Abstract
Background
There is an increasing evidence that psychological stress and depression trigger changes in various biochemical parameters in animals and human subjects. Chronic stress in rats, and psychosocial stress in humans, is implicated in the pathophysiology of mood and anxiety disorders.
Purpose
The current study was been designed to investigate the behavioral, anatomical status of rat brain and expression of serotonin receptor (5HT2A) mRNA related to pathophysiology of stress after high light (HL) illumination and to evaluate the effect of Phyllanthus amarus (PA) over such stress induced changes in mid brain and prefrontal cortex region of rat brain.
Methods
Bright light illumination was used to induce stress in wistar rats. Established methods were used to analyse biochemical and histopathological tests.
Results
PA administration to HL stressed animals for 7 days prevented stress-induced oxidative damage, as evidenced by significant enhancement of key endogenous antioxidant defense components. HL stress also caused reduced activities of membrane bound enzymes in synaptic membrane along with reduced levels of lipid profile and glycoprotein in the midbrain region. Stress induced changes in the locomotor activity, time spent for exploring the center of the arena, frequency of rearing and grooming, and frequency of facing the corner of the arena, were altered on PA administration.
Conclusion
The findings suggest that the therapeutic efficacy of PA may be mediated, atleast partially, via reversal of oxidative damage. Further the study demonstrates the promising intervention by PA on the mRNA expression of 5HT 2A in the brain. These preliminary results pave the way for further validation of PA against stress.
Keywords: High light illumination, Chronic stress, Depression, Phyllanthus amarus
Introduction
Stress is inevitable in every walk of life and reverberates in any individual’s life, proving detrimental. Stress is a major contributor of depression and by the year 2020, it may rank second in morbidity among all illnesses worldwide. Depression may arise when neuronal systems do not exhibit appropriate, adaptive plasticity in response to external stimuli such as stress. Stress/depression has been shown to increase neuronal apathy/death. Stress exerts detrimental effects on several cell functions, through impairment of antioxidant defenses, leading to oxidative damage, which is central to many diseases.1 Free-radical damage by reactive oxygen species has been suggested to play a critical role in the pathophysiology of diseases, neuropsychiatric disorders and stress induced depression. Although clinical depression, depressive symptoms and psychological stress should be distinguished, they are closely related with one another2 and play an important role in the development of affective disorders. Repeated chronic stress has been associated with the development and manifestation of depression.3
Serotonergic neurotransmission in the central nervous system (CNS) is believed to be involved in this pathogenesis and recovery from depression.4 Clinical findings suggest that enhancement of serotonergic neurotransmission underlies the antidepressant response associated with most agents presently available to treat depression.5 Studies have shown that rats subjected to chronic stress exhibit behavioral, biochemical and physiological impairments.
Phyllanthus amarus (PA) is a rich source of plant chemical and all of its parts constitute biologically active compounds. It has a long back history documented in reducing pain and cholesterol maintanence. Some of the earlier studies showed that it possesses antioxidant, antidiabetic, antitumor properties and that it suppresses the expression of hepatitis B viral mRNA in cell lines.6 Traditionally, PA also has been used to treat jaundice, gonorrhea, uncontrolled menstruation, dysentery.7 The aqueous extract of PA had been employed for treatment of nervous debility, epilepsy, as medhya(intellect promoting) and in vata disorders antiamnesic effects,8 and thus the plant with multipotentiated effects was selected for an evaluation as an antistress drug.
Light is an important environmental factor for regulation of mood. As rats are nocturnal and are sensitive to extreme bright light environment, light illumination was selected in the current study as a stressor for the stress-induced depression models. Serotonergic neurons may play a particularly important role in the facilitation of anxiety-related physiological or behavioral responses. A wealth of evidence supports an association between the neuronal activity of brainstem serotonergic neurons and the level of somatic motor activity or behavioral arousal on exposure to uncontrollable stress or other anxiety related stimuli including anxiogenic drugs and social defeat. Bright light can be used as an effective aversive stimulus leading to an increase in avoidance behaviors. Exposure of rats to the HL(400–500 lux) condition performed in the current study was comparable to previous behavioral studies using a similar behavioral test arena for an alternative anxiety paradigm, i.e. the social interaction test which will be a suitable model for analyzing the light induced variations in the brain.9 The aim of the investigation is to analyze light induced variations in the brain of rats and the potential of Phyllanthus amarus in targeting the variations.
Methods
Animals. Adult Male albino rats of Wistar strain weighing about 150–200g were obtained from the Tamilnadu Veterinary and Animal Science University, Chennai, India. The animals were acclimatized to animal house conditions, fed commercial pellet rat chow (Hindustan Lever Ltd., Bangalore, India) and had free access to water. This study was conducted according to the ethical norms approved by Ministry of Social Justices and Empowerment, Government of India and by Animal Ethics Committee Guidelines of our Institution (360/01/a CPCSEA).
Stress Induction
To induce stress, rats were exposed to bright light illumination. In the present study, we attempted to manipulate the aversiveness of the test conditions by comparing undisturbed control and gently handled Wistar rats, that were exposed to bright light (400–500 lx) illumination in the box.
Each experimental animal was randomly assigned to one of four treatment groups: i.e. Group I -control (CO), Group II- high-light test (HL: 400–500 lx throughout the box), Group III- light and PA(200 mg/kg bwt) treated (LPA) and Group IV- drug alone (PA 200 mg/kg bwt)10 treated groups. CO rats were left undisturbed on the test day. An hour before the stress induction treatment drug (PA) is given to LPA group. After acclimatization altogether 4 groups, HL and LPA groups were stressed per day (for a total of 7 days) between 9:00 a.m. and 1:00 p.m. During the stress procedure, the experimental group of rats were deprived of food and water, each group consisting of six animals.
Histopathological analysis
The brain tissue was excised and fixed in 10% phosphate buffered formalin. The tissue sections were embedded in paraffin wax and sectioned at 5–6 μm thickness. Sections were then stained with hematoxylin and eosin to evaluate the cellular pattern in the brain tissue of control and experimental groups.
Biochemical profile
100mg of the whole brain tissue was weighed, uniformly homogenized with 1 ml of 0.1 M phosphate buffer, pH 7.4 and the homogenate was used for the following biochemical assays. Part of the assays was also performed using the synaptosomal plasma membrane isolated by gradient centrifugation techniques. 0.1 ml of the whole homogenate and 20 ml of synaptic plasma membrane diluted to 0.1 ml was used for the assay.
Isolation of synaptosomes
Fresh brain tissue was suspended in ice cold 5.0 mM Tris Hcl buffer containing 0.32 M sucrose (pH 7.4). The suspended tissue was gently disrupted in a Teflon glass homogenizer; w/v ratio 1:10. The homogenate was centrifuged at 1000 g for 5 minutes at 4°C. The supernatant was taken and centrifuged at 17,000 g for 15 minutes at 4°C.
The pellet thus obtained was the synaptosomal fraction. It was suspended in 10.0 mM Tris Hcl buffer containing 136.0 mM sodium chloride, 5.0 mM potassium chloride, 0.16 mM calcium chloride, 0.1 mM ethylene diamine tetra acetic acid, 1.3 mM magnesium chloride and 10.0 mM glucose at a protein concentration of 1.0 mg/ml.
Isolation of synaptosomal plasma membrane
Synaptic plasma membrane was prepared by hypo-osmotic lysis of the synaptosomes, homogenization, and separation of the synaptic plasma membrane fron myelin and mitochondria by sucrose gradient centrifugation following the method of C.W. Cotman.11
Total protein was estimated by the method of.12 Induction of oxidative damage was ascertained by measuring the extent of LPO in brain tissue extract. LPO was estimated by the method of Ohkawa et al.13 Superoxide dismutase (SOD) was estimated by method of Markland.14 Catalase (CAT) was estimated by the method of Sinha.15 Glutathione-S-transferase (GST) was estimated by the method of Habig.16 Glutathione peroxidase (GPx) was estimated by method of Rotruck.17 Reduced glutathione (GSH) was estimated by the method of Moron18 and vitamin C was estimated by the method of Omaye.19
Assay of ATP ases
The enzyme analyses were performed in the synaptic membrane of brain cells from mid brain of stress induced, drug treated and control rats. Na-k2+ ATP ase was estimated by the method of Bonting.20 Mg2+ ATP ase was estimated by the method of Ohinishi.21 Ca2+ ATP ase was estimated by the method of Desai.22
Assays of enzymes
The enzyme analyses were performed in the synaptic membrane of brain cells from mid brain of stress induced, drug treated and control rats. Activities of 5’ND by the method of Fini,23 AChE was estimated by method of Ellman.24
Lipid profile
Estimation of lipid profile Total lipid was extracted from the brain sample according to the method of Folch.25 Tissue cholesterol content was estimated by the method of Parekh and Jung.26 Free cholesterol was estimated by the method of Leffler and Mc Dougald.27 [Phospholipids were estimated by the method of Rouser, after digesting the lipid extract with perchloric acid. Triglycerides were estimated by the method of Rice.28 Free fatty acid content was estimated by the method of Horn and Menahan.29
Estimation of PBCs
Hexose was estimated by the method of Neibes.30 Hexosamine was estimated by the method of Wagner.31 Fucose content was estimated by the method of Winzler.32 The estimation of sialic acid was done by the method of Warren.33
RT-PCR analysis
Total RNA was extracted with the method of guanidine isothiocyanate and phenol. The content of creceptor mRNA was determined by reverse transcription-PCR presenting a modification of the method described elsewhere.34 For reverse transcription 1 μg total RNA, 180 ng random primer, and 16 μl sterile 2.25 μmol KCl were mixed in a 0.5 ml tube, denaturated at 94°C for 5 min and annealed at 41°C. A buffer (15 l) μ containing 200 U M-MulV reverse transcriptase (Biosan), 0.225 μ mol Tris-HCl (pH 8.1-8.3), 0.015 μmol each dNTP, 0.225 μmol dithiothreitol, and 0.03 μmol MnCl was added to the tube. The mixture (final volume 31 μl) was incubated at 41°C for 60 min. The cDNA obtained was stored at –20°C. An aliquot (1 μl) was mixed with 2.5 μl mixture of 5HT2A -receptor gene-specific primers(5'-TGCAGAATGCCACCAACTT and 5'-TGCCACAAAAGAGCCTATGAG, 5 pmol each) and 14 μl buffer containing 0.8 U Taq-polymerase (Medigen), 1 μmol KCl, 0.2 μmol Tris-HCl (pH 9.0), 0.004 μmol dNTP, 0.15% Triton X-100, and 0.03 μmol MgCl (final volume 17.53 μl). The mixture was denaturated at 94°C, for 4 min and amplified (10 cycles: 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C). β-Actin primers (5 GGGAACCGCTCATTGCC and 5 – ACCCACACTGTGCCCATCTA, 2.5 μl, 5 pmol each) were added to tubes, denaturated at 94°C for 4 min and amplified in additional 25 cycles. Preliminary experiments under the same conditions revealed linear amplification of β-actin and 5HT2A receptor gene fragments within 27 and 38 cycles, respectively. Amplification products (15 μl) were mixed with 5 μl 0.35% orange G in 30% sucrose and analyzed by electrophoresis in 1 agarose with 0.5x TBE buffer. After ethidium bromide staining, the gels were scanned in UV and band intensity was determined with the help of Scion Image Program (Scion Corporation).
Statistical analysis
All statistical computations were performed using SPSS tool. Values of experimental groups are given as mean, (±) SD. Two-way ANOVA with Bonferroni post test analysis was used to determine statistical significance. P<0.05 was considered to be statistically significant.
Results and Discussion
Exposure of rats to the high light condition increased multiple measures of anxiety-related behavior of group II rats. Increasing the intensity of illumination of the open-field arena reduced locomotor activity and increased avoidance of the center of the arena. In addition, rearing and grooming, sleep cycle were reduced under the condition while the duration of time spent in the corners of the apparatus and the frequency of a stereotypical behavior of facing the corners of the apparatus were increased. Facing the corner is interpreted as a coping style to avoid the exposure to the bright light and the open surface of the arena. Further, stress exposed Group II rats exhibited decreased ambulation and rearing which indicated reduced exploration and apathy respectively in these animals. In the Group III experimental rats showed better improvement in their behavioural alteration.
Figure 1 shows the brain tissue histopathology of (a) control group, (b) stress induced, (c) stress+drug treated, (d) PA treatment alone. Histopathology results from the midbrain region of rats under the group control (a) and (d) PA treated shows the normal architecture of the cells.In the group (b) stress induced group shows damaged neuron in the edematous cortical region. This damage to the neuron cells was apparently rescued to near normal in stress induced PA treated group.
Fig. 1:
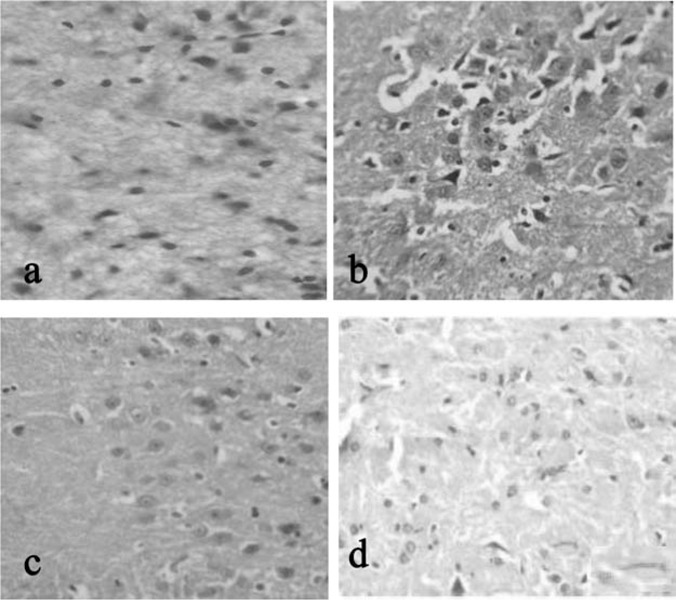
Histopathological tissue section from mid brain of rat stained with H & E in 40X. a (CONTROL): Normal architecture of brain observed. b (STRESS INDUCED): rare normal cells present in edematous cortical region with scattered damaged neurons.c(STRESS+DRUG TREATED): more normal cells are present in edematous appearing cortical region when compared to that of group II. d(drug treated): drug control animal showing normal architecture of cell as that of control.
In the present study, exposure to stress by light, appears to enhance lipid peroxidation in the animal system which could be due to the activation of immune cells by proinflammatory cytokines that leads to over production of ROS interfering with structure and ratio of PUFA,35 that causes loss of fluidity in the biological membrane. Similar to the observation which showed that PA cause reduction in LPO, group III rats in the study registered a reduced LPO (p<0.05) in comparison to stress group thus preventing the impairment neural activity.
LPO levels in cortex of brain are negatively correlated with GSH in the depression. Depression causes a significant decrease in cysteine and cystine in the brain because cysteine is the rate-limiting precursor for glutathione synthesis. An alteration in GST activity in various tissues in response to chronic stress is accompanied by significant decrease in GSH content, which could be attributed to the increase in conjugation reactions.36
Depression is characterized with increased production of procytokines. Further, proinflammatory cytokine-induced increase production of ROS. Probably, this mechanism triggered during depression causes reduction in the activities of super oxide dismutase and catalase in stress induced rats in our study. Figure 2, b, c reflects the antioxidants activity in the brain tissue of control and experimental rats. In line with these experimental findings, clinical studies on patients with affective disorders have also revealed lower levels of superoxide dismutase37 and catalase.38 The protective activities can be mediated, at least partially, by their inductive effect on antioxidant enzymes such as CAT, SOD and GST (endogenous scavengers of ROS) and that could be accomplished in group III rats by PA treatment.
Fig. 2:
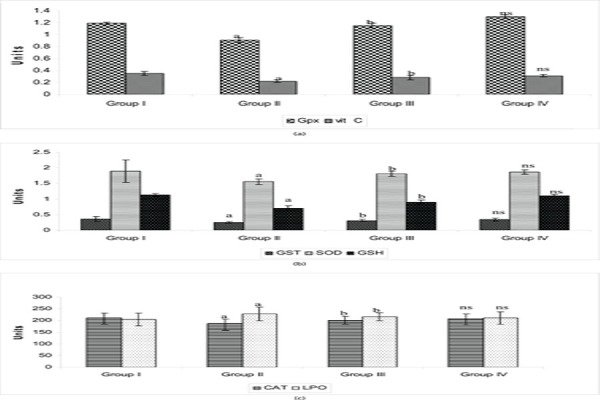
Effect of PA on the activity of antioxidants in the brain tissue of control and experimental groups of rats. SOD, units/min/mg protein; CAT, m mol of H2O2 consumed/min/mg protein; GPx, m mol of GSH oxidized/min/mg protein; GST, m mol of 1-chloro-2,4 dinitrobenzene conjugated/min/mg protein; GSH, m g/mg protein; LPO, nmol of MDA released/mg protein. Vitamin C, m g/mg protein. Statistical significance: * p<0.05. a: Group II compared with Group I. b: Group II compared with Group III. NS-not significant. Group IV compared with Group I.
Similar alteration was reflected in the study showing reduced activities of GST, GPX, reduced levels GSH and Vit C in group II rats. The mechanism under stress condition might be depletion in GR activity which catalyses NADPH-dependent conversion of GSSG to GSH which could be due to a decrease in NADPH levels, a secondary manifestation of cellular free radical stress. But the stress induced and PA treated group III rats showed different picture of increase in the enzyme activity and GSH levels strengthening the antioxidant defense which depicts the drug’s ability to detoxify lipid per oxidation products accumulated during chronic stress. These findings are similar to results of other investigators studying GSH in relation to risk factors in depression illness in rats.39
Thus, in the Group III the protective effect of PAs may be observed which may be due to its antioxidant property by virtue of susceptible brain cells getting exposed to less oxidative stress resulting in reduced brain damage and improved neuronal function.
Figure 3 reflects the ATPases in the brain tissue of control and experimental groups of rats. The activities of synaptosomal membrane ATPases were assayed which showed a reduction in the stress induced group of rats. It was observed that these activities were reversed towards normal values (p<0.05) to certain extent because of potent activity of PA thus challenging stress induced variation. The function of brain is based on ion homeostasis and ATPases are crucial for magnifying ionic gradients in neurons. The enzymes are involved in buffering after a period of hyperstimulation when the physiology is excited to hyperstate. Stress-induced disturbances were reported to alter ATP levels by the altered number of pumps, which in turn may be due to an increase or decrease in protein synthesis or degradation.40 The above mentioned observations could be explained on the basis of depleted ATP levels on light-induced stress followed by reduction in the activities of ATPases which were found to be altered on PA treatment.
Fig. 3:
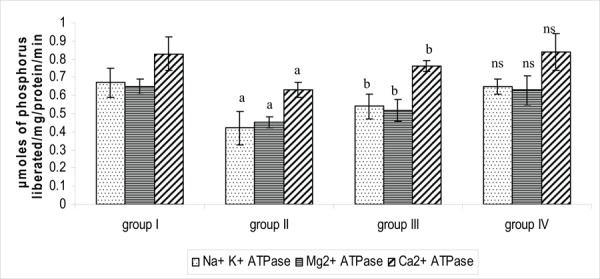
Effect of PA on the activity of ATPases in the brain tissue of control and experimental groups of rats. Each value is expressed as mean_S.D. for six rats in each group. ATPases are expressed as micromoles of phosphorus liberated/mg/ protein/min. Statistical significance: * p<0.05. a: Group II compared with Group I. b: Group II compared with Group III. NS-not significant. Group IV compared with Group I.
Figure 4 reflects the glycoprotein levels in the brain tissue of control and experimental groups of rats. Protein bound carbohydrates (PBC) such as hexose, hexose amine, sialic acid and fucose levels were found to be increased in the stress induced rats (p<0.05) when compared to control while PA treatment changed such clinical scenario.43 It is evidenced that there is increase in the levels of PBC during stress thus arguing that the glycomoieties which involve in chief functions like brain from memory, sleep to anxiety/depression counteracted by PA may be due to its regulatory action on mechanism of glycoprotein release.
Fig. 4:
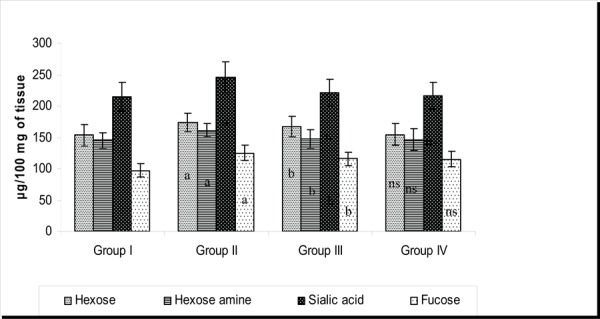
Effect of PA on the levels of PBCs in the brain tissue of control and experimental groups of rats. Each value is expressed as mean_S.D. for six rats in each group. Glycoprotein levels are expressed as μg/100 mg of tissue. Statistical Significance expressed as *P<0.05. a: Group II compared with Group I. b: Group II compared with Group III. NS- not Significant. Group IV compared with Group I.
Figure 5 reflects the levels of lipid in the brain tissue of control and experimental groups of rats. Lipid profile showed variation among the experimental groups which is more related to the 5HT receptor function. A decreased content of cholesterol may induce a relatively increased membrane fluidity with increase in presynaptic 5-HT reuptake and decreased postsynaptic 5-HT function, resulting in antiaggressive depression. The connection between affective disorders and hypocholesterolemia may be associated most closely with alteration of cytokine mediators of inflammation produced by the brain which in turn, create changes in mood and behavior.44 The decrease in cholesterol, triglycerides, free fatty acids and phospholipids were observed in stress induced rats that might be involved in modulating neurotransmitter function and these altered level of the lipids were found to be restored towards a normal in group treated with PA, highlighting that the drug might have an influence on serotonin utilization.
Fig. 5:
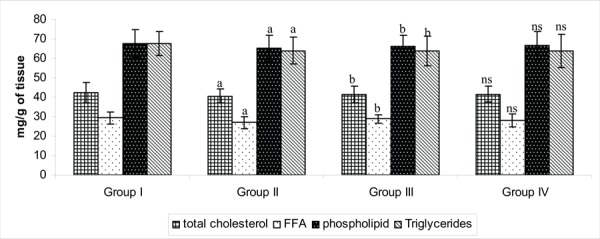
Effect of PA on the levels of Lipid profile in the brain tissue of control and experimental groups of rats. Each value is expressed as mean±S.D for six rats in each group. Lipid levels are expressed as mg/g of tissue. Statistical significance expressed as *P<0.05. a: Group II compared with Group I. b: Group II compared with Group III. NS- not significant. Group IV compared with Group I.
Figure 6 reflects the 5’ND and ACHe levels in the brain tissue of control and experimental groups of rats. The activities of enzymes namely acetylcholine esterase and 5’nucleotidase were found to be decreased in the synaptosomal plasma membrane. The activity of ACH was reported to be widely varying in different regions of brain after undergoing stress, suggesting that reduced cholinergic transmission might be partly responsible for the cognitive deficits. The result obtained in LPA can be viewed on the basis of decreased cholinergic transmission after stress-exposure.41 However, such result was not encountered in LPA groups suggesting the potential of PA.
Fig. 6:
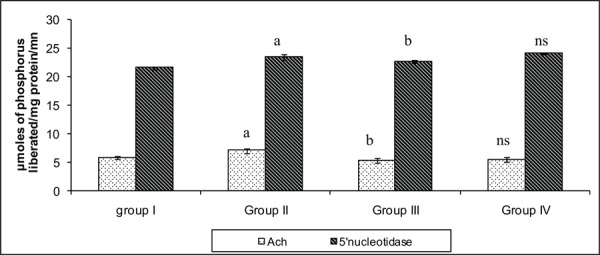
Effect of PA on the activity of marker enzymes in the brain tissue of control and experimental groups of rats. Each value is expressed as mean±S.D. for six rats in each group. Marker enzymes are expressed as MicroMoles of phosphorus liberated/mg/ protein/min. Statistical significance: *P<0.05. a: Group II compared with Group I. b: Group II compared with Group III. NS- not significant. Group IV compared with Group I.
The activity of 5’nucleotidase alters with variations in membrane fluidity and on stress. The lowered activity of 5’ND was found in stress induced group when compared to control. Further, creatine kinase, on the other hand, showed an enhancement in the activity when compared to control as it might undergo rapid changes which is an essential feature as a part of buffering system to avoid fluctuations in ATP/ADP ratios.42 Such an enhanced activity was almost towards the normal value on PA treatment again depicting attenuation of stress on PA administration.
Figure 7 shows that 5HT2A expression showed a decreased expression in stress induced depression when compared to that of control group and drug treated group. Regulation of 5-HT release in the context of stressful and arousing stimuli via mPFC seems to be an important mechanism for effectively dealing with a stressor and is associated with the cessation of fear-related behaviour.45 Studies show down regulation of 5HT2A receptors in prefrontal cortex of depressed patients and suicide victims. During the stress condition, 5-HT2A receptor mediates excitatory effects of 5-HT release on mPFC projection neurons that, in turn, facilitate regulation of amygdala reactivity and associated emotional behaviors.
Fig. 7:
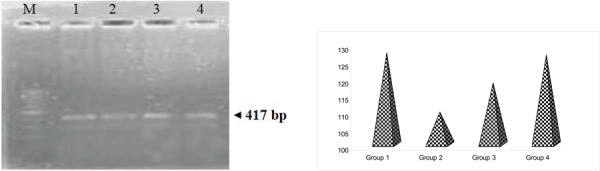
RT-PCR analysis of the effect of PA on the expression levels of 5HT2A in control and experimental groups of rats. and their corresponding densitometric analysis. Lane 1: control (group 1); Lane 2: stress induced (group 2); Lane 3: drug treated (group 3); Lane 4: drug control (group 4).
It was noted that a decrease in total cholesterol as observed in the stress induced group II may induce a relative increase in brain cell membrane fluidity with increased presynaptic 5-HT reuptake and decreased postsynaptic 5-HT function, resulting in an antiaggressive, depression-promoting effect.46 The observed decrease in mRNA level in 5HT2A in the suggests that the drug might have a positive impact on coping-up mechanisms against stressors as 5-HT is involved in regulation of stress-related behaviour.
PA seems to establish a protective strategy against light induced stress in rats by virtue of its ability to alter biochemical, histopathological and 5HT2A mRNA expression as observed in the current study.
Footnotes
Competing interests – None, Source of Funding – None
References
- 1.Torres IL, Buffon A, Dantas G. Acute and chronic stress alter ecto- nucleotidase activities in synaptosomes from the rat hippocampus. Pharmacology Biochemistry and Behavior. 2004;vol no?:341–347. doi: 10.1016/j.pbb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Tsuboi H, Tatsumi A, Yamamoto K et al. Possible connections among job stress, depressive symptoms, lipid modulation and antioxidants. J. Affect. Disorder. 2006;91:63–70. doi: 10.1016/j.jad.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Checkley S. The neuroendocrinology of depression and chronic stress. Br. Med. Bull. 1996;52:597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- 4.Mahdi AA, Tripathi S, Neerja J et al. Aluminium Mediated oxidative stress: Possible Relationship to Cognitive Impairment of Alzheimer’s type. Annals of Neurosciences. 2006;13:18–24. [Google Scholar]
- 5.Poirier MF, Boyer P. Venlafaxine and paroxetine in treatment- resistant depression. Double-blind, randomised comparison. British Journal of Psychiatry. 1999;175(1):12–16. doi: 10.1192/bjp.175.1.12. [DOI] [PubMed] [Google Scholar]
- 6.Joseph B, Raj SJ. An Overview: Pharmacognostic properties of Phyllanthus amarus Linn. Int. J. Pharmacol. 2010;7:40–45. [Google Scholar]
- 7.Kassuya CA, Silvestre A, Menezes-de-Lima OJ et al. Anti-inflammatory and antiallodynic actions of the lignan niranthin isolated from Phyllanthus amarus. Evidence for interaction with platelet activating factor receptor. Eur J Pharmacoly. 2006;546(1-3):182–188. doi: 10.1016/j.ejphar.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Karuna R, Reddy SS, Baskar R et al. Antioxidant potential of aqueous extract of Phyllanthus amarus in rats. Indian J Pharmacol. 2009;41:64–7. doi: 10.4103/0253-7613.51342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adriaan BJ, Francesca S, Daniel RS et al. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: Relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Research Bulletin. 2007;72:32–43. doi: 10.1016/j.brainresbull.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi Hanumanthachar, Milind Parle. Pharmacological evidences for antiamnesic potentials of Phyllanthus amarus in mice. African Journal of Biomedical Research. 2007;10:165–173. [Google Scholar]
- 11.Cotman CW, Matthews DA. Synaptic plasma membranes from rat brain synaptosomes: Isolation and partial characterization. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1971;249(2–3):380–394. doi: 10.1016/0005-2736(71)90117-9. [DOI] [PubMed] [Google Scholar]
- 12.Lowry OH, Rosebrough NJ, Farr AL et al. Protein measurement with the Folin phenol reagent. J. Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Marklund S, Marklund GJ. Biochem Catalytic activity of superoxide dismutase: A method based on its concentration-dependent constant decrease in rate of autoxidation of pyrogallol. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 15.Sinha AK. Coiorimetric assay of catalase Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 16.Habig WH, Pabst MJ, Jakpoby WB. Glutathione transferase : A first enzymatic step in mercapturic acid formation. 1974. J. Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 17.Rotruck JT, Pope AL, Ganther HE. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 18.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 19.Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol. 1971;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 20.Bonting SC. In: Membranes and ion transport. Bittar E, editor. E(ed) Wiley – Inter science. 1970;1:257–263. [Google Scholar]
- 21.Ohinishi A comparative study of plasma membrane magnesium ion ATPase activities in normal, regenerating and malignant cells. Biochem. Biophys. Acta. 1982;684:67–74. doi: 10.1016/0005-2736(82)90050-5. [DOI] [PubMed] [Google Scholar]
- 22.Desai ID. Vitamin E analysis methods for animal tissues. Methods Enzymol. 1984;105:138–143. doi: 10.1016/s0076-6879(84)05019-9. [DOI] [PubMed] [Google Scholar]
- 23.Fini C, Ipata PL, Palmerini CA et al. 5'-nucleotidase from bull seminal plasma. Biochem Biophys Acta. 1983;14:405–412. doi: 10.1016/0167-4838(83)90186-3. [DOI] [PubMed] [Google Scholar]
- 24.Ellman GL, Courtney KD, Andres VJ et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 25.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 26.Parekh AC, Jung DH. Cholesterol determination with ferric acetateuranium acetate and sulfuric acid-ferrous sulfate reagents. Anal Chem Invest New Drugs. 1970;42:1423–1427. [Google Scholar]
- 27.Leffler HH, Mc Dougald CH. Estimation of cholesterol in serum by means of improved technics. Tech Bull Regist Med Technol. 1963;33:19–23. [PubMed] [Google Scholar]
- 28.Rice EW. Triglycerides (“neutral fats”) in serum. In Standard Methods of Clinical Chemistry. 1970;6:215–222. [Google Scholar]
- 29.Hron WT, Menahan LA. A sensitive method for the determination of free fatty acids in plasma. J Lipid Res. 1981;22:377–381. [PubMed] [Google Scholar]
- 30.Neibes P. Determination of enzymes and degradation products of glycosaminoglycans metabolism in the serum of healthy and varicose subjects. Clin Chim Acta. 1972;42:399–408. [Google Scholar]
- 31.Wagner WD. A more sensitive assay discriminating galactosamine and glucosamine in mixtures. Anal Biochem. 1979;15:394–396. doi: 10.1016/0003-2697(79)90379-8. [DOI] [PubMed] [Google Scholar]
- 32.Winzler RJ. Determination of Serum Glycoproteins. In: Glick D (ed) Methods of Biochemical Analysis. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]
- 33.Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234:1971–1975. [PubMed] [Google Scholar]
- 34.Kulikov AV, Tikonova MA, Barkina NN et al. Effect of chronic administration of Imipramine on 2A-Serotonin receptor mRNA in brain cortex of rats predisposed and resistant to catalepsy. Bulliten of experimental biology and medicine. 2002;134:194–196. doi: 10.1023/a:1021144500515. [DOI] [PubMed] [Google Scholar]
- 35.Whanger PD. Selenium and the brain: a review. Nutr Neurosci. 2001;4:81–97. doi: 10.1080/1028415x.2001.11747353. [DOI] [PubMed] [Google Scholar]
- 36.Alptekin N, Seckin S, Yelkenci F et al. Lipid peroxides, glutathione, glutamylcysteine synthetase and g-lutamyltranspeptidase activities in several tissues of rats following water-immersion stress. Pharmacol Res. 1996;34:167–169. doi: 10.1006/phrs.1996.0084. [DOI] [PubMed] [Google Scholar]
- 37.Saraf M. Memory–mechanisms, tools and aids. Annals of Neurosciences. 2009;16:119–122. [Google Scholar]
- 38.Ozcan ME, Gulec M, Ozerol E et al. Antioxidant enzyme activities and oxidative stress in affective disorders. Int. Clin. Psychopharmacol. 2004;19:89–95. doi: 10.1097/00004850-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Kaushik Susmita, Kaur Jyotdeep. Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clinica Chimica Acta. 2003;333:69–77. doi: 10.1016/s0009-8981(03)00171-2. [DOI] [PubMed] [Google Scholar]
- 40.Paula Ana. Santana de Vasconcellos. Na+,K+-ATPase activity is reduced in hippocampus of rats submitted to an experimental model of depression: Effect of chronic lithium treatment and possible involvement in learning deficits. Neurobiology of Learning and Memory. 2005;84:102–110. doi: 10.1016/j.nlm.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Giovannini MG, Bartolini L, Kopf SR et al. Acetylcholine release from the frontal cortex during exploratory activity. Brain Res. 1998;784:218–227. doi: 10.1016/s0006-8993(97)01161-x. [DOI] [PubMed] [Google Scholar]
- 42.Makino S, Smith MA. Increased expression of corticotropin- releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- 43.Nandadeve Mukesh, Ojha SK, Kaur Ranjit. Changes in levels of serum glycoproteins in major depressive disorders Ind Jour of Clinical Biochemistry. 2005;20(2):154–157. doi: 10.1007/BF02867417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kane Patricia, Millville Understanding the biochemical and biobehavioral nexus of Depression. The Journal of Orthomolecular Medicine. 1997;14:38–42. [Google Scholar]
- 45.Amat J, Baratta MV, Paul E et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 46.Logan AC. Neurobehavioral Aspects of Omega-3 Fatty Acids: possible mechanisms and therapeutic value in major depression. Alternative Medicine Review. 2003;8(4):410–425. [PubMed] [Google Scholar]


