). Thus, in these patients a clonal evolution or a relapse of a subclone of the initial leukemia is very likely. Considering this aspect, monitoring a second, stable molecular marker, e.g. DNMT3A,33 or monitoring MRD by an alternative method, e.g. flow cytometry, may improve the relapse prediction rate and lower the rate of false negative MRD results.
In conclusion, our results showed the prognostic impact of NPM1 MRD monitoring by RT-PCR. MRD monitoring can identify patients at high risk of relapse, especially in the clinically relevant subgroup of the ELN favorable-risk patients. Particularly high MRD levels above our estimated cut-off after induction therapy were strongly associated with a high cumulative incidence of relapse. This and data previously published by others demonstrate that, in addition to pre-therapeutic prognostic factors, the individual course of MRD should be used as new prognostic factor for the guidance of treatment and patients with high or increasing levels of MRD should undergo HSCT, if eligible.
Acknowledgments
We thank all participating centers of the AMLCG trials. This work was supported by the Deutsche Krebshilfe e.V. and Collaborative Research Center 684 Molecular Mechanisms of Normal and Malignant Hematopoiesis, projects A12.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74 [DOI] [PubMed] [Google Scholar]
- 2.Estey EH. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88(4):318–27 [DOI] [PubMed] [Google Scholar]
- 3.Rollig C, Bornhauser M, Thiede C, Taube F, Kramer M, Mohr B, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29(20):2758–65 [DOI] [PubMed] [Google Scholar]
- 4.Paietta E. Minimal residual disease in acute myeloid leukemia: coming of age. Hematology Am Soc Hematol Educ Program. 2012;2012:35–42 [DOI] [PubMed] [Google Scholar]
- 5.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruggemann M, Raff T, Flohr T, Gokbuget N, Nakao M, Droese J, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116–23 [DOI] [PubMed] [Google Scholar]
- 7.Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27(22):3650–8 [DOI] [PubMed] [Google Scholar]
- 8.Kern W, Haferlach C, Haferlach T, Schnittger S. Monitoring of minimal residual disease in acute myeloid leukemia. Cancer. 2008;112(1):4–16 [DOI] [PubMed] [Google Scholar]
- 9.Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27(31):5195–201 [DOI] [PubMed] [Google Scholar]
- 10.Kronke J, Schlenk RF, Jensen KO, Tschurtz F, Corbacioglu A, Gaidzik VI, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29(19):2709–16 [DOI] [PubMed] [Google Scholar]
- 11.Shayegi N, Kramer M, Bornhauser M, Schaich M, Schetelig J, Platzbecker U, et al. The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood. 2013;122(1):83–92 [DOI] [PubMed] [Google Scholar]
- 12.Yin JA, O’Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120(14):2826–35 [DOI] [PubMed] [Google Scholar]
- 13.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352(3):254–66 [DOI] [PubMed] [Google Scholar]
- 14.Falini B, Sportoletti P, Martelli MP. Acute myeloid leukemia with mutated NPM1: diagnosis, prognosis and therapeutic perspectives. Curr Opin Oncol. 2009;21(6):573–81 [DOI] [PubMed] [Google Scholar]
- 15.Thiede C, Koch S, Creutzig E, Steudel C, Illmer T, Schaich M, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood. 2006;107(10):4011–20 [DOI] [PubMed] [Google Scholar]
- 16.Gorello P, Cazzaniga G, Alberti F, Dell’Oro MG, Gottardi E, Specchia G, et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia. 2006;20(6):1103–8 [DOI] [PubMed] [Google Scholar]
- 17.Schnittger S, Kern W, Tschulik C, Weiss T, Dicker F, Falini B, et al. Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood. 2009;114(11):2220–31 [DOI] [PubMed] [Google Scholar]
- 18.Papadaki C, Dufour A, Seibl M, Schneider S, Bohlander SK, Zellmeier E, et al. Monitoring minimal residual disease in acute myeloid leukaemia with NPM1 mutations by quantitative PCR: clonal evolution is a limiting factor. Br J Haematol. 2009;144(4):517–23 [DOI] [PubMed] [Google Scholar]
- 19.Schnittger S, Schoch C, Kern W, Mecucci C, Tschulik C, Martelli MF, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106(12):3733–9 [DOI] [PubMed] [Google Scholar]
- 20.Buchner T, Berdel WE, Schoch C, Haferlach T, Serve HL, Kienast J, et al. Double induction containing either two courses or one course of high-dose cytarabine plus mitoxantrone and postremission therapy by either autologous stem-cell transplantation or by prolonged maintenance for acute myeloid leukemia. J Clin Oncol. 2006;24(16):2480–9 [DOI] [PubMed] [Google Scholar]
- 21.Braess J, Spiekermann K, Staib P, Gruneisen A, Wormann B, Ludwig WD, et al. Dose-dense induction with sequential high-dose cytarabine and mitoxantone (S-HAM) and pegfilgrastim results in a high efficacy and a short duration of critical neutropenia in de novo acute myeloid leukemia: a pilot study of the AMLCG. Blood. 2009;113(17):3903–10 [DOI] [PubMed] [Google Scholar]
- 22.Buchner T, Hiddemann W, Wormann B, Loffler H, Gassmann W, Haferlach T, et al. Double induction strategy for acute myeloid leukemia: the effect of high-dose cytarabine with mitoxantrone instead of standard-dose cytarabine with daunorubicin and 6-thioguanine: a randomized trial by the German AML Cooperative Group. Blood. 1999;93(12):4116–24 [PubMed] [Google Scholar]
- 23.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9 [DOI] [PubMed] [Google Scholar]
- 24.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988:1141–54 [Google Scholar]
- 25.Ommen HB, Schnittger S, Jovanovic JV, Ommen IB, Hasle H, Ostergaard M, et al. Strikingly different molecular relapse kinetics in NPM1c, PML-RARA, RUNX1-RUNX1T1, and CBFB-MYH11 acute myeloid leukemias. Blood. 2010;115(2):198–205 [DOI] [PubMed] [Google Scholar]
- 26.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18 [DOI] [PubMed] [Google Scholar]
- 27.Schneider F, Hoster E, Unterhalt M, Schneider S, Dufour A, Benthaus T, et al. The FLT3ITD mRNA level has a high prognostic impact in NPM1 mutated, but not in NPM1 unmutated, AML with a normal karyotype. Blood. 2012;119(19):4383–6 [DOI] [PubMed] [Google Scholar]
- 28.Zhu HH, Zhang XH, Qin YZ, Liu DH, Jiang H, Chen H, et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood. 2013;121(20):4056–62 [DOI] [PubMed] [Google Scholar]
- 29.Sockel K, Wermke M, Radke J, Kiani A, Schaich M, Bornhauser M, et al. Minimal residual disease-directed preemptive treatment with azacitidine in patients with NPM1-mutant acute myeloid leukemia and molecular relapse. Haematologica. 2011;96(10):1568–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26(3):381–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholl S, Loncarevic IF, Krause C, Kunert C, Clement JH, Höffken K. Minimal residual disease based on patient specific Flt3-ITD and -ITT mutations in acute myeloid leukemia. Leuk Res. 2005;29(7):849–53 [DOI] [PubMed] [Google Scholar]
- 32.Cloos J, Goemans BF, Hess CJ, van Oostveen JW, Waisfisz Q, Corthals S, et al. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia. 2006;20(7):1217–20 [DOI] [PubMed] [Google Scholar]
- 33.Kronke J, Bullinger L, Teleanu V, Tschurtz F, Gaidzik VI, Kuhn MW, et al. Clonal evolution in relapsed NPM1-mutated acute myeloid leukemia. Blood. 2013;122(1):100–8 [DOI] [PubMed] [Google Scholar]