Abstract
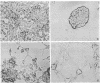
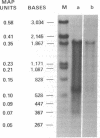
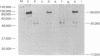
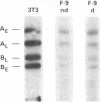
Infection of differentiated mouse embryo cells by simian virus 40 (SV40) leads to the production of the early mRNAs and the tumor (T) antigens that they encode. In contrast, undifferentiated F-9 murine teratocarcinoma cells do not support these early stages of the SV40 cycle. This block results from the inability to accumulate stable processed early SV40 mRNAs. It has recently been shown that vitamin A and its derivatives can induce in vitro differentiation of stem cells. Undifferentiated F-9 cells, upon treatment with a low concentration of retinoic acid, exhibited pronounced morphologic changes as well as the appearance of the H-2 surface antigens. After differentiation, the susceptibility of F9 cells to SV40 infection could be demonstrated by the appearance of large T and small T antigens, as shown by immunofluorescence and immunoprecipitation. Furthermore, SI nuclease mapping of early SV40 transcripts confirmed the presence of the two spliced early mRNAs. These results indicate that the undifferentiated F-9 stem cells contain the genetic information needed for generating stable processed early SV40 mRNAs but are blocked in the production of functional species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstine E. G., Hooper M. L., Grandchamp S., Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanov I., Solter D. Experimental teratoma. Curr Top Pathol. 1974;59:69–130. doi: 10.1007/978-3-642-65857-0_2. [DOI] [PubMed] [Google Scholar]
- Ferdinand F. J., Brown M., Khoury G. Characterization of early simian virus 40 transcriptional complexes: late transcription in the absence of detectable DNA replication. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5443–5447. doi: 10.1073/pnas.74.12.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand F. J., Brown M., Khoury G. Synthesis and characterization of late lytic simian virus 40 RNA from transcriptional complexes. Virology. 1977 May 1;78(1):150–161. doi: 10.1016/0042-6822(77)90087-3. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Mousset S. Isolation and partial characterization of a nuclear RNA polymerase - SV40 DNA complex. FEBS Lett. 1975 Aug 1;56(1):149–155. doi: 10.1016/0014-5793(75)80130-x. [DOI] [PubMed] [Google Scholar]
- Gruss P., Lai C. J., Dhar R., Khoury G. Splicing as a requirement for biogenesis of functional 16S mRNA of simian virus 40. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4317–4321. doi: 10.1073/pnas.76.9.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- KLEINSMITH L. J., PIERCE G. B., Jr MULTIPOTENTIALITY OF SINGLE EMBRYONAL CARCINOMA CELLS. Cancer Res. 1964 Oct;24:1544–1551. [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Khoury G. Deletion mutants of simian virus 40 defective in biosynthesis of late viral mRNA. Proc Natl Acad Sci U S A. 1979 Jan;76(1):71–75. doi: 10.1073/pnas.76.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas as a model system for the study of embryogenesis and neoplasia. Cell. 1975 Jul;5(3):229–243. doi: 10.1016/0092-8674(75)90098-7. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Wiley L. M., Damjanov I. The development of cystic embryoid bodies in vitro from clonal teratocarcinoma stem cells. Dev Biol. 1977 Dec;61(2):230–244. doi: 10.1016/0012-1606(77)90294-9. [DOI] [PubMed] [Google Scholar]
- May E., May P., Weil R. "Early" virus-specific RNA may contain information necessary for chromosome replication and mitosis induced by Simian Virus 40. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1654–1658. doi: 10.1073/pnas.70.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Smith A. E. The sequences between 0.59 and 0.54 map units on SV40 DNA code for the unique region of small t antigen. Cell. 1978 Nov;15(3):1011–1020. doi: 10.1016/0092-8674(78)90285-4. [DOI] [PubMed] [Google Scholar]
- Segal S., Levine A. J., Khoury G. Evidence for non-spliced SV40 RNA in undifferentiated murine teratocarcinoma stem cells. Nature. 1979 Jul 26;280(5720):335–338. doi: 10.1038/280335a0. [DOI] [PubMed] [Google Scholar]
- Sherman M. I., Miller R. A. F9 embryonal carcinoma cells can differentiate into endoderm-like cells. Dev Biol. 1978 Mar;63(1):27–34. doi: 10.1016/0012-1606(78)90110-0. [DOI] [PubMed] [Google Scholar]
- Sherman M. I., Strickland S., Reich E. Differentiation of early mouse embryonic and teratocarcinoma cells in vitro: plasminogen activator production. Cancer Res. 1976 Nov;36(11 Pt 2):4208–4216. [PubMed] [Google Scholar]
- Shih T. Y., Khoury G. Isolation of viral specific RNA from SV40 infected cells by viral DNA chemically linked to a cellulose matrix. Biochemistry. 1976 Feb 10;15(3):487–493. doi: 10.1021/bi00648a005. [DOI] [PubMed] [Google Scholar]
- Solter D., Shevinsky L., Knowles B. B., Strickland S. The induction of antigenic changes in a teratocarcinoma stem cell line (F9) by retinoic acid. Dev Biol. 1979 Jun;70(2):515–521. doi: 10.1016/0012-1606(79)90043-5. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Swartzendruber D. E., Friedrich T. D., Lehman J. M. Resistance of teratocarcinoma stem cells to infection with simian virus 40: early events. J Cell Physiol. 1977 Oct;93(1):25–30. doi: 10.1002/jcp.1040930105. [DOI] [PubMed] [Google Scholar]
- Swartzendruber D. E., Lehman J. M. Neoplastic differentiation: interaction of simian virus 40 and polyoma virus with murine teratocarcinoma cells in vitro. J Cell Physiol. 1975 Apr;85(2 Pt 1):179–187. doi: 10.1002/jcp.1040850204. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp W., Hall J. D., Rifkin D., Levine A. J., Pollack R. The characterization of SV40-transformed cell lines derived from mouse teratocarcinoma: growth properties and differentiated characteristics. J Cell Physiol. 1977 Nov;93(2):269–276. doi: 10.1002/jcp.1040930212. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Feunteun J., Crawford L. V., Berg P., Fiers W. Nucleotide sequence deletions within the coding region for small-t antigen of simian virus 40. J Virol. 1979 Jun;30(3):674–682. doi: 10.1128/jvi.30.3.674-682.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]