Chromosomal translocations of the mixed-lineage leukemia (MLL) gene are common genetic events in acute leukemias. In acute myeloid leukemia (AML), the translocation t(9;11)(p22;q23) [subsequently referred to as t(9;11)], resulting in the MLL-MLLT3-fusion protein, is the most common translocation involving MLL. Translocation (9;11) can be found in both de novo and therapy-related AML (t-AML) and the current WHO classification separates these two subtypes into distinct categories.1 De novo and t-AML generally differ in terms of treatment response and survival with therapy-related cases being associated with inferior outcome and abnormal and more complex cytogenetics.1,2 However, little is known about the biological and clinical difference between de novo and therapy-related MLL-MLLT3-rearranged AML.
The exposure to chemo-/radiation therapy as it occurs in t-AML is hypothesized to alter the number of copy number alterations (CNAs) that could potentially reflect biological differences between these AML subtypes. Furthermore, cooperating genetic events have been suggested to be required for MLL-leukemogenesis, since knock-in mice that express the MLL-MLLT3-fusion gene under control of the MLL promoter develop AML with a leukemia onset that ranges between four months to over one year.3
To explore number and type of cooperating genetic lesions in AML with t(9;11), we performed SNP-array based genotyping of 40 diagnostic leukemia samples from de novo and t-AML patients (de novo, n=22; t-AML, n=16; unknown, n=2) (Online Supplementary Table S1). Intra-individual germline DNA from remission bone marrow or peripheral blood was available for paired analysis in 15 cases. In order to minimize false positive calls, lesions from the unpaired cohort were considered truly somatically required, only if they were found to be altered in at least one sample from the paired cohort. SNP-array (GeneChip Human Mapping 6.0; Affymetrix)-based genotyping and data analysis were performed as previously described.4 Raw data were made publicly available at the NCBI Gene Expression Omnibus (series accession number GSE46745).
Paired analysis of the 15 cases revealed few somatic genetic alterations with only 1.73 CNAs per case (range 0–13). Deletions were more common than gains (de novo: 0.75 losses/case vs. 0.38 gains/case; t-AML: 2.29 losses/case vs. 0.14 gains/case, all mean) and 53% of all cases lacked any detectable CNAs (de novo: 50%; t-AML: 57%). Notably, no significant difference was observed in the mean number of CNAs between de novo and t-AML (de novo: 1.13/case vs. t-AML: 2.43/case; P=0.78). Only one single t-AML case exhibited 13 CNAs (case MLL#3; Online Supplementary Table S2). These findings clearly distinguish t-AML with t(9;11) from other t-AML entities that frequently exhibit additional chromosomal alterations,2 although the statistical power of this analysis might be limited by the small collection of paired samples. The relatively small number of CNAs in de novo cases is consistent with prior reports on distinct types of de novo AML5 and MLL-AF4-rearranged acute lymphoblastic leukemia (ALL)6 but stands in clear contrast to most other ALL subtypes.7
Despite the small number of CNAs identified, we were able to detect several recurrent lesions (Table 1 and Online Supplementary Tables S2 and S3). The most common cytogenetic event was trisomy 8 detected in 20% of the 40 cases (de novo: 27%; t-AML: 6%; P=0.21) (Table 1 and Figure 1A). Besides focal recurrent deletions and gains adjacent to the genes involved in t(9;11) (Table 1), we identified two novel minimally altered regions (MARs) at chromosomal bands 7q36.1-q36.2 (loss: 5%; de novo: 5%; t-AML: 6%) and 13q21.33-q22.1 (gain: 5%; de novo: 5%; t-AML: 6%) (Table 1). Both regions contain genes whose alterations may cooperate with the translocation-encoded MLL-MLLT3-fusion protein to induce overt leukemia. Del(7q) is a recurrent alteration frequently seen in t-AML and complex karyotype AML but also occurs in other AML subtypes such as the Core-Binding Factor (CBF)-AMLs.4,8 The identified MAR at 7q36 contains only 6 genes (Table 1 and Figure 1B) and is partly overlapping with the MAR that we previously described at 7q36.1 in CBF-AMLs.4 Of note, the overlapping region contained only the MLL3 gene. MLL3 is one of five members of the MLL gene family that encode H3K4 methyltransferases with homology to the Drosophila-trithorax gene and have all been implicated in cancer.9 The gain(13)(q21.33-q22.1) affected six genes (Table 1 and Figure 1C). Of these, the two genes DIS3 and KLF5 were previously implicated in oncogenesis. KLF5 is a member of the krüppel-like factor transcription factor family critically involved in regulation of cell proliferation and differentiation.10 It promotes cell proliferation in various tissue types such as gastrointestinal epithelial cells and embryonic stem cells11 and is found to be over-expressed in gastric carcinoma.12 To explore the role of KLF5 gene expression in MLL-MLLT3-rearranged AML, we performed Affymetrix HGU133 plus 2.0 microarray analysis in 20 cases (10 cases with and 10 cases without available SNP-array data). Cases were dichotomized into high and low KLF5 expression cases based on the median expression of KLF5. In the retrospective survival analysis of this highly selected sample collection, high KLF5 expression was associated with inferior overall survival (Online Supplementary Table S4 and Figure S1). Inactivation mutations of DIS3, another gene located within the gain(13q), were recently reported in relapsed AML.13 Notably, in the total cohort of 40 cases, no single region of CN-LOH was identified. This finding is in clear contrast to other AML subtypes where CN-LOH is more common14 and suggests that the mechanism of CN-LOH seems not to play a critical role in MLL-MLLT3-rearranged AML. Sequence analysis of selected AML candidate disease genes revealed mutations in FLT3 (ITD, 2 of 36, 6%; TKD: 3 of 29, 10%; NPM1: 2 of 31, 7%) at a low incidence, whereas mutations in IDH1/2 (0 of 29), DNMT3A (0 of 19), TET2 (0 of 15), and NRAS (0 of 6) were absent. In contrast, deregulated EVI1 expression was a frequent event affecting 53% of t(9;11)-positive AML patients.
Table 1.
Recurrent copy number alterations (CNAs) in 40 de novo and therapy-related acute myeloid leukemia cases with t(9;11).
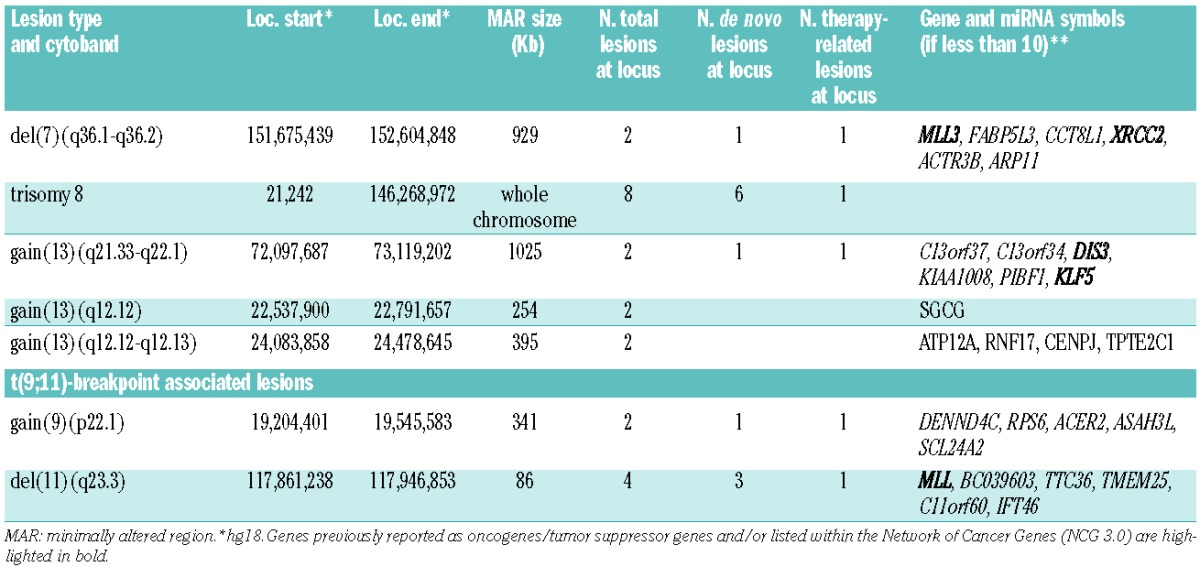
Figure 1.
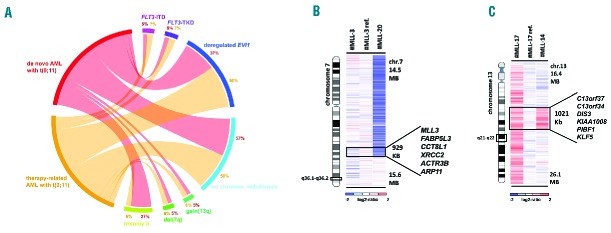
(A) Circos-plot illustration of recurrent secondary genetic alterations in 22 de novo (red) and 16 t-AML cases (orange) with t(9;11). Frequencies of respective lesions are indicated in percent [deregulated EVI1 = over-expressed EVI1; no chromos. imbalances = cases lacking any CNAs]. (B and C) Heat map of recurrently altered regions in t(9;11)-positive AML. (B) Log2-ratio SNP copy number data of deletion 7q36.1-q36.2 involving MLL3 showing one large and one micro-deletion [ref.: paired normal control; minimally altered region (MAR) containing 6 genes as indicated]. (C) Gain of 13q21.33-q22.1 involving KLF5 and DIS3 [ref.: paired normal control; MAR is defined by case #MLL-14 including 6 genes].
This finding is consistent with recently reported data demonstrating EVI1 to be essential for tumor growth in a subset of MLL-MLLT3-rearranged AML.15 However, with regard to number or type of CNA, we did not find any substantial differences between EVI1 high and low expression cases (Online Supplementary Tables S2 and S3). Based on this observation, we cannot propose an increase in genomic complexity as the consequence of the interaction between MLL and EVI1. In summary, our study demonstrates that CNAs occur at a very low frequency in t(9;11)-positive AML. However, we discovered novel recurrently altered regions that point to potential cancer disease genes. With regard to CNAs, we found no differences in number or type of lesions between de novo and t-AML with t(9;11), calling into question the current WHO classification which separates them into different categories. Further clinical outcome data are needed to address this issue.
Acknowledgments
For excellent technical assistance we thank Juliane Gohlke and Karin Müller. We also thank all the members of the German-Austrian AMLSG for their participation in the clinical trials and for providing leukemia samples.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 4th ed Lyon: IARC Press; 2008 [Google Scholar]
- 2.Kayser S, Döhner K, Krauter J, Kohne CH, Horst HA, Held G, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–45 [DOI] [PubMed] [Google Scholar]
- 3.Corral J, Lavenir I, Impey H, Warren AJ, Forster A, Larson TA, et al. An Mll-AF9 fusion gene made by homologous recombination causes acute leukemia in chimeric mice: a method to create fusion oncogenes. Cell. 1996;85(6):853–61 [DOI] [PubMed] [Google Scholar]
- 4.Kühn MWM, Radtke I, Bullinger L, Goorha S, Cheng J, Edelmann J, et al. High-resolution genomic profiling of adult and pediatric core-binding factor acute myeloid leukemia reveals new recurrent genomic alterations. Blood. 2012;119(10):e67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullinger L, Fröhling S. Array-based cytogenetic approaches in acute myeloid leukemia: clinical impact and biological insights. Semin Oncol. 2012;39(1):37–46 [DOI] [PubMed] [Google Scholar]
- 6.Chen K, Wallis JW, Kandoth C, Kalicki-Veizer JM, Mungall KL, Mungall AJ, et al. BreakFusion: targeted assembly-based identification of gene fusions in whole transcriptome paired-end sequencing data. Bioinformatics. 2012;28(14):1923–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullighan CG, Downing JR. Genome-wide profiling of genetic alterations in acute lymphoblastic leukemia: recent insights and future directions. Leukemia. 2009;23(7):1209–18 [DOI] [PubMed] [Google Scholar]
- 8.Döhner K, Brown J, Hehmann U, Hetzel C, Stewart J, Lowther G, et al. Molecular cytogenetic characterization of a critical region in bands 7q35-q36 commonly deleted in malignant myeloid disorders. Blood. 1998;92(11):4031–5 [PubMed] [Google Scholar]
- 9.Gu DL, Chen YH, Shih JH, Lin CH, Jou YS, Chen CF. Target genes discovery through copy number alteration analysis in human hepatocellular carcinoma. World J Gastroenterol. 2013;19(47):8873–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nandan MO, McConnell BB, Ghaleb AM, Bialkowska AB, Sheng H, Shao J, et al. Kruppel-like factor 5 mediates cellular transformation during oncogenic KRAS-induced intestinal tumorigenesis. Gastroenterology. 2008;134(1):120–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ema M, Mori D, Niwa H, Hasegawa Y, Yamanaka Y, Hitoshi S, et al. Kruppel-like factor 5 is essential for blastocyst development and the normal self-renewal of mouse ESCs. Cell Stem Cell. 2008;3(5):555–67 [DOI] [PubMed] [Google Scholar]
- 12.Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61(5):673–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyybakinoja A, Elonen E, Vauhkonen H, Saarela J, Knuutila S. Single nucleotide polymorphism microarray analysis of karyotypically normal acute myeloid leukemia reveals frequent copy number neutral loss of heterozygosity. Haematologica. 2008;93(4):631–2 [DOI] [PubMed] [Google Scholar]
- 15.Gröschel S, Schlenk RF, Engelmann J, Rockova V, Teleanu V, Kühn MW, et al. Deregulated Expression of EVI1 Defines a Poor Prognostic Subset of MLL-Rearranged Acute Myeloid Leukemias: A Study of the German-Austrian Acute Myeloid Leukemia Study Group and the Dutch-Belgian-Swiss HOVON/SAKK Cooperative Group. J Clin Oncol. 2013;31(1):95–103 [DOI] [PubMed] [Google Scholar]


