Abstract
Background
Parkinson's disease (PD) is the most common neurodegenerative disorder, characterized by loss of dopaminergic neurons in substantia nigra and depletion of dopamine in striatum due to mitochondrial dysfunction, oxidative stress, excitotoxicity, apoptosis, inflammation and proteasome failure.
Purpose
The present study deals with the neuroprotective effect of resveratrol, a wine polyphenol (50 mg/kg body weight) against MPTP (30mg/kg body weight as i.p. administration) induced mice model of idiopathic Parkinson's disease.
Methods
A combination of behaviour tasks and biochemical parameters were tested using standard molecular tools.
Results
Pretreatment of resveratrol significantly reversed toxic effects of MPTP by increasing the levels of dopamine, its metabolites, GSH and activities of GPx and reducing levels of TBARS, catalase and SOD activities along with enhanced behavior performance.
Conclusion
The multifactorial etiology of these diseases suggests that drugs with multiple targets such as resveratrol could have therapeutic potential for these pathologies.
Keywords: Parkinson's disease, MPTP, Resveratrol, Oxidative stress, Behaviour, Dopamine
Introduction
Parkinson's disease (PD) is the most common neurodegenerative disorder; it affects 1% of the population aged 65 years of age, and its incidence increases to 3% over 80 years of age. The clinical symptoms of PD include tremor at rest, rigidity, bradykinesia, postural abnormalities and the freezing phenomenon.1,2 The causes of the degeneration of dopamine neurons are largely unknown. To explore the disease mechanisms and develop new therapies for PD, different animal models treated with a number of neurotoxins, such as cyanide, carbon monoxide, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA), 3-nitropropionic acid (3-NP), malonic acid (MA), rotenone, paraquat and azide,3 have been developed. MPTP is a potent neurotoxin, capable of producing PD-like symptoms in humans, non-human primates and some non-primate animals such as mice.4
Epidemiological studies have shown that moderate consumption of wine and smoking can be protective against neurological disorders.5,6 Moreover, in vitro and in vivo preclinical studies have shown a neuroprotective effect of lyophilized red wine,7 grape polyphenols8 and trans-resveratrol.9 Resveratrol (3, 4, 5-tri-hydroxy-trans-stilbene), a non-flavonoid polyphenol, is naturally present at high concentrations in red wine, grape seeds, berries, knot weed, peanuts and other plants10 and is reported to exert strong neuroprotective effects against many neurological diseases such as ischemia, epilepsy, Alzheimer's disease (AD), PD and Huntington's disease.11,12 Resveratrol was found to protect neuronal cells from 6-hydroxydopamine,13 1 -methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP),14 MPP+,15 nitric oxide and B-amyloid, 16 polyglutamine toxicity,17 kainate 18 and global ischemia.19
Motor function is normally measured by performing a number of behavioral tests, specifically, rotarod performance, 20 stride length, 21 the hang test, the open field test and narrow beam walking.22 These methods are reported to be sensitive enough to detect functional impairments in MPTP-administered PD mouse models and to quantify the potential efficacy of treatments designed to restore dopaminergic function. There is increasing evidence indicating that oxidative stress may contribute to several CNS pathologies, including PD, aging and AD.23 The present work was carried out to determine to what extent resveratrol modulates the behavioral deficits and oxidative stress produced by MPTP administration in C57BL/6 black mice, a commonly used experimental PD model.
Methods
Animals and standard housing conditions
Inbred adult male albino C57BL/6 mice (30-35 g) from the National Institute of Nutrition, Hyderabad, were used in the present study. The animals were kept under 12 h light/dark cycles, at 22°C and 60% humidity with food and water ad libitum. The experimental protocols met with the National Guidelines on the Proper Care and Use of Animals in Laboratory Research(Indian National Science Academy, New Delhi, 2000) and were approved by the Animal Ethics Committee of the Institute (Approval No. 34-/12-281/2008).
Chemicals
MPTP, resveratrol, thiobarbituricacid (TBA), reduced glutathione and 5, 5-Dithiobis[2-nitrobenzoic acid (DTNB)] were procured from Sigma Chemical Co. (St. Louis, MO, USA). All other reagents were of analytical grade and were procured locally.
Experimental procedures
The mice were divided into four groups of twelve animals. The first group of mice was kept as a control. The second group was injected with MPTP for four days (i.p., 30 mg/kg body weight).24 The third group received resveratrol orally (50 mg/kg body weight)14 for seven days and from the fourth day onwards, MPTP (as for group II) was injected i.p. until the seventh day of the experiment. The fourth group received only resveratrol (as for group III) for seven days. At the end of the experiment (eighth day), the following behavioral tests were performed.
Behavioral assessment
Open field test
The mice were placed into one corner of an open field chamber (W100 cmxDIOO cmxH40 cm) made of wood and resin. The floor of this chamber was gridded into 25 cm (5x5) squares and the number of squares crossed (in 5 min) was counted manually in triplicate using a forepaw crossing of a grid line as the criterion (horizontal activity). Vertical activity was measured using the number of observations of grooming and rearing (5 min). The whole study was performed as blind study.22
Rotarod Test
Mice were allowed to adjust their posture in order to maintain their balance on a rotating rod at speeds of 5,10,15 and 20 rpm. The average retention time on the rod was calculated as described previously.25
Hang test
Mice were placed on a horizontal grid and were supported until they held the grid. Then, the grid was inverted so that the mice were allowed to hang upside down. Staying time was measured as described previously.26
Narrow beam walking test
Animals were trained to walk on a narrow flat stationary wooden beam (L100 cmxWI cm) placed at a height of 100 cm from the floor. The time taken to cross the beam from one end to the other was counted as described previously.22 The mice were sacrificed using cervical dislocation, and the brain was dissected to procure striatum and midbrain using the method described by Glowinski and Iversen.27 These tissues were used in the following biochemical analysis.
Biochemical estimations
HPLC analysis of dopamine, Dopamine and its metabolites 3,4-dihydroxy phenylacetic acid (DOPAC) and Homovanillic acid (HVA)
Dopamine and its metabolites (DOPAC and HVA) were analyzed using reversed phase ion-pair chromatography combined with electrochemical detection under isocratic conditions. The mobile phase (0.6 mM 1-octanesulfonic acid, 0.27 mM Na.EDTA, 0.043 M triethylamine and 35 ml acetonitrile/L, adjusted to pH 2.95 with H3PO3) was delivered at a flow rate of 0.65 ml/min at 22°C onto the reversed phase column filled with Nucleosil 120-3 C18 (Knaur, Berlin, Germany). Data was calculated by an external standard calibration.28
Thiobarbituric acid reactive substances (TBARS)
The activity of TBARS was determined as described previously.29 Briefly, the tissue extracts were incubated with 0.2 ml phenyl methosulfate at 37°C in metabolic water bath shaker. After 1 h of incubation, 0.4 ml of 5% tricarboxylic acid and 0.4 ml of 0.67% thiobarbituric acid were added. The reaction mixture was centrifuged at 4000 rpm for 15 min, and the supernatant was boiled for 10 min. After cooling, the samples were read at 535 nm. The rate of lipid peroxidation was expressed as nmol of TBARS formed/h/g tissue.
Superoxide dismutase (SOD)
SOD activity was assayed using an indirect inhibition assay, in which xanthine and xanthine oxidase serve as a superoxide generator, and nitro blue tetrazolium (NBT) is used as a superoxide indicator. The assay mixture consisted of 960 μl of 50 mM sodium carbonate buffer (pH 10.2) containing 0.1 mM xanthine, 0.025 mM NBT, and 0.1 mM EDTA, 20 μl of xanthine oxidase and 20 μl of the brain supernatant. Changes in absorbance were observed spectrophotometrically at 560 nm. The activity was expressed as units/min/mg protein.30
Catalase
Catalase activity was assayed by measuring the rate of decomposition of hydrogen peroxide at 240 nm.31 The assay mixture consisted of 50 μ1 of M Tris-HCI buffer (pH 8.0) containing 5 mM EDTA, 900 £<l of 10 mM H202.30 μl of MQ water and 20 μl of the brain tissue supernatant. The rate of decomposition of hydrogen was observed spectrophotometrically at 240 nm. The enzyme activity was expressed as nmol of hydrogen peroxide decomposed/min/mg protein.
Glutathione peroxidase (GPx)
The GPx assay mixture consisted of 100 μl of M Tris-HCI (pH 8.0) containing 5 mM EDTA, 20 μl of 0.1 M GSH, 100 μl of GSH reductase solution (10 units/ml), 100 μl of 2 mM NADPH, 650 μl of distilled water, 10 μl of 7 mM tert-butyl hydroperoxide and 10 μl of the brain supernatant. Oxidation of NADPH was determined spectrophotometrically at 340 nm. One unit of activity was defined as the amount of GPx required to oxidize 1 μmol of NADPH per min.32
Reduced glutathione
The level of GSH in the brain homogenate was measured by the method described by Jollow etal.33 Brain tissue homogenate was centrifuged at 16,000xg for 15 min at 4°C. The supernatant (0.5 ml) was added to 4 ml of ice-cold 0.1 mM solution of 5,5-Dithiobis [2-nitrobenzoic acid (DTNB)] in 1 M phosphate buffer (pH8).The optical density was read at 412 nm in a spectrophotometer.
Statistical analysis
All the data were expressed as mean±SD of the number of experiments (n = 6). Statistical significance was evaluated using one-way analysis of variance (ANOVA) using SPSS version 10.0 software, and individual comparisons were obtained using Duncan's Multiple Range Test (DMRT). Values were considered statistically significant if p < 0.05.
Results
Figure 1 shows the levels of dopamine and its metabolites (DOPAC and HVA) in corpus striatum of the normal and experimental groups. The levels of these metabolites in the striatum were significantly depleted in PD mice (group II) as compared to control animals. Oral administration of resveratrol to MPTP-exposed mice (group III) significantly improves DOPAC and HVA levels as compared to group II animals. Table I depicts the levels of TBARS and GSH and the activities of SOD, catalase and GPx in the substantia nigra of the normal and experimental groups. The levels of TBARS and the activities of SOD and CAT in corpus striatum were significantly elevated, and the levels of GSH and the activities of GPx were significantly diminished in MPTP-treated animals (group II) as compared to control. Prior administration of resveratrol to MPTP-treated mice (group III) tends to reverse the oxidative stress.
Fig. 1:
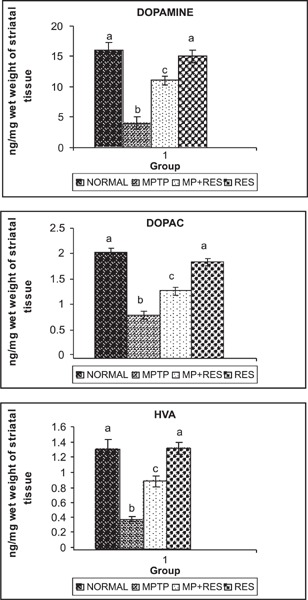
(a,b,c) shows the levels of neurochemicals in control and experimental mice.
Table 1. Changes in the levels of TBARS, GSH and activities of SOD, catalase and GPx in midbrain of control and experimental mice.
| Groups/Variables | Control | MPTP | MPTP+RES | RES |
|---|---|---|---|---|
| a= nmol NADPH oxidised/minute/mg protein; B= amount of enzyme required to inhibit 50% of NBT reduction; c= nmol H202 consumed/minute/mg protein; Values are given as means ±SD for six mice in each group. Values not sharing a common superscript letter differ significantlyatp<0.05(DMRT). | ||||
| TBARS (nmoles /g of wet tissue) | 1.55±0.10a | 3.78±0.30b | 2.31±0.22c | 1.49±0.14a |
| GSH (g/ g of wet tissue) | 12.5+1.21a | 6.19±0.60b | 9.11±0.93c | 12.7±1.2a |
| GPx (UA/mg protein) | 12.1±1.32a | 7.79±.70b | 9.01±0.85c | 13.31±1.12a |
| SOD (UB/mg protein) | 1.41 ±0.12a | 4.15±0.40b | 3.55±0.35c | 1.46±0.21a |
| Catalase (Uc/mg protein) | 1.8±0.19a | 2.6±0.28b | 2.19±0.17c | 1.86±0.10a |
Figure 2 shows the significant reduction (p<0.05) in peripheral movements, rearing and grooming in MPTP-injected animals (group II) when compared to controls. Administration of resveratrol (group III) to the MPTP-lesioned mice significantly increased the activity of the mice in the open field test (p < 0.05).
Fig. 2:
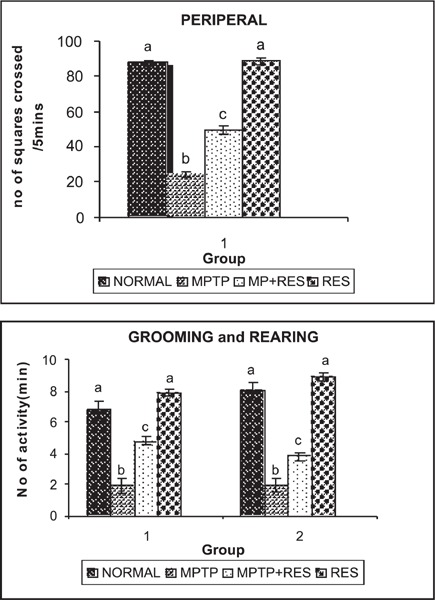
(a,b) shows the behaviour of control and experimental mice in open field test.
Figure 3 shows that MPTP treatment increased beam-crossing duration and reduced hang time as compared with control mice (group I). Administration of resveratrol (group III) to the lesioned animals significantly reduced crossing time and increased hang time (p<0.05), which indicates an improvement in motor function.
Fig. 3:
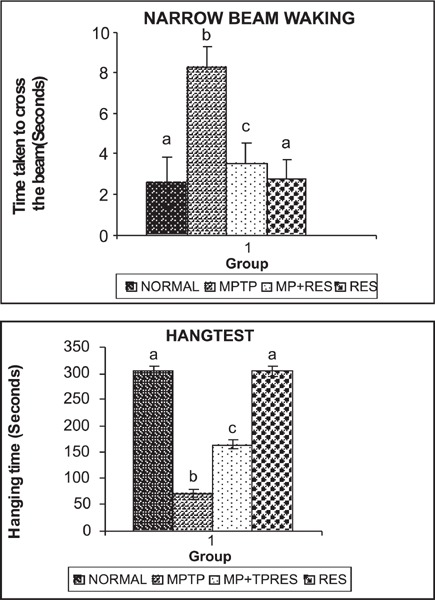
(a,b) shows the time taken to cross beam and hang.
PD mice showed a significant reduction in retention time on the rotarod compared to controls. Administration of resveratrol to the PD mice for 7 days improved the retention time in the PD mice compared to the resveratrol-untreated PD mice (Figure 4).
Fig. 4:
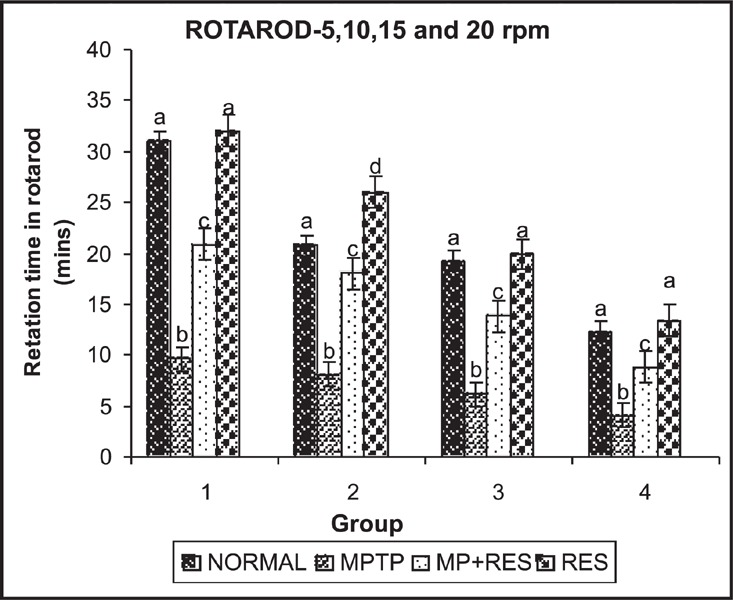
shows the retention time of mice in rotorod at different rpm (5,10,15,20).
Discussion
The hallmark of PD is a severe reduction in dopamine and its metabolites in all regions of the striatum, basal ganglia, substantia nigra pars compacta and reticulata. Moderate losses of dopamine are found in the lateral hypothalamus, medial olfactory region and amygdaloid nucleus.34 In the non-degenerative neurons in patients with PD, dopamine turnover appears to be greatly increased, judging from the concentrations of HVA in the nerve terminals in the striatum which is consistent with our results and the cell bodies and dendrites in the substantia nigra.35
The present results showed that MPTP treatment increased lipid peroxidation in the brain and increased the activities of SOD and catalase. Acute treatment of MPTP caused a significant enhancement in the levels of TBARS and increased the specific activity of SOD and CAT in both striatum and midbrain. 36,37 Elevations in oxidative products, such as lipid peroxides,38 protein carbonyls 39 and products of nucleic acid 8-hydroxyguanosine 40 have been observed in the substantia nigra of PD patients.
Human postmortem analysis has revealed increased lipid peroxidation and SOD activities along with elevated free iron levels in the substantia nigra of Parkinsonian patients.41 This increase may reflect an adaptive response due to a leakage of superoxide anion resulting from mitochondrial respiration impairment, as suggested by42 Thiffault ef al. In the present study, MPTP-treated animals showed marked depletion of GSH in the midbrain, which is in support of previous findings in other strains of mice.28,43,44 The activity of GPx was found to be reduced in our experiment, which is consistent with previous experiments. 45 The decreased levels of GSH and GPx in MPTP-lesioned mice in our study also confirm that MPTP induces a state of oxidative stress in the brain. Interestingly, treatment of PD mice with resveratrol produces a notable improvement in the levels of GSH and GPx in the PD mouse.
The behavioral effects of PD are closely linked to the degree of neuronal dysfunction. 46 Studying behavioral deficits in animal models of PD is of particular interest in order to investigate the relationship among neuronal degeneration, the effectiveness of potential therapeutics, brain recovery processes and corresponding behavioral changes. Rotarod performance, 25 stride length 21 and the pole test 47 have also been reported as sensitive methods that measure more complex motor function in both acute and chronically administered MPTP mouse models. The rotarod test, which requires animals to balance and walk on a rotating cylinder, is widely used to measure coordinated motor skills,48 and it has also been employed in the MPTP mouse model. MPTP-treated mice cannot maintain their balance on a rotating rod, two days 49 or even hundred days after treatment.20,25 In the majority of open field studies, decreases in locomotion and/or rearing following treatment with MPTP were found, which indicates hypokinesia. Such hypoactivity was found within 30 min after treatment with MPTP 50 and persisted 40 weeks after treatment. 51 The beam-walking task has the ability to assess fine motor initiation, coordination and postural balance of an individual animal. Previously, the beam-walking apparatus has been used to detect sensorimotor impairments in rat models of stroke, 52 transgenic mouse models of Parkinson's and Huntington's disease 53,54 and aged rats with altered dopaminergic function.55 The hang test is the indicator for neuromuscular strength. 26 A significant reduction in hang time was found in MPTP-injected animals. Pre-administration of resveratrol to the lesioned animals modulates these behavioral changes significantly. The cells of the midbrain use dopamine (a neurotransmitter, or chemical messenger between brain and nerve cells) to communicate with the cells in another region of the brain called the striatum. Thus, a reduction in nigral dopamine levels results in a decrease in stratial dopamine that is believed to cause PD symptoms. 23 Pretreatment with resveratrol tends to recover the behavior of MPTP-exposed mice by restoring dopamine levels.
The pathogenesis of Parkinson's disease is multifactorial with toxic reactions including inflammation, glutamatergic toxicity, dysfunction of mitochondrial activity and of the ubiquitin/proteasome system, activation of apoptosis pathways, elevation of iron and nitric oxide and alteration of the homeostasis of antioxidants/oxidation 56 found that a resveratrol-rich diet prevented the depletion of striatal DA in an acute MPTP mice model of PD. Recent evidence indicates that inhibition of oxidative stress and neuroinflammation by resveratrol can prevent, in part, the degeneration of DA neurons in mixed glial-neuron cell cultures 57 In cerebellar neurons, the antiapoptotic actions of resveratrol are mainly mediated by its antioxidant properties, 58,59 which may impact regulatory systems, such as mitochondrial superoxide dismutase. 60 In resveratrol and MPTP-treated mice, we noted a substantial decrease in oxidative stress and an improvement in behavior patterns and neurochemical levels. Finally, our present findings and other data 61,62 demonstrate that naturally occurring and pharmacologically active molecules like resveratrol might be regarded as powerful complementary and/or preventive therapies for neurodegenerative diseases.
Acknowledgment
Financial assistance in the form of a major research project from the University Grant Commission, New Delhi, is gratefully acknowledged.
Footnotes
Competing interests - None, Source of Funding - UGC
References
- 1.Patel S, Sinha A, Parmar D et al. An update on the role of environmental factors in Parkinson's disease. Annals of Neurosciences. 2005;12:79–86. [Google Scholar]
- 2.Hamani C, Lozano AM. Physiology and pathophysiology of Parkinson's disease. Ann NY Acad Sci. 2003;991:15–21. doi: 10.1111/j.1749-6632.2003.tb07459.x. [DOI] [PubMed] [Google Scholar]
- 3.Alexi T, Borlongan CV, Faull RLM et al. Neuroprotective strategies for basal ganglia degeneration: Parkinson's and Huntington's diseases. Prog Neurobiol. 2000;60:409–70. doi: 10.1016/s0301-0082(99)00032-5. [DOI] [PubMed] [Google Scholar]
- 4.Przedborski S, Tieu K, Perier C et al. MPTP as a mitochondrial neurotoxic model of Parkinson's disease. J Bioenerg Biomembr. 2004;36:375–79. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- 5.Orgogozo JM, Dartigues JF, Lafont S et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeauxarea. Rev Neurol. 1997;3:185–92. [PubMed] [Google Scholar]
- 6.Prabhakar S, Vinish M, Das CP et al. Occurrence of PARK2 Mutations in a Never-Smoker Population with Parkinson's Disease in North India. Neuroepidemiology. 2010;35:152–159. doi: 10.1159/000313855. [DOI] [PubMed] [Google Scholar]
- 7.De Ruvo C, Amodio R, Algeri S et al. Nutritional antioxidants as antidegenerative agents. Int J DevNeurosci. 2000;18:359–66. doi: 10.1016/s0736-5748(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 8.Sun GY, Xia J, Draczynska-Lusiak B et al. Grape polyphenols protect neurodegenerative changes induced by chronic ethanol administration. Neuroreport. 1999;10:93–96. doi: 10.1097/00001756-199901180-00018. [DOI] [PubMed] [Google Scholar]
- 9.Virgili M, Contestabile A. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neurosci Lett. 2001;281:123–26. doi: 10.1016/s0304-3940(00)00820-x. [DOI] [PubMed] [Google Scholar]
- 10.Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53:115–28. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- 11.Markus MA, Morris BJ. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging. 2008;3:331–39. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F, Liu J, Shi JS. Anti-inflammatory activities of resveratrol in the brain: Role of resveratrol in microglial activation. Eur J Pharmacol 2010 (in press) doi: 10.1016/j.ejphar.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Khan MM, Ahmad A, Ishrat T et al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson's disease. Brain Res. 2010;1328:139–51. doi: 10.1016/j.brainres.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Blanchet J, Longpre F, Bureau G et al. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog Neuro-Psychopharmacol Bio Psychiatry. 2008;32:1243–50. doi: 10.1016/j.pnpbp.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Gelinas S, Martinoli MG. Neuroprotective effect of estradiol and phytoestrogens on MPP+-induced cytotoxicity in neuronal PC12 cells. J Neurosci Res. 2002;70:906–13. doi: 10.1002/jnr.10315. [DOI] [PubMed] [Google Scholar]
- 16.Bastianetto S, Zheng WH, Quirion R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br J Pharmacol. 2000;131:711–20. doi: 10.1038/sj.bjp.0703626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker JA, Arango M, Abderrahmane S et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–50. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Yu S, Simonyi A et al. Resveratrol protects against neurotoxicity induced by kainic acid. Neurochem Res. 2004;29:2105–12. doi: 10.1007/s11064-004-6883-z. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Xu J, Rottinghaus GE et al. Resveratrol protects against global ischemic injury in gerbils. Brain Res. 2002;958:439–47. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 20.Banik A, Anand A. Loss of learning in mice when exposed to rat odor: A water maze study.; Behav Brain Res; 10.1016/j.bbr.2010.07035; 2000. [DOI] [PubMed] [Google Scholar]
- 21.Fernagut PO, Diguet E, Labattu B et al. A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J Neurosci Meth. 2002;113:123–30. doi: 10.1016/s0165-0270(01)00485-x. [DOI] [PubMed] [Google Scholar]
- 22.Rajasankar S, Manivasagam T, Surendran S. Ashwagandha leaf extract: A potential agent in treating oxidative damage and physiological abnormalities seen in a mouse model of Parkinson's disease. Neurosci Lett. 2009;454:11–15. doi: 10.1016/j.neulet.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 23.Jenner P. The MPTP-treated primate as a model of motor complications in PD: primate model of motor complications. Neurology. 2003;23:4–11. doi: 10.1212/wnl.61.6_suppl_3.s4. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Xu HM, Yang HD et al. Rg1 reduces nigral iron levels of MPTP-treated C57BL6 mice by regulating certain iron transport proteins. Neurochem Int. 2009;54:43–8. doi: 10.1016/j.neuint.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Anand A, Saraf MK, Prabhakar S. Sustained inhibition of brotizolam induced anterograde amnesia by norharmane and retrograde amnesia by L-glutamic acid in mice. Behavioral Brain Research. 2007;182:12–20. doi: 10.1016/j.bbr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Mohanasundari M, Srinivasan MS, Sethupathy S et al. Enhanced neuroprotective effect by combination of bromocriptine and Hypericum perforatum extract against MPTP-induced neurotoxicity in mice. J Neurol Sci. 2006;249:140–44. doi: 10.1016/j.jns.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Glowinski J, Iversen LL. Regional studies of cateholamines in the rat brain. J Neurochem. 1966;13:655–69. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 28.Muralikrishnan D, Mohanakumar KP. Neuroprtotection by bromocriptine against 1- methyl 4-pheny1 1,2,3,6 tetrahydrophyridine induced neurotoxicity in mice. FASEB J. 1998;12:905–12. doi: 10.1096/fasebj.12.10.905. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharya A, Ghosal S, Bhattacharya SK. Antioxidant effect of Withania somnifera glycowithanolides in chronic foot-shock stress-induced perturbations of oxidative free radical scavenging enzymes and lipid peroxidation in rat frontal cortex and striatum. J Ethnopharmacol. 2001;74:1–16. doi: 10.1016/s0378-8741(00)00309-3. [DOI] [PubMed] [Google Scholar]
- 30.Oberley LW, Spitz DR. Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol. 1984;105:457–64. doi: 10.1016/s0076-6879(84)05064-3. [DOI] [PubMed] [Google Scholar]
- 31.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–26. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto Y, Takahashi K. Glutathione peroxidase isolated from plasma reduces phospholipid hydroperoxide. Arch Biochem Biophys. 1993;305:541–45. doi: 10.1006/abbi.1993.1458. [DOI] [PubMed] [Google Scholar]
- 33.Jollow DJ, Mitchell JR, Zampaglione N et al. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–69. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 34.Yurek DM, Sladek JR. Dopamine cell replacement: Parkinson's disease. Annu Rev Neurosci. 1990;13:415–40. doi: 10.1146/annurev.ne.13.030190.002215. [DOI] [PubMed] [Google Scholar]
- 35.Agid Y, Ruberg M, Agid JF et al. Are dopaminergic neurons selectively vulnerable to Parkinson's disease? Adv Neurol. 1993;60:148–64. [PubMed] [Google Scholar]
- 36.Rajasankar S, Manivasagam T, Krishnamurti A et al. The neuroprotective effect of Withania somnifera root extract in MPTP-intoxicated mice: An analysis of behavioral and biochemical variables. J Cell Tissue Res. 2007;7:473–81. doi: 10.2478/s11658-007-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajeswari A. Curcumin protects mouse brain from oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Eur Rev Med Pharmacol Sci. 2006;10:157–61. [PubMed] [Google Scholar]
- 38.Groc L, Bezin L, Foster JA et al. Lipid peroxidation-mediated oxidative stress and dopamine neuronal apoptosis in the substantia nigra during development. Neurochem Int. 2001;39:127–33. doi: 10.1016/s0197-0186(01)00013-4. [DOI] [PubMed] [Google Scholar]
- 39.Alam ZI, Daniel SE, Lees AJ et al. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem. 1997;69:1326–29. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 40.Vinish M, Milstein J. Non motor aspects of Parkinson's disease. Annals of Neurosciences. 2009;16(4):176–179. [Google Scholar]
- 41.Sian J, Gerlach M, Youdim MH et al. Parkinson's disease: A major hypokinetic basal ganglia disorder. J Neural Transm. 1999;106:443–76. doi: 10.1007/s007020050171. [DOI] [PubMed] [Google Scholar]
- 42.Thiffault C, Anumount N, Quirion R et al. Effect of MPTP and L-deprenyl on antioxidant enzymes and lipid peroxidation levels in mouse brain. J Neurochem. 1995;65:2725–33. doi: 10.1046/j.1471-4159.1995.65062725.x. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Zhou Y, Chen Y. Ginsenoside Rgz 1 reduces MPTP induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol Sin. 2005;26:56–62. doi: 10.1111/j.1745-7254.2005.00019.x. [DOI] [PubMed] [Google Scholar]
- 44.Munoz A, Rey P, Guerra MJ et al. Reduction of dopaminergic degeneration and oxidative stress by inhibition of angiotensin converting enzyme in a MPTP model of parkinsonism. Neuropharmacology. 2006;51:112–20. doi: 10.1016/j.neuropharm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Gene S, Akhisaroglu M, Kuralay F et al. Erythropoietin restores glutathione peroxidase activity in 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine-induced neurotoxicity in C57BL mice and stimulates murine astroglial glutathione peroxidase production in vitro. Neurosci Lett. 2002;321:73–76. doi: 10.1016/s0304-3940(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 46.Schwarting RK, Bonatz AE, Carey RJ et al. Relationships between indices of behavioral asymmetries and neurochemical changes following mesencephalic 6-hydroxydopamine injections. Brain Res. 1991;554:46–55. doi: 10.1016/0006-8993(91)90170-z. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa N, Hirose Y, Ohara S et al. A simple quantitative bradykinesia test in MPTP-treated mice. Res Commun Chem Pathol Pharmacol. 1985;50:435–41. [PubMed] [Google Scholar]
- 48.Kelly MA, Rubinstein M, Phillips TJ et al. Locomotor activity in D2 dopamine receptor deficient mice is determined by gene dosage, genetic background and development adaptations. J Neurosci. 1998;18:3470–79. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colotla VA, Flores E, Oscos A et al. Effects of MPTP on locomotor activity in mice. Neurotoxicol Teratol. 1990;12:405–47. doi: 10.1016/0892-0362(90)90061-g. [DOI] [PubMed] [Google Scholar]
- 50.Platel A, Strolin-Benedetti M, Guffroy C. In: MPTP-induced decrease in motor activity in two strains of mice: its reversal by different monoamine oxidase inhibitors (MAOIs). In: Markey SP, Castagnoli N Jr, Trevor AJ, Kopin IJ, editors. MPTP: a Neurotoxin Producing a Parkinsonian Syndrome. Orlando, FL: Academic Press; 1986. pp. 443–47. [Google Scholar]
- 51.Ferger B, Spratt C, Earl CD et al. Effects of nicotine on hydroxyl free radical formation in vitro and on MPTP-induced neurotoxicity in vivo. Naunyn-Schmiedeberg'sArch Pharmacol. 1998;358:351–59. doi: 10.1007/pl00005264. [DOI] [PubMed] [Google Scholar]
- 52.Virely DJ, Beech JS, Smart SC et al. A temporal MRI assessment of neuropathology after transient middle cerebral artery occlusion in the rat: correlations with behaviour. J Cereb Blood Flow Metab. 2000;20:563–82. doi: 10.1097/00004647-200003000-00015. [DOI] [PubMed] [Google Scholar]
- 53.Carter RJ, Lione LA, Humby T et al. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci. 1999;19:3248–57. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang DY, Fleming SM, Ardayfio P et al. 3,4-Dihydroxyphenylalanine reverses the motor deficits in pitx3-deficient aphakia mice: behavioural characterization of a novel genetic model of Parkinson's disease. J Neurosci. 2005;25:2132–37. doi: 10.1523/JNEUROSCI.3718-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drucker-Colin R, Garcia-Hernandez F. A new motor test sensitive to aging and dopaminergic function. J Neurosci Meth. 1991;39:153–61. doi: 10.1016/0165-0270(91)90081-a. [DOI] [PubMed] [Google Scholar]
- 56.Anderson DW, Bradbury KA, Schneider JA. Neuroprotection in Parkinson's models varies with toxin administration protocol. Eur J Neurosci. 2006;24:3174–82. doi: 10.1111/j.1460-9568.2006.05192.x. [DOI] [PubMed] [Google Scholar]
- 57.Bureau G, Longpre F, Martinoli MG. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced byneuroinflammation. J Neurosci Res. 2007;86:403–10. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- 58.Alvira D, Yeste-Velasco M, Folch J et al. Comparative analysis of the effects of resveratrol in two apoptotic models: inhibition of complex I and potassium deprivation in cerebellar neurons. Neurosci. 2007;147:746–756. doi: 10.1016/j.neuroscience.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 59.Patri M, Padmini A, Babu PP. Polyclinic aromatic hydrocarbons in air and their neurotoxic potency in association with oxidative stress. A brief perspective. Annals of Neurosciences. 2009;16(1):22–30. [Google Scholar]
- 60.Robb EL, Page MM, Wiens BE et al. Molecular mechanisms of oxidative stress resistance induced by resveratrol: specific and progressive induction of MnSOD. Biochem Biophys Res Commun. 2008;367:406–412. doi: 10.1016/j.bbrc.2007.12.138. [DOI] [PubMed] [Google Scholar]
- 61.Anekonda TS. Resveratrol a boon for treating Alzheimer's disease? Brain Res Rev. 2006;52:316–326. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Baur JA, Pearson KJ, Price NL et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]


