Abstract
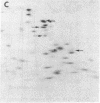
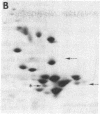
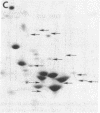
Hybrid cell lines formed by fusion of mouse 3T3 cells and Chinese hamster ovary (CHO) cells resistant to emetine, which have an altered 40S ribosomal protein, are generally sensitive to emetine. From most hybrid lines it was possible to select sublines resistant to emetine. The ribosomal components of three lines were studied: A34, an emetine-sensitive hybrid; A34/R3, an emetine-resistant derivative of A34; and A72, an emetine-sensitive hybrid that did not give rise to emetine-resistant sublines. Genetic and biochemical evidence suggests that in A34 both the mouse emetine sensitivity gene and the hamster emetine resistance gene are active, whereas in A34/R3 only the hamster emetine resistance gene is active and in A72 only the mouse emetine sensitivity gene is active. The ribosomes of all three sublines contained both mouse and hamster RNA, predominantly mouse. However, the 60S subunits had roughly equal amounts of the three mouse and hamster proteins that could be distinguished by two-dimensional electrophoresis, suggesting the association of mouse RNA with hamster ribosomal proteins. The emetine-resistant and emetine-sensitive 40S subunits could be separated by sedimentation in 0.5 M KCl. Resistant subunits contained predominantly mouse RNA, presumably associated with the hamster protein conferring emetine resistance. We conclude that hybrid cells can form hybrid ribosomes and that the amounts of ribosomal RNA and ribosomal protein of each species are not closely coupled.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boersma D., McGill S., Mollenkamp J., Roufa D. J. Emetine resistance in Chinese hamster cells. Analysis of ribosomal proteins prepared from mutant cells. J Biol Chem. 1979 Jan 25;254(2):559–567. [PubMed] [Google Scholar]
- Bramwell M. E., Handmaker S. D. Ribosomal RNA synthesis in human-mouse hybrid cells. Biochim Biophys Acta. 1971 Mar 25;232(3):580–583. doi: 10.1016/0005-2787(71)90611-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L. The ribosomal RNA of hamster-mouse hybrid cells. J Cell Biol. 1972 Apr;53(1):177–184. doi: 10.1083/jcb.53.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T., Eliceiri G. L. Ribosomal proteins of hamster, mouse and hybrid cells. Biochim Biophys Acta. 1975 Aug 21;402(2):238–243. doi: 10.1016/0005-2787(75)90043-x. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Mutants of CHO cells resistant to the protein synthesis inhibitor emetine: genetic and biochemical characterization of second-step mutants. Somatic Cell Genet. 1978 Jan;4(1):77–94. doi: 10.1007/BF01546494. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The isolation and preliminary characterization of somatic cell mutants resistant to the protein synthesis inhibitor-emetine. Cell. 1976 Oct;9(2):213–219. doi: 10.1016/0092-8674(76)90112-4. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The molecular basis of emetine resistance in Chinese hamster ovary cells: alteration in the 40S ribosomal subunit. Cell. 1977 Jan;10(1):61–66. doi: 10.1016/0092-8674(77)90140-4. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Lawrence J. B., Ruddle F. H. A sequential staining technique for the chromosomal analysis of the interspecific mouse/hamster and mouse/human somatic cell hybrids. Exp Cell Res. 1977 Mar 1;105(1):109–117. doi: 10.1016/0014-4827(77)90156-2. [DOI] [PubMed] [Google Scholar]
- Kuter K. J., Rogers A. The synthesis of ribosomal protein and ribosomal RNA in a rat-mouse hybrid cell line. Exp Cell Res. 1975 Mar 15;91(2):317–325. doi: 10.1016/0014-4827(75)90110-x. [DOI] [PubMed] [Google Scholar]
- Madjar J. J., Arpin M., Buisson M., Reboud J. P. Spot position of rat liver ribosomal proteins by four different two-dimensional electrophoreses in polyacrylamide gel. Mol Gen Genet. 1979 Mar 20;171(2):121–134. doi: 10.1007/BF00269998. [DOI] [PubMed] [Google Scholar]
- McConkey E. H., Bielka H., Gordon J., Lastick S. M., Lin A., Ogata K., Reboud J. P., Traugh J. A., Traut R. R., Warner J. R. Proposed uniform nomenclature for mammalian ribosomal proteins. Mol Gen Genet. 1979 Jan 16;169(1):1–6. doi: 10.1007/BF00267538. [DOI] [PubMed] [Google Scholar]
- Mets L. J., Bogorad L. Two-dimensional polyacrylamide gel electrophoresis: an improved method for ribosomal proteins. Anal Biochem. 1974 Jan;57(1):200–210. doi: 10.1016/0003-2697(74)90065-7. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Tantravahi R., Dev V. G., Miller O. J. Q- and C-band chromosome markers in inbred strains of Mus musculus. Genetics. 1976 Sep;84(1):67–75. doi: 10.1093/genetics/84.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. J., Dev V. G., Miller D. A., Tantravahi R., Eliceiri G. L. Transcription and processing of both mouse and Syrian hamster ribosomal RNA genes in individual somatic hybrid cells. Exp Cell Res. 1978 Sep;115(2):457–460. doi: 10.1016/0014-4827(78)90309-9. [DOI] [PubMed] [Google Scholar]
- Nesbitt M. N., Francke U. A system of nomenclature for band patterns of mouse chromosomes. Chromosoma. 1973;41(2):145–158. doi: 10.1007/BF00319691. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Schibler U., Huebner K., Croce C. M. Selective suppression of the transcription of ribosomal genes in mouse-human hybrid cells. J Cell Physiol. 1979 Mar;98(3):553–559. doi: 10.1002/jcp.1040980313. [DOI] [PubMed] [Google Scholar]
- Pontecorvo G. Production of mammalian somatic cell hybrids by means of polyethylene glycol treatment. Somatic Cell Genet. 1975 Oct;1(4):397–400. doi: 10.1007/BF01538671. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Basilico C. Complementation of a defect in the production of ribosomal RNA in somatic cell hybrids. Nature. 1974 Mar 29;248(447):411–413. doi: 10.1038/248411a0. [DOI] [PubMed] [Google Scholar]
- Warner J. R. In the absence of ribosomal RNA synthesis, the ribosomal proteins of HeLa cells are synthesized normally and degraded rapidly. J Mol Biol. 1977 Sep 25;115(3):315–333. doi: 10.1016/0022-2836(77)90157-7. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]