Abstract
Millions of people suffer from spinal cord injury (SCI) with little known effective clinical therapy. Neuropathic pain (NP) is often accompanied with SCI, making clinical treatment challenging. Even though the key mediators in the development of NP have been discovered, the pathogenesis is still unclear. Some of the key mediators in the sustenance of NP include the inflammatory processes, cannabinoid receptors, matrix metalloproteases, and their tissue inhibitors. Animal models have shown promising results with modulation of these mediators, yet the clinical models have been unsuccessful. One such study with matrix metalloproteases (MMPs) has yielded encouraging results. The relationship between MMPs and their tissue inhibitors (TIMPs) plays a significant role in the pathogenesis and recovery of SCI and the CNS. Key factors that lead to the functional consequences of MMP activity are cellular localization, tissue distribution, and temporal pattern of MMP expression. Studies concluding that MMPs can be seen as contributors of tissue damage and as contributors in the repair mechanisms have provided a need to reexamine their roles after acute and chronic neuropathic pain
Keywords: Spinal cord injury, Neuropathic pain, MMPs, TIMPs
Introduction
There are a vast array of outcomes associated with spinal cord injury (SCI) shown to produce motor and sensory complications, including the formation of chronic neuropathic pain (NP). The formation of NP can be more debilitating than any symptom associated with SCI.1 For all of these effects of SCI, there is little known effective clinical therapy and advances have been slow. Yet, promising developments have given insight into the cellular and molecular mechanisms. Recent improvements in the field give insight into SCI and associated pain pathways to better possible treatments. In this review, we explore recent advances in the field of treatment and pathogenesis of SCI and chronic NP.
Inflammatory Response in SCI
As in many human injury processes, there are invoked characteristic systems that have detrimental and beneficial effects. One such system that has been identified is the inflammatory response. For our purposes the role of the inflammatory process in the pathogenesis of neuropathic pain following SCI has garnered significant attention in recent years. Work has implicated the role of cytokines, chemokines, prostaglandins and other inflammatory factors in the pathogenesis of pain.2,1 For all of these effects of SCI, there is little known effective clinical therapy and advances have been slow. Yet, promising developments have given insight into the cellular and molecular mechanisms. Recent improvements in the field give insight into SCI and associated pain pathways to better possible treatments. In this review, we explore recent advances in the field of treatment and pathogenesis of SCI and chronic NP.
Inflammatory Response in SCI
As in many human injury processes, there are invoked characteristic systems that have detrimental and beneficial effects. One such system that has been identified is the inflammatory response. For our purposes the role of the inflammatory process in the pathogenesis of neuropathic pain following SCI has garnered significant attention in recent years. Work has implicated the role of cytokines, chemokines, prostaglandins and other inflammatory factors in the pathogenesis of pain.2,3
Although shared features between CNS and non-CNS tissue to injury and the inflammatory process, there are important differences between the two types. The CNS contains several highly specialized cell types (i.e. central neurons, astrocytes, oligodendrocytes, microglial cells) which are absent in systemic tissues. Also there are anatomical differences e.g. absence of a lymphatic drainage system and the spatial constraints during injury induced swelling process. These features expose the CNS to substantial risk from increased tissue pressure, ischemia, and damage secondary to inflammation. The blood-brain barrier also significantly changes peripheral leukocyte kinetics, such that their migration into central neural tissue is significantly delayed.4 Spinal nerve injury, however, has been demonstrated to induce maximal blood-spinal cord permeability two weeks following injury. Persistent elevation of permeability was still evident at ten weeks.
Inflammation has important beneficial effects following injury: removing offending pathogens and necrotic tissue, and repair of injured tissue. Activated leukocytes phagocytose necrotic cells, release cytotoxic factors that induce further tissue damage. Recent studies illustrate the importance of microglial and astrocytic release of neurtrophic factors. These factors have neuroprotective effects, but at the same time are pro-inflammatory.5-7 However, these neurotrophic factors are also known to play a central role in the development of chronic pain syndromes.8,9 Clear examples are brain-derived and nerve growth factors which are now known to be key components in the pathogenesis of NP via the modulation of pain afferent excitability and nociceptor sensitization.10-13
Damage to the CNS due to inflammation has made modulation of inflammation a therapeutic method. Inflammatory response is known to cause further damage to injured and non-injured tissue. The secondary damage due to acute inflammation involves mediators including nitric oxide (NO), bradykinins, prostaglandins, and tumor necrosis factor alpha (TNF-α).14 TNF-α will remain an important therapeutic target as lesion size post-SCI is significantly larger than original lesion size pre-SCI.15
Novel targets for Spinal Cord Injury Neuropathic Pain
Non-inflammatory mediators of chronic NP are also key components to central and peripheral hypersensitization. Of interest is interaction between the cannabinoid and matrix metalloprotease systems.16 Recent work has demonstrated that there may be spinal cord injury mediated induction of natural downregulators of matrix metalloproteases.17 Importantly, these natural downregulators are activated by cannabinoid stimulation. Understanding how these two systems are interrelated will afford clinicians with an additional therapeutic option for neuropathic pain.
MMPs and Spinal Cord Injury
Matrix metalloproteases (MMPs) are a large family of zinc-dependent extracellular proteases that have the ability to digest extracellular matrix components such as collagen, proteoglycan and laminin. They are able to digest a variety of cell surfaces and soluble proteins including growth factors, adhesion molecules, receptors, and most notably cytokines and chemokines.18 (Figure 1)
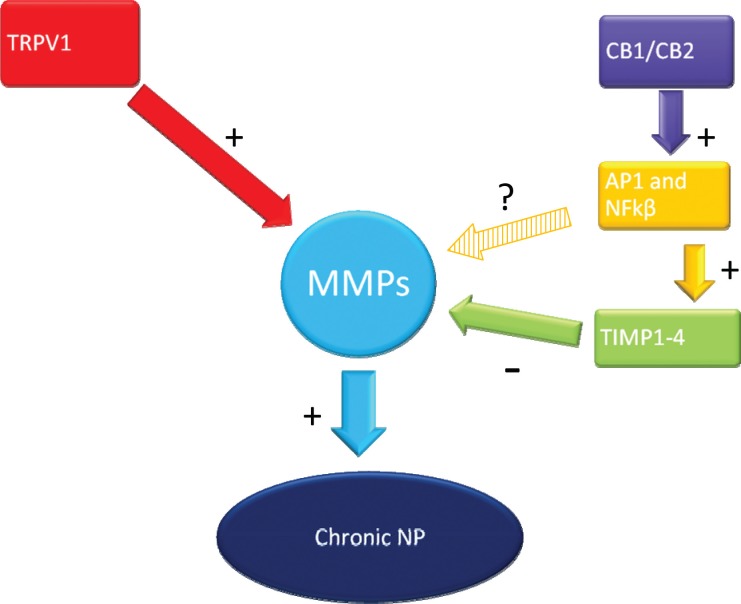
Fig. 1:
Inhibition of MMPs through TRPV1 and Cannabinoid Receptors. TRPV1 upregulates MMP expression leading to chronic NP. CB1/CB2 upregulate AP1 and NFkβ, which then further upregulates TIMP 1-4. TIMP1-4 will in turn downregulate MMP expression. The relationship between AP1 and NFkβ and MMP is still unclear.
In recent years, studies have demonstrated that MMPs are overexpressed in injured spinal cord tissue. Its role following injury is linked to several degenerative and protective processes ranging from inflammation, demyelination, to angiogenesis, and axonal growth.19-23 Here we examine the potentially beneficial and detrimental roles of two members of this family, MMP-2 and MMP-9, in the context of the pathogenesis of SCI induced neuropathic pain.
Temporal and spatial differences are seen in MMP-2 and MMP-9 regulation following injury. MMP-9 overexpression is seen in the acute phase following injury, while MMP-2 induction is seen in the late phase of injury.8,19,24,25 In uninjured spinal cord, MMP-9 has some basal expression, albeit low levels. MMP-9 is expressed in uninjured meninges and motorneurons.26 This tissue localization can be changed within 24 hours following injury to show expression in peri-lesional blood vessels, macrophages, and astrocytes.27 Additionally, MMP-9 expression is proportional to degree of injury.19 These findings demonstrate that MMP-9 plays an important role in early changes to spinal cord injury.
MMP-9 seems to play a key role altering homeostasis in the cellular environment of the acute phase of injury. Detrimental effects of MMP-9 have implicated this protein plays a role in the disruption of the blood-spinal cord barrier (BSCB). Disruption of the BSCB allows for subsequent neutrophil invasion which can cause additional tissue damage and demyelination. BSCB breakdown also assists in the access of inflammatory components to healthy spinal cord tissue. Infiltrating cells are able to activate and interact with resident microglia and astrocytes, as well as pro-inflammatory cytokines.19,23 nvasion of neutrophils can induce extension tissue necrosis which will lead to lesion expansion. Additionally, reactive oxygen species, proteases, nitric oxide, and pro-inflammatory cytokines are generated by a majority of neutrophils in white matter within the lesion.23,27,28 Temporally, neutrophil infiltration coincided with MMP-9 upregulation at 3 days post-injury.28 Inflitrating macrophages expressing MMP-9 were also found to contribute to the demyelination of uninjured neurons.27 Although the effects of neutrophils have degenerative traits, they are important for sterilization and debridement of the injury site. Myelin basic protein is a cleavage substrate for MMP-9 supporting its role as in demyelination.
In contrast to MMP-9 expression patterns, MMP-2 exhibits delayed upregulation following injury. Its expression begins approximately 5 days post-injury and peaks about 7 to14 days post-injury.19,20,24 Examination of injured human spinal cord tissue reported a transient early induction of MMP-2 in macrophages epicenter of injury.12 However, MMP-2 activity was found in reactive astrocytes bordering the lesion within the spinal cord.20 Knowledge of the role of MMP-2 in the chronic phase of injury has much ground to gain with respect our current understanding of MMP-9.
Knockout studies in mice and specific MMP inhibitors have been undertaken to better understand the function of MMPs. These studies support MMP-9 as playing a beneficial and detrimental role in SCI. MMP-9 deficient mice demonstrate improved BSCB integrity, subsequently diminished neutrophils, and macrophages infiltration. Additionally, there is improved locomotor recovery.19,23,27 Atorvastatin (MMP-9 inhibitor) post-SCI is neuroprotective.23 Atorvastatin treated rats also showed diminished breakdown of the BSCB and reduced neutrophil and macrophage infiltration to the spinal cord compared to controls. Pharamcological inhibition of MMP-9 reduced secondary tissue damage and increased functional recovery. Taken together, these data support inhibition of MMP-9 as neuroprotective following spinal cord injury.23
It is also important to recognize the studies that have implicated MMP-9 as neuroprotective. MMP-9 is expressed in migrating growth cones during the acute phase of SCI.21 Moreover, MMPs may also play a key role in ECM remodeling, glial scar formation, and revascularization. These are key events in the wound healing process that occur within the first two weeks after injury 19. Importantly, during this time MMP-2 expression is increased.19
The formation of a glial scar can serve both positive and negative effects. It can reestablish a physical barrier at the site of injury to reduce further damage, but the cells within the scar can also prevent neuroregeneration by secreting inhibitory molecules. Chondroitin sulfate proteoglycans (CSPG) are a family of molecules found in glial scars and can have negative effects on axonal growth.29 MMP-2 is able to degrade CSPGs19,20 and it has been shown that glial scarring was more common in MMP-2 knockout mice.20 Gelatinase activity was also found most prevalent in the epicenter of the injury site, and gradually decreased peripherally. In contrast, astrocytes were found on the border of injury site, with gelatinase activity being seen immediately surrounding the epicenter.19 Therefore, we can see a correlation between the gelatinase activity within the glial scar and the lack of regeneration and recovery following SCI. The molecules associated with glial scarring may be responcible for the gelatinase activity. MMP-2 may be able to reduce the glial scarring and ultimately improve functional recovery.
Transcriptional Regulation of MMPs
Following injury to the spinal cord, there is a complex release of inflammatory cytokines (such as IL-1β and TNF-α) from infiltrating macrophages and neurotransmitters from neuronal cells.30 These molecules induce molecular pathways that alter the expression of genes that account for the pathogenesis of neuropathic pain. Of particular interest in this review is the gene regulation of MMPs and TIMPs.31
Noxious substances such as reactive oxygen species (ROS) and mechanical stimuli are capable of activating intracellular transcription factors such as activator protein 1 (AP-1) and nuclear factor-κB (NF-κB). These transcription factors are responsible for direct increase in the transcription of inflammatory mediators (IL-1β, TNF-α, MMPs, etc). AP-1 is a heterodimeric protein derived from the c-Fos and c-Jun families. AP-1 transcription factor sites are found in the MMP-2, MMP-9 and TIMP-1 genes. Moreover, their expression is enhanced by active AP-1.Furthermore, NF-κB upregulates MMP-2 and MMP-9 during the inflammation response of microglia and astrocytes.32,33
Mitogen activated protein kinase (MAPK) pathways also activate AP-1 and NF-κB, eventually inducing MMP expression. Activation of the cannabinoid (CB), IL-1β, transient receptor potential vanilloid 1 (TRPV1), and TNF-α receptors have the capacity to induce the MAPK pathways (figure 2). Extracellular signal-regulated kinase (ERK 1/2) and p38 MAPK are upregulated following mechanical and ischemic brain injury.34 Interestingly, inhibition of ERK 1/2 and p38 kinase pathways diminishes MMP-9 expression following injury. Additionally, inhibition of p38 and ERK in rat cortical astrocyte culture decreases TNF-α-induced MMP-9 expression.1 It has also been shown that IL-1β stimulates neuronal MMP-9 levels following mouse CNS trauma.35
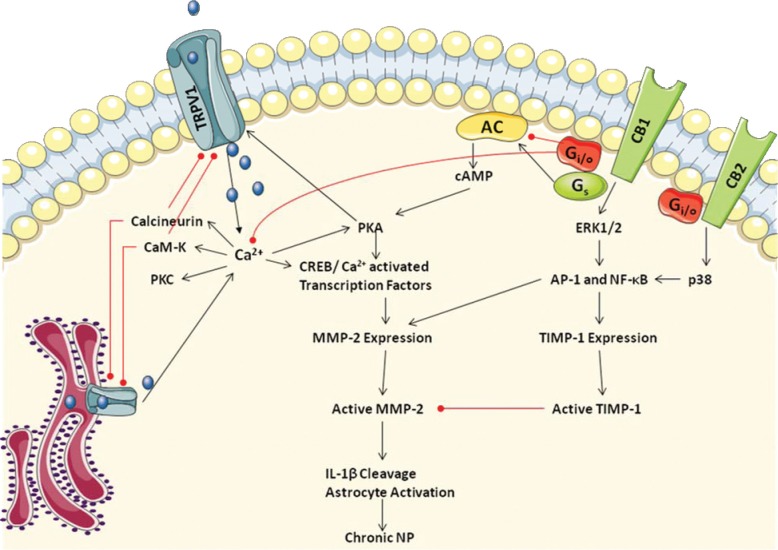
Fig.2:
Proposed mechanism of MMP and TIMP-1 transcriptional regulation though TRPV1 and CB1/2 signaling receptors involved in the formation of chronic neuropathic pain. TRPV1 and CB1/2 modulate Ca2+ -dependent and MAPK intracellular signaling pathways which are capable of activation CREB, AP-1 and NF-κB transcription factors which control expression of MMP-2 and TIMP-1. Reproduced from Pearce, SM, Ramsey, MA, Miranpuri, GS, Resnick, DK. 2008. Annals of Neurosciences 15:94-105.
The cannabinoid system receptors CB1 and CB2, homologous, are G-protein coupled receptors. Their activation stimulates adenyl cyclase mediated cAMP production, intracellular Ca-dependent pathways and MAPK activity. Activation of the CB1 receptor reduces Ca2+ levels in the cell and increases inward-rectifying K+ currents through direct coupling with inward-rectifying K+ channels Ca2+-Q.36,37 Lowering the potassium potential reduces neuronal excitability and neurotransmitter release in the CNS. This is an interesting effect; however, more work must be done to determine electrophysiological changes following spinal cord injury. Through Gs and Gi/o proteins, CB receptors have effects on cAMP levels. Namely, CB1 couples with Gs which will lower cAMP, while CB2 couples with Gi/o which will decrease cAMP levels.37
CB receptors also modulate the MAPK pathways. Agonist binding to CB1 stimulates both p38 and ERK1/2 MAPK pathways, while CB2 receptor only induces p38 MAPK. There are well established links between MAPK activation and the expression c-Fos and c-Jun or the AP-1 transcription factor family.38 More directly, CB receptor activation has been shown to increase MAPK activation which is implicated in MMP and TIMP gene regulation (Figure 1). Unstimulated cells have low MMP expression, but expression can be increased by MAPK modulation of c-Jun and c-Fos. Additionally, TIMP is upregulated by these transcription factors.39 Fibroblasts treated with an inflammatory cytokine, Oncostatin M (OSM), increased TIMP-1 mRNA expression. When p38 and ERK1/2 phosphorylation was inhibited, TIMP-1 levels did not increase. This demonstrates that MAPK phosphorylation is needed for OSM-induced TIMP-1 expression. More direct evidence is increased expression of c-Jun following OSM stimulation.39,40 In NIH 3T3 cells, c-Fos increases TIMP-1 promoter activity.41
Using a non-specific endocannabinoid of CB1/2 receptors, anandamide cancer cell invasiveness was reduced by elevated TIMP-1/ 2 expression.42 We conclude that TIMP-1/2 expression induced by the cannabinoid system is an area that needs greater attention given the specificity and knowledge known about how to manipulate the cannabinoid system pharmacologically (Figure 1).
MMP Inhibition by TIMPs via Cannabinoid Receptor Activation
Localization of the CB receptors has been shown to be in the presynaptic membranes. This location allows the receptors to inhibit the release of neurotransmitters.37 Following saphenous nerve injury CB1 and CB2 receptors are upregulated in the DRG and ipsilateral spinal cord.43 Administration of endogenous or synthetic cannabinoids decrease hyperalgesia in both central and peripheral injury models of chronic pain.39,44-48 However, of particular interest here, CB receptors are able to induce the production of TIMPs.
There is currently increased attention in the anti-hyperalgesic effect of CB receptor activation. Activation of these different receptor isoforms hypothetically may be able to reduce TRPV1 dependent expression of MMP-2 via CB dependent TIMP activation. More work is needed to determine the differential effects of CB1 and CB2 receptor activation on TIMP activation (Figure 1). This theoretical framework affords a unique strategy in modulating SCI induced neuropathic pain, namely stimulation of the cannabinoid system. However, central and peripheral models of neuropathic pain offer conflicting results regarding CB receptor which is responsible for the analgesic effects of the cannabinoid system. Outlining the molecular pathway specifically upregulated following injury is an area of work that we believe will have tremendous implications in the field of pharmacological treatment of neuropathic pain.
Pharmacological inhibition of Cannabinoid receptors
Recent studies have shown that cannabinoids (CB) affects the formation of NP.51 Although, CB activates both CB1 and CB2 receptors and the ionotropic receptor, TRPV1, the way in which it modulates NP is poorly understood. TRPV1 receptors play a role in the development of pain during thermal hyperalgesia (TH).49,50 The G-protein receptors, CB1/2, are expressed intracellularly after SCI [72] and when given intrathecally, CB is able to reduce hyperalgesia in NP models.45,51 Understanding that CB affects nociception in a NP model through the CB1 receptors following peripheral nerve injury, Pol et al. (2006) noticed that the CB1 receptors found at the spinal and peripheral level did not play a role in the adaptive responses occurring.56 This is most likely due to the upregulation of CB1 receptors in the brain or spine located away from the site of injury. The CB1 receptors are located in the dorsal root ganglia, peripheral terminals of C fibers, and the spinal cord (Figure 2).18,52
While the TRPV1 receptors seem to correlate with pain during TH, CB1 receptors have been found to either inhibit TRPV1-mediated nociception or enhance it. The activation of the cAMP-signaling pathway is the determinant.52 It is through these signaling events that we might be able to understand TRPV1-induced endogenous biological effects by observing which of the two receptors would be initially activated. The CB1 receptor agonists, Win 55, 212-2, and the TRPV1 agonist, Anandamide, were found to significantly reduce TH during NP.53,54 Sagar et al. has even proposed that CB inhibition of TRPV1 is CB1 and CB2 independent.55 While advancements within the field have been considerable, more research is still required for any clinical or pharmacological developments of the CB system during NP. The signaling processes are involved with the desensitization of TRPV1 by CB.
MMPs and the Neuropathic Pain Model
MMP-2 and MMP-9 are linked to the late and early phase of NP respectively.14 After spinal nerve ligation (SNL), there was an upregulation of MMP-2 and MMP-9 with these phases of NP. IL-1β has been found to play an important role in the upregulation of the MMPs following SNL. Both MMP-2 and MMP-9 are activated through IL-1β. MMP-2 also mediates NP during the chronic phase of NP through astrocyte activation while MMP-9 mediates NP during the acute stage though microglial activation.26 Both MMP-2 and MMP-9 are inhibited through the tissue inhibitors, TIMPs. TIMP-1 was upregulated in rats experiencing NP compared to the rats with inflammatory pain.56 Given exogenously, TIMP-2 reduced MMP-2 and TH, and ultimately NP during the chronic phase of injury.14,42,57,58
TIMPs as Pain Mediators
The TIMP family includes TIMP-1 through TIMP-4, which all inhibit MMP protease activity. The different isoforms each have unique expression patterns, regulation, and efficiency of MMP inhibition.2 MMPs are regulated primarily by TIMPs. The temporal and spatial expression patterns of these two protein families may modulate many pathophysiological changes post-injury. TIMPs induce a conformational change in the catalytic domain of MMPs which abolishes MMP proteolytic activity.59 Namely, TIMP-4, inhibits MMP-1, -2, -3, -7, and -9. Additionally, TIMP-1 and -2 bind the hemopexin domain of pro-MMP-9 and -2, which results in complex effects MMP activation.11 The balance between MMPs and their endogenous inhibtion by TIMPs is a key component to understanding the environment created post-spinal cord injury.
MMP activity has been linked to the production of chronic NP following SCI. Inhibition of MMP could result in functional recovery. The balance between tissue formation and degradation by MMPs is usually sustained through an interaction with TIMPs. Following SCI, TIMPs have been seen to reduce the upregulation of MMPs in the tissue surrounding the impaction site.59 Likewise, in both animal and human models, SCI has limited the production of TIMPs in comparison to the production of MMP. The most efficient way to prevent additional damage by secondary pathogenesis is to examine the post-injury inflammatory response.28 TIMPs have been reported to reduce the inflammatory response, improve locomotor recovery, and decrease tissue damage post-injury.19,27,60-62 In addition to the benefits to the injury site of TIMPs, neuropretective effects have been seen following cerebral damage.34,63-65 With all of the novel findings regarding MMPs, TIMPs has generated substantial interest as pain mediators.
Even though studies have shown many exciting benefits to TIMPs, clinical studies have not shown the same results. One possible reason as to why TIMPs aren’t succeeding in a clinical setting is because MMPs can control a range of other processes and their inhibition could affect other essential functions, like the cleavage of the IL-6 receptor or the discharge of TNFα.66 The effects from inhibiting the proteases could be very harmful to the system being studied. Liver injury has been seen as a result from inhibiting the MMPs to release TNFα.67 Additionally, joint pain has resulted from the use of TIMPs in some patients.68 These findings only intensify the complexity behind TIMPs. The pain mediating effects of TIMPs may be in part to non-MMP involved processes.69,70 In order for TIMPS to offer clinical benefits by mediating NP and improving locomotive abilities, we need to more closely examine the spatial and temporal patterns of MMP expression. When dealing with TIMPs and locating the specific isoform of MMP that should be inhibited, a lot of attention and detail is demanded.
Conclusions
The present review gives insight the involvement of CB, MMP and TIMP in the formation of NP. The information should be used as a base work to construct further hypotheses to be researched. There is still much to be discovered in the SCI field. Even though SCI may result in extreme physical and emotional insufficiencies, the formation of chronic NP can be even more devastating. Great strides have been made, yet the molecular and cellular mechanisms involved in the formation of NP are still poorly understood. To help fill this gap, current NP research has been reviewed and associated with noteworthy advances in the field of the pathology and treatment of SCI and chronic NP.
Footnotes
The article complies with International Committee of Medical Journal Editor’s uniform requirements for the manuscripts.
Competing interests – None
Source of Funding – None
References
- 1.Arai K, Lee SR, Lo EH. Essential role for ERK mitogen-activated protein kinase in matrix metalloproteinase-9 regulation in rat cortical astrocytes. Glia. 2003;43(3):254–264. doi: 10.1002/glia.10255. [DOI] [PubMed] [Google Scholar]
- 2.Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol. 2002;15:355–60. doi: 10.1097/00019052-200206000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147:S232–40. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 5.Bracken MB, Shepard MJ, Collins WF et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–11. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 6.Goss JR, O’Malley ME, Zou L et al. Astrocytes are the major source of nerve growth factor upregulation following traumatic brain injury in the rat. Exp Neurol. 1998;149:301–9. doi: 10.1006/exnr.1997.6712. [DOI] [PubMed] [Google Scholar]
- 7.Heese K, Hock C, Otten U. Inflammatory signals induce neurotrophin expression in human microglial cells. J Neurochem. 1998;70:699–707. doi: 10.1046/j.1471-4159.1998.70020699.x. [DOI] [PubMed] [Google Scholar]
- 8.Miletic G, Miletic V. Increases in the concentration of brain derived neurotrophic factor in the lumbar spinal dorsal horn are associated with pain behavior following chronic constriction injury in rats. Neurosci Lett. 2002;319:137–40. doi: 10.1016/s0304-3940(01)02576-9. [DOI] [PubMed] [Google Scholar]
- 9.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–38. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 10.Coull JA, Beggs S, Boudreau D et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 11.Guo W, Robbins MT, Wei F et al. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–37. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hathway GJ, Fitzgerald M. Time course and dose-dependence of nerve growth factor-induced secondary hyperalgesia in the mouse. J Pain. 2006;7:57–61. doi: 10.1016/j.jpain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Matayoshi S, Jiang N, Katafuchi T et al. Actions of brain-derived neurotrophic factor on spinal nociceptive transmission during inflammation in the rat. J Physiol. 2005;569:685–95. doi: 10.1113/jphysiol.2005.095331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasaki Y, Xu ZZ, Wang X et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008;14(3):331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–78. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 16.Finnerup NB, Jensen TS. Mechanisms of disease: mechanism-based classification of neuropathic pain-a critical analysis. Nat Clin Pract Neurol. 2006;2:107–15. doi: 10.1038/ncpneuro0118. [DOI] [PubMed] [Google Scholar]
- 17.Tachibana T, Noguchi K, Ruda MA. Analysis of gene expression following spinal cord injury in rat usuing complementary DNA microarray. Neurosci Lett. 2002;327(2):133–7. doi: 10.1016/s0304-3940(02)00375-0. [DOI] [PubMed] [Google Scholar]
- 18.Pearce SM, Ramsey MA et al. Regulation and fuction of Matrix Matalloproteins in nervous system injury and neuropathic pain. Annals of Neurosciences. 2008;15:94–105. [Google Scholar]
- 19.Goussev S, Hsu JY, Lin Y et al. Differential temporal expression of matrix metalloproteinases after spinal cord injury: relationship to revascularization and wound healing. J. Neurosurg. 2003;99(2 Suppl):188–197. doi: 10.3171/spi.2003.99.2.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu JY, McKeon R, Goussev S et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J. Neurosci. 2006;26(39):9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones TB, Ankeny DP, Guan Z et al. Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J Neurosci. 2004;24:3752–61. doi: 10.1523/JNEUROSCI.0406-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klusman I, Schwab ME. Effects of pro-inflammatory cytokines in experimental spinal cord injury. Brain Res. 1997;762:173–84. doi: 10.1016/s0006-8993(97)00381-8. [DOI] [PubMed] [Google Scholar]
- 23.Pannu R, Christie DK, Barbosa E et al. Post-trauma Lipitor treatment prevents endothelial dysfunction, facilitates neuroprotection, and promotes locomotor recovery following spinal cord injury. J. Neurochem. 2007;101(1):182–200. doi: 10.1111/j.1471-4159.2006.04354.x. [DOI] [PubMed] [Google Scholar]
- 24.Ho A, Blum M. Regulation of astroglial-derived dopaminergic neurotrophic factors by interleukin-1 beta in the striatum of young and middle-aged mice. Exp Neurol. 1997;148:348–59. doi: 10.1006/exnr.1997.6659. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39(3):279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 26.Conti A, Miscusi M, Cardali S et al. Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and inflammation. Brain Res Rev. 2007;54:205–18. doi: 10.1016/j.brainresrev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Noble LJ, Donovan F, Igarashi T et al. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J. Neurosci. 2002;22(17):7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurlbert RJ. Strategies of medical intervention in the management of acute spinal cord injury. Spine. 2006;31:S16–21. doi: 10.1097/01.brs.0000218264.37914.2c. [DOI] [PubMed] [Google Scholar]
- 29.Pizzi MA, Crowe MJ. Matrix metalloproteinases and proteoglycans in axonal regeneration. Exp. Neurol. 2007;204(2):496–511. doi: 10.1016/j.expneurol.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Mun-Bryce S, Lukes A, Wallace J et al. Stromelysin-1 and gelatinase A are upregulated before TNF-alpha in LPS-stimulated neuroinflammation. Brain Res. 2002;933(1):42–49. doi: 10.1016/s0006-8993(02)02303-x. [DOI] [PubMed] [Google Scholar]
- 31.Bracken MB, Collins WF, Freeman DF et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45–52. [PubMed] [Google Scholar]
- 32.Del Zoppo GJ, Milner R, Mabuchi T et al. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38(2 Suppl):646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 33.Yezierski RP. Spinal cord injury: a model of central neuropathic pain. Neurosignals. 2005;14:182–93. doi: 10.1159/000087657. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Mori T, Jung JC et al. Secretion of matrix metalloproteinase-2 and -9 after mechanical trauma injury in rat cortical cultures and involvement of MAP kinase. J. Neurotrauma. 2002;19(5):615–625. doi: 10.1089/089771502753754082. [DOI] [PubMed] [Google Scholar]
- 35.Vecil GG, Larsen PH, Corley SM et al. Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. J. Neurosci. Res. 2000;61(2):212–224. doi: 10.1002/1097-4547(20000715)61:2<212::AID-JNR12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Hagenacker T, Ledwig D, Busselberg D. Feedback mechanisms in the regulation of intracellular calcium ([Ca2+]i) in the peripheral nociceptive system: role of TRPV-1 and pain related receptors. Cell Calcium. 2008;43(3):215–227. doi: 10.1016/j.ceca.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 37.Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008;153(2):319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karin M, Zheng-gang L, Ebrahim Z. AP-1 function and regulation. Current Opinion in Cell Biology. 1997;9(2):240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 39.Reunanen N, Li SP, Ahonen M et al. Activation of p38 alpha MAPK enhances collagenase-1 (matrix metalloproteinase (MMP)-1) and stromelysin-1 (MMP-3) expression by mRNA stabilization. J. Biol. Chem. 2002;277(35):32360–32368. doi: 10.1074/jbc.M204296200. [DOI] [PubMed] [Google Scholar]
- 40.Botelho FM, Edwards DR, Richards CD. Oncostatin M stimulates c-Fos to bind a transcriptionally responsive AP-1 element within the tissue inhibitor of metalloproteinase-1 promoter. J Biol Chem. 1998;273(9):5211–8. doi: 10.1074/jbc.273.9.5211. [DOI] [PubMed] [Google Scholar]
- 41.Tong L, Smyth D, Kerr C et al. Mitogen-activated protein kinases Erk1/2 and p38 are required for maximal regulation of TIMP-1 by oncostatin M in murine fibroblasts. Cell. Signal. 2000;16(10):1123–1132. doi: 10.1016/j.cellsig.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Ramer R, Hinz B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J. Natl. Cancer Inst. 2008;100(1):59–69. doi: 10.1093/jnci/djm268. [DOI] [PubMed] [Google Scholar]
- 43.Walczak JS, Pichette V, Leblond F et al. Characterization of chronic constriction of the saphenous nerve, a model of neuropathic pain in mice showing rapid molecular and electrophysiological changes. J. Neurosci. Res. 2006;83(7):1310–1322. doi: 10.1002/jnr.20821. [DOI] [PubMed] [Google Scholar]
- 44.Costa B, Colleoni M, Conti S et al. Repeated treatment with the synthetic cannabinoid WIN 55,212-2 reduces both hyperalgesia and production of pronociceptive mediators in a rat model of neuropathic pain. Br. J. Pharmacol. 2004;141(1):4–8. doi: 10.1038/sj.bjp.0705587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Croxford JL. Therapeutic potential of cannabinoids in CNS disease. CNS Drugs. 2003;17(3):179–202. doi: 10.2165/00023210-200317030-00004. [DOI] [PubMed] [Google Scholar]
- 46.Guindon J, Walczak JS, Baulieu P. Recent advances in the pharmacological management of pain. Drugs. 2007;67(15):2121–33. doi: 10.2165/00003495-200767150-00002. [DOI] [PubMed] [Google Scholar]
- 47.Herzberg U, Eliav E, Bennett GJ et al. The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci. Lett. 1997;221(2-3):157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- 48.Patwardhan AM, Jeske NA, Price TJ et al. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc. Natl. Acad. Sci. U.S.A. 2006;103(30):11393–11398. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DomBourian MG, Turner NA, Gerovac TA et al. B1 and TRPV-1 receptor genes and their relationship to hyperalgesia following spinal cord injury. Spine. 2006;(31):2778–82. doi: 10.1097/01.brs.0000245865.97424.b4. [DOI] [PubMed] [Google Scholar]
- 50.Rajpal S, Gerovac TA, Turner NA et al. Antihyperalgesic effects of vanilloid-1 and bradykinin-1 receptor antagonists following spinal cord injury in rats. J Neurosurg Spine. 2007;6:420–4. doi: 10.3171/spi.2007.6.5.420. [DOI] [PubMed] [Google Scholar]
- 51.Rice AS, Farquhar-Smith WP, Nagy I. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66(2–3):243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- 52.Hermann H, De Petrocellis L, Bisogno T et al. Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor-mediated response. Cell Mol Life Sci. 2003;60:607–16. doi: 10.1007/s000180300052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hama A, Sagen J. Antinociceptive effect of cannabinoid agonist WIN 55, 212–2 in rats with a spinal cord injury. Exp Neurol. 2007;204:454–7. doi: 10.1016/j.expneurol.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajpal S, Gerovac TA, Turner NA et al. Selective inhibition of thermal hyperalgesia following spinal cord injury with selective B1 receptor antagonists versus TRPV1 antagonists. J Neurosurg Spine. 2007 doi: 10.3171/spi.2007.6.5.420. [DOI] [PubMed] [Google Scholar]
- 55.Sagar DR, Smith PA, Millns PJ et al. TRPV1 and CB(1) receptor-mediated effects of the endovanilloid/endocannabinoid N-arachidonoyl-dopamine on primary afferent fibre and spinal cord neuronal responses in the rat. Eur J Neurosci. 2004;20:175–84. doi: 10.1111/j.1460-9568.2004.03481.x. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez Parkitna J, Korostynski M, Kaminska-Chowaniec D et al. Comparison of gene expression profiles in neuropathic and inflammatory pain. J. Physiol. Pharmacol. 2006;57(3):401–414. [PubMed] [Google Scholar]
- 57.Cao J, Zucker S. Biology and chemistry of metalloproteinases (MMPs). http://www.abcam.com/index.html?pageconfig=resource&rid=11034&pid=10628. 2008. http://www.abcam.com/index.html?pageconfig=resource&rid=11034&pid=10628 Retrieved 2008, from Abcam plc:
- 58.Sommer C, Schmidt C, George A et al. A metalloprotease-inhibitor reduces pain associated behavior in mice with experimental neuropathy. Neurosci. Lett. 1997;237(1):45–48. doi: 10.1016/s0304-3940(97)00813-6. [DOI] [PubMed] [Google Scholar]
- 59.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J. Biol. Chem. 1999 Jul 30;274(31):21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 60.Fleming JC, Norenberg MD, Ramsay DA et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129(Pt 12):3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 61.Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience. 2004;129:767–77. doi: 10.1016/j.neuroscience.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 62.Yang JY, Kim HS, Lee JK. Changes in nitric oxide synthase expression in young and adult rats after spinal cord injury. Spinal Cord. 2007 doi: 10.1038/sj.sc.3102036. [DOI] [PubMed] [Google Scholar]
- 63.Campbell LG, Ramachandran S, Liu W et al. Different Roles for Matrix Metalloproteinases-2 and Matrix Metalloproteinase-9 in the Pathogenesis of Cardiac Allograft Rejection. American Journal of Transplantaion. 2004;5:517. doi: 10.1111/j.1600-6143.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Deng XL, Xiao XH et al. A non-steroidal anti-inflammatory agent provides significant protection during focal ischemic stroke with decreased expression of matrix metalloproteinases. Curr. Neurovasc Res. 2007;4(3):176–183. doi: 10.2174/156720207781387187. [DOI] [PubMed] [Google Scholar]
- 65.Yamaguchi M, Jadhav V, Obenaus A et al. Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgically-induced brain injury. Neurosurgery. 2007;61(5):1067–75. doi: 10.1227/01.neu.0000303203.07866.18. [DOI] [PubMed] [Google Scholar]
- 66.Hooper NM, Karran EH, Turner AJ. Membrane protein secretases. Biochem. J. 1997;321(Pt 2):265–279. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solorzano CC, Ksontini R, Pruitt JH et al. Involvement of 26-kDa cell-associated TNF-alpha in experimental hepatitis and exacerbation of liver injury with a matrix metalloproteinase inhibitor. J. Immunol. 1997;158(1):414–419. [PubMed] [Google Scholar]
- 68.Wojtowicz-Praga S. Clinical potential of matrix metalloprotease inhibitors. Drugs R.D. 1999;1(2):117–129. doi: 10.2165/00126839-199901020-00001. [DOI] [PubMed] [Google Scholar]
- 69.Dittmar M, Kiourkenidis G, Horn M et al. Cerebral ischemia, matrix metalloproteinases, and TNF-alpha: MMP inhibitors may act not exclusively by reducing MMP activity. Stroke. 2004;35(7):e338–40. doi: 10.1161/01.STR.0000135294.08862.5d. [DOI] [PubMed] [Google Scholar]
- 70.Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34(8):2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]