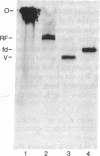
Abstract
The double-stranded replicative form (RF) DNA of the autonomous parvovirus H-1 can be isolated from infected cells in a covalent complex with protein. The protein is present on most or all of the RF DNA, including actively replicating molecules, and is associated with the 5'-terminal endonuclease Hae III fragments of both the viral and complementary strands of RF. The size of the protein is estimated to be 60,000-70,000 daltons from its effect on buoyant density of DNA. DNA with covalently bound protein has not been found in H-1 virions.
Full text
PDF
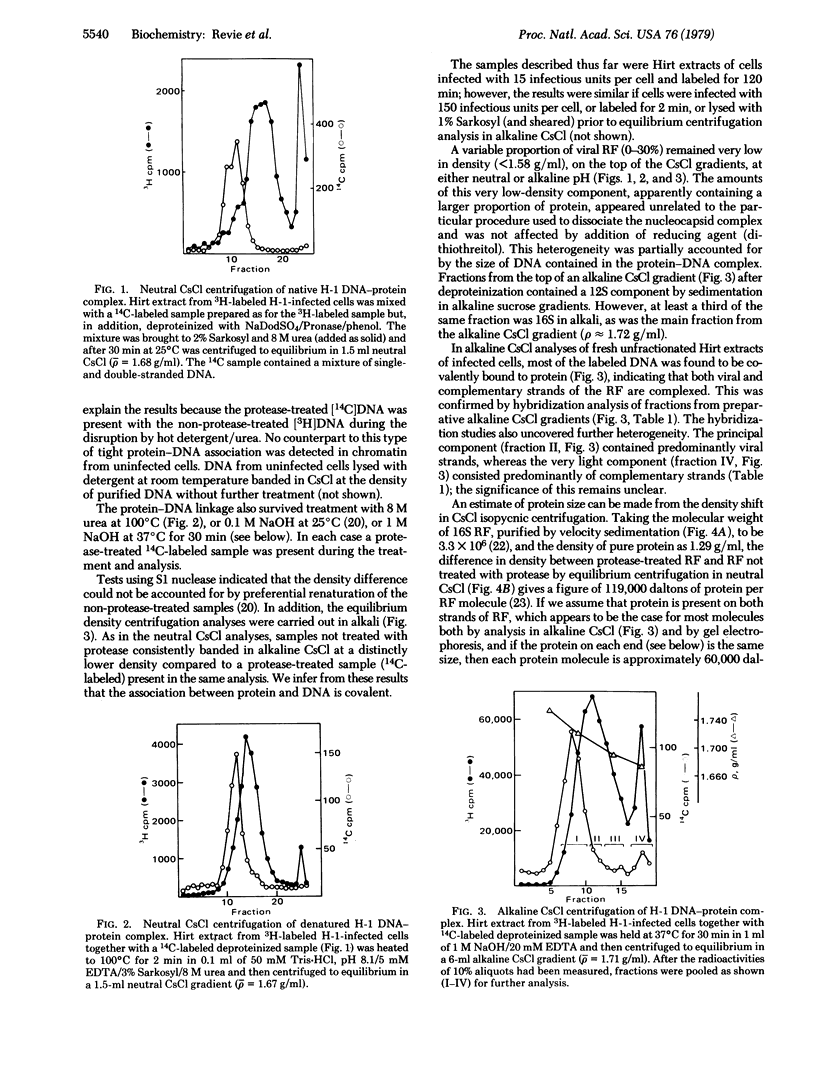
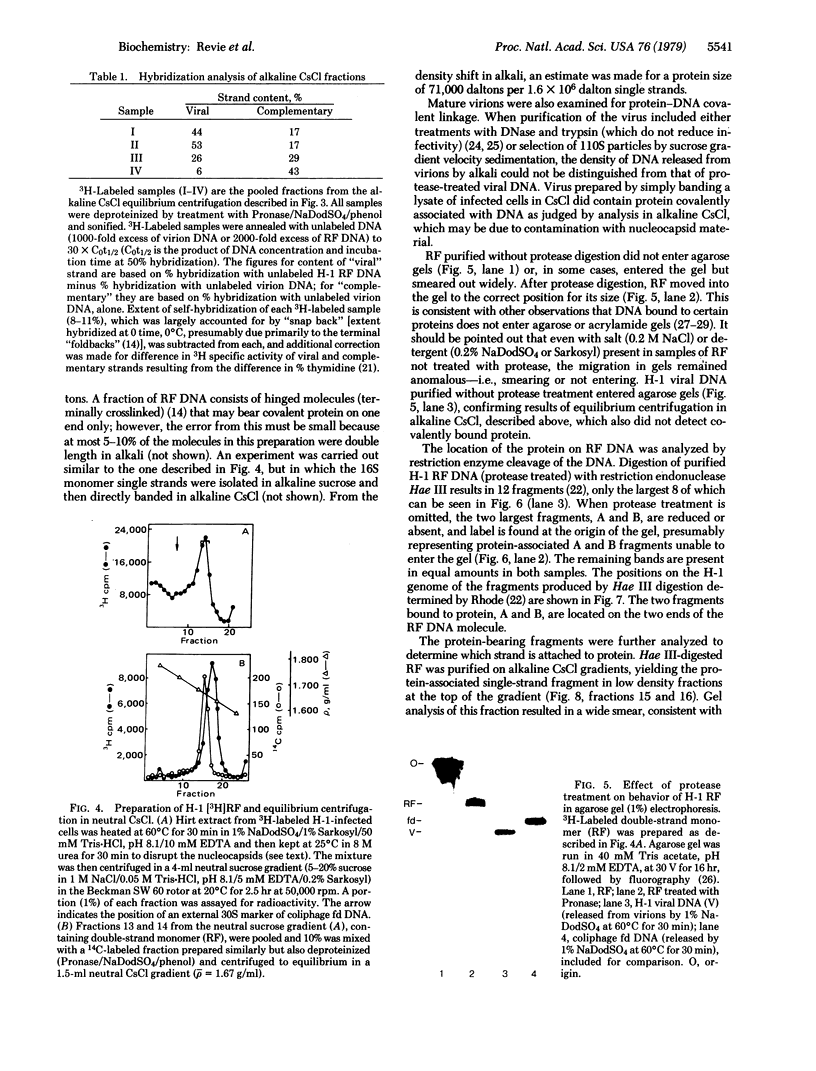
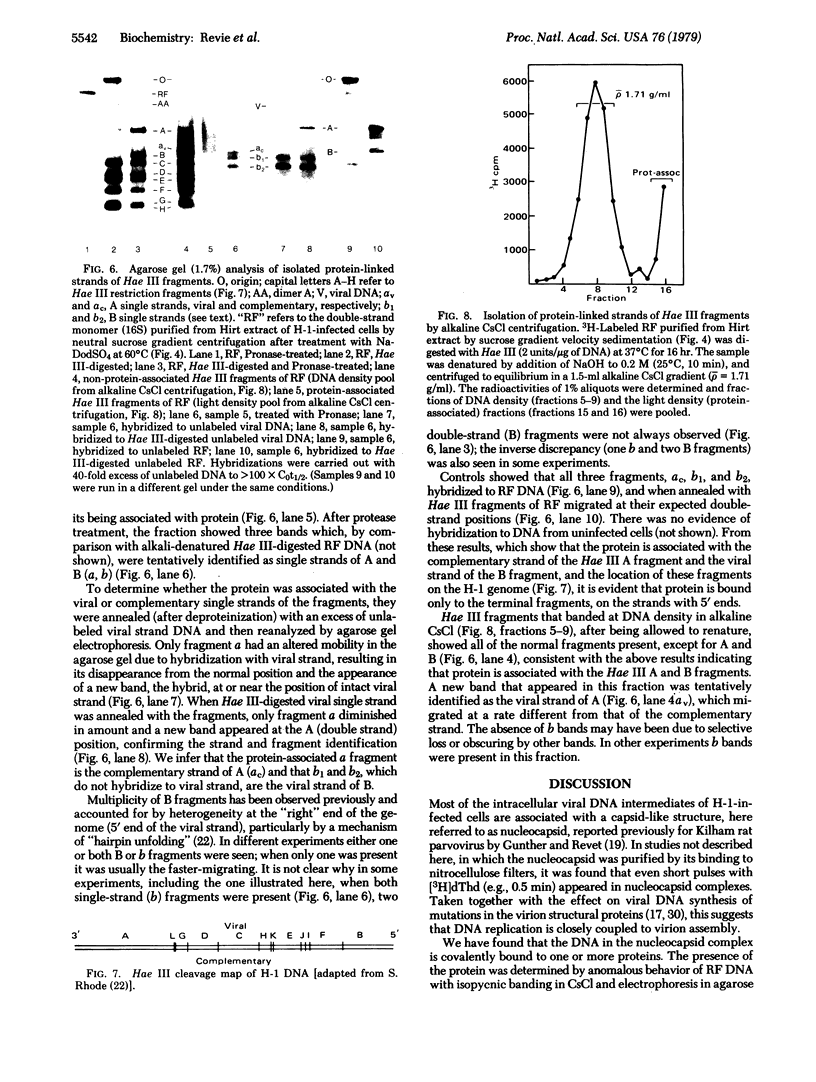

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourguignon G. J., Tattersall P. J., Ward D. C. DNA of minute virus of mice: self-priming, nonpermuted, single-stranded genome with a 5'-terminal hairpin duplex. J Virol. 1976 Oct;20(1):290–306. doi: 10.1128/jvi.20.1.290-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J. Strand breakage by the DNA untwisting enzyme results in covalent attachment of the enzyme to DNA. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3800–3804. doi: 10.1073/pnas.74.9.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Hayashi M. The parvovirus MVM: a comparison of heavy and light particle infectivity and their density conversion in vitro. Virology. 1976 Oct 1;74(1):57–63. doi: 10.1016/0042-6822(76)90127-6. [DOI] [PubMed] [Google Scholar]
- Depew R. E., Liu L. F., Wang J. C. Interaction between DNA and Escherichia coli protein omega. Formation of a complex between single-stranded DNA and omega protein. J Biol Chem. 1978 Jan 25;253(2):511–518. [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golini F., Nomoto A., Wimmer E. The genome-linked protein of picornaviruses. IV. Difference in the VPg's of encephalomyocarditis virus and poliovirus as evidence that the genome-linked proteins are virus-coded. Virology. 1978 Aug;89(1):112–118. doi: 10.1016/0042-6822(78)90045-4. [DOI] [PubMed] [Google Scholar]
- Guiney D. G., Helinski D. R. Relaxation complexes of poasmid DNA and protein. III. Association of protein with the 5' terminus of the broken DNA strand in the relaxed complex of plasmid ColE1. J Biol Chem. 1975 Nov 25;250(22):8796–8803. [PubMed] [Google Scholar]
- Harding N. E., Ito J., David G. S. Identification of the protein firmly bound to the ends of bacteriophage phi 29 DNA. Virology. 1978 Feb;84(2):279–292. doi: 10.1016/0042-6822(78)90248-9. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Roberts W. K. Encephalomyocarditis virus RNA. III. Presence of a genome-associated protein. J Virol. 1978 Jan;25(1):413–415. doi: 10.1128/jvi.25.1.413-415.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciarte M. R., Lázaro J. M., Salas M., Vińuela E. Physical map of bacteriophage phi29 DNA. Virology. 1976 Oct 15;74(2):314–323. [PubMed] [Google Scholar]
- Ito J., Kawamura F., Yanofsky S. Analysis of phi 29 and phi 15 genomes by bacterial restriction endonucleases, EcoR1 and Hpal. Virology. 1976 Mar;70(1):37–51. doi: 10.1016/0042-6822(76)90234-8. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Wu M. Structure of a nicked DNA-protein complex isolated from simian virus 40: covalent attachment of the protein to DNA and nick specificity. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1945–1949. doi: 10.1073/pnas.73.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Morris N. R. Assembly of SV40 chromatin in a cell-free system from Xenopus eggs. Cell. 1977 Feb;10(2):237–243. doi: 10.1016/0092-8674(77)90217-3. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Relaxation complexes of plasmid DNA and protein. II. Characterization of the proteins associated with the unrelaxed and relaxed complexes of plasmid ColE1. J Biol Chem. 1975 Nov 25;250(22):8790–8795. [PubMed] [Google Scholar]
- McGeoch D. J., Crawford L. V., Follett E. A. The DNAs of three parvoviruses. J Gen Virol. 1970 Jan;6(1):33–40. doi: 10.1099/0022-1317-6-1-33. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Ambros V., Baltimore D. Identification of a protein linked to nascent poliovirus RNA and to the polyuridylic acid of negative-strand RNA. J Virol. 1978 Aug;27(2):357–365. doi: 10.1128/jvi.27.2.357-365.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1 V. Isolation and characterization of temperature-sensitive H-1 mutants. J Virol. 1976 Feb;17(2):659–667. doi: 10.1128/jvi.17.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. II. Isolation and characterization of H-1 replicative form DNA. J Virol. 1974 Feb;13(2):400–410. doi: 10.1128/jvi.13.2.400-410.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. IX. Physical mapping studies of the H-1 genome. J Virol. 1977 May;22(2):446–458. doi: 10.1128/jvi.22.2.446-458.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. VI. Characterization of a replication terminus of H-1 replicative-form DNA. J Virol. 1977 Feb;21(2):694–712. doi: 10.1128/jvi.21.2.694-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. X. Isolation of a mutant defective in replicative-form DNA replication. J Virol. 1978 Jan;25(1):215–223. doi: 10.1128/jvi.25.1.215-223.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. J., Bellett J. D. A circular DNA-protein complex adenoviruses and its possible role in DNA replication. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):523–531. doi: 10.1101/sqb.1974.039.01.064. [DOI] [PubMed] [Google Scholar]
- Salas M., Mellado R. P., Viñuela E. Characterization of a protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1978 Feb 25;119(2):269–291. doi: 10.1016/0022-2836(78)90438-2. [DOI] [PubMed] [Google Scholar]
- Salzman L. A., Koczot F. Isolation of nucleoprotein from the parvovirus KRV. Virology. 1978 Feb;84(2):434–442. doi: 10.1016/0042-6822(78)90260-x. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Replication process of the parvovirus H-1. VIII. Partial denaturation mapping and localization of the replication origin of H-1 replicative-form DNA with electron microscopy. J Virol. 1977 Feb;21(2):724–731. doi: 10.1128/jvi.21.2.724-731.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Rottier P., Davies J. W., Zabel P., Van Kammen A. A protein linked to the 5' termini of both RNA components of the cowpea mosaic virus genome. Nucleic Acids Res. 1978 Dec;5(12):4505–4522. doi: 10.1093/nar/5.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Sebring E. D., Rose J. A. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Ward D. C. Rolling hairpin model for replication of parvovirus and linear chromosomal DNA. Nature. 1976 Sep 9;263(5573):106–109. doi: 10.1038/263106a0. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Grafstrom R. H., Revie D., Oertel W., Goulian M. Studies on early intermediates in the synthesis of DNA in animal cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):263–270. doi: 10.1101/sqb.1979.043.01.032. [DOI] [PubMed] [Google Scholar]
- Wu M., Davidson N., Wimmer E. An electron microscope study of the proteins attached to polio virus RNA and its replicative form (RF). Nucleic Acids Res. 1978 Dec;5(12):4711–4723. doi: 10.1093/nar/5.12.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]