Abstract
The antigen presentation to lymphocytes in brain occurs in two steps. Initially it happens at perivascular spaces by perivascular microglia/macrophage population and finally at the site of inflammation deep into brain parenchyma by the resident microglia. But recent evidence challanges the existing notion of involvement of distinct and different cells at these sites. Studies have shown that many of these microglial cells show dendritic cell phenotype in pathogenic and cytokine driven environment. Different subsets of the cell show wide range of myeloid lineage functions indicating a pre-differentiated status of the cell. Monocytic CD34+/B220+ precursor cells have been transformed to microglial cells in vitro and transplantation of these cells show Iba-1 or F4/80 positivity with microglial phenotypes in vivo in adults. Even they can be converted into dendritic cell like forms. The interconvertability among macrophage-microglia-dendritic cells and final effector maturation according to the microenvironmental cues indicates existence of a pre-mature myeloid cell population concerned with antigen presentation and related functions in brain. With the substantial recent observation this article sketches the idea that brain APCs appearing as macrophage/microglia/DC like forms are derivatives of the same stock in response to their position and microenvironment. And also microglia is never any distinct cells, both in neonatal stage and adults.
Keywords: Macrophage, Microglia, Dendritic Cells, Mononuclear phagocytic system (MPS), Differentiation
Introduction
From the beginning it was unequivocally accepted that microglia in brain tissue are the resident macrophage or equivalent population of cells having phenotypic similarities and antigen presenting ability in CNS. When a century earlier Cajal, first histologically identified the cell in brain parenchyma later characterized by Pio del Rio Hortega with silver staining, the cells appeared as short rod like structure embedded in CNS parenchyma and smaller than the other glial partners, hence named microglia.12 In normal brain they constitute not more than 5-10% of brain cells, but during neuropathogenesis they jump in number and infest the site of pathogenesis.34 Plenty of research activities are now surfacing every day, mostly addressing the functional aspects of microglia in different neurodegenerative diseases, brain trauma, tumor and even in behavioral disorders. The cells present antigens, produce variety of neurotrophic factors, chemokines, cytokines and other signaling molecules which have profound influence in controlling CNS microenvironment.5-9 A simple search in ‘PubMed’ for articles containing microglia in last two decades yields nearly 11,500 results. Such huge data indicates that microglia possess a double edged (both beneficial and detrimental) contribution for CNS.
However, the controversy over its origin and cellular identity still remains a pertinent issue. A cluster of recent observations may evaluate the issue from the broader perspective which considers the cell as an integral part of mononuclear phagocytic system (MPS) cells. Many of the microglial research groups hold a tendency of distinguishing it into distinct subsets. But the dynamic variations of its phenotypes and functional activities mostly make those attempts inconsistent. Rather changeability of its cellular morphology and function can offer a consistent reply of this polymorphic appearance of brain APC. In this review, with the new experimental findings and from a fast developing outlook of cellular dynamics/differentiation property, the issue has been addressed to resolve this apparent contradiction regarding the existence of different brain APCs.
What are brain APCs?
Microglia is the only exceptional cell population of CNS that has its origin out of the brain. Though with other glial members and neurons they reside deep into the brain parenchyma, these cells possess the mesodermal hematopoietic lineage. Ling and colleagues while performing their carbon-particle tracing experiments found movement of blood-borne cells in brain at embryonic stage which promoted the idea of mesodermal/monocytic lineage of microglia.10 But, it was challenged in experiments using chimeric rats with OX-27 positive cells of hemopoietic lineage. Then the cells were found present in subarachnoid space and spinal cord CSF, but none entered in brain parenchyma to form ramified microglia.11 On the contrary, contemporary studies on microglia ontogeny using cell surface antigen RMG-1 and RMG-2 suggested migration of small population of cells from blood to deep into CNS parenchyma and transformed into ramified form.12 Some recent workers defy the existing concept that microglia originate from circulating blood monocytes that take residence in brain at some point in gestation and in early post-natal period. A few studies raise the controversy claiming that prenatal microglia are derived from mesodermal progenitors distinct from that of monocytic lineage.13 Therefore, microglial identity crisis is still provoking debates. Another aspect is the morphological heterogeneity of the cell namely distinguished as ‘ramified’ and ‘amoeboid’ forms. This change of forms is termed as ‘reactive microgliosis’ and correlated with the functional plasticity of microglia. Conventionally, ‘ramified’ microglia represents the resting phase and ‘amoeboid’ form hints to its activation and phagocytic state.1415
In contrast to the misty microglia there are others of monocytic lineage around brain at the interface with blood, which are also efficient in presenting antigen. They are blood borne macrophages at perivascular spaces. These cells are phagocytic and monitor their location at the interface between blood and brain with MHC class II and B7 expression.16-18 They are ready to capture CNS antigens, cellular components or debris or anything abnormal leaked from inside out in perivascular space CSF and then phagocytose them to present antigen to the circulating lymphocytes in the CSF surrounding the brain.18-21 As the ependymal lining of the ventricles lacks ‘tight junctions’ that characteristically separate brain from blood, the area is porous and the Virchow-Robin space is also common with such porosity. These areas clear some interstitial fluids from brain parenchyma with several antigens.22 Now different distinct passages for cells from blood to perivascular space through postcapillary venules and from there to brain parenchyma, crossing the BBB and other porous windows have been identified.23 Disagreement occurs regarding the issues whether these cells are tissue macrophages or microglia or only the blood borne macrophages, how efficiently they can cross the BBB and infest brain parenchyma or to what extent they participate in turnover of microglia in neuropil etc. In literature the term ’perivascular macrophage/microglia’ or ‘brain macrophage/microglia’ is being used frequently and these terms are frequent in functional studies on the cells, irrespective of the demarcation between microglia and macrophages in brain.
Several studies on chimera and transgenic experiments have shown that bone- marrow progenitors can be traced in adult CNS. Majority of them are located around perivascular spaces generating perivascular macrophages and a few perivascular microglia but not the true representative of endogenous microglial population.2425 This distinction between perivascular macrophage-microglia population and seemingly ‘true’ microglia is also very feeble. It depends on their location, apparent morphology at a single time point and immunophenotype, mostly on the expression of few surface markers.26 One such study distinguished microglia from perivascular macrophages depending on low level of CD45 expression in comparison with others.27 Any definite markers are lacking for microglia and their immunophenotypes are overlapping with the other monocytic lineage cells. So it seems that the distinction is over-conscious and the flexibility of cellular response behavior according to location, microenvironment and need has been overlooked. Recently, when GFP-transfected bone marrow precursors have been reported to repopulate the brain parenchyma with ramified morphology and co-expression of microglial markers Iba-1 and CD11c, the ideas of distinguishing perivascular and parenchymal microglia/macrophages are at stake.28
For long, it was assumed that brain is devoid of professional antigen presenting dendritic cells (DC) and it was one of the key reasons of immune deprivation in brain.2930 But that became invalid when McMenamin followed by Fischer and Reichmann claimed the existence of DC into the brain and they are phenotypically related to microglia.3132 The CD11c+ cells are considered as DCs which were found to proliferate in brain with its counterpart CD11c- cells, but expressing other co-markers CD11b and CD45.32 This CD11c positivity and microglial connection with DC has been identified for many occasions from then onwards. Another marker commonly found on some DC is CD4, which also has been found on microglia.3334 For bone marrow derived myeloid DCs injected into brain parenchyma and CSF, the DCs from CSF efficiently reached to cervical lymph node; whereas, others move little in brain parenchyma with some restricted movement.35 These connections of DC with microglia thus indicate a serious link between the myeloid monocytic lineage cells, all concerning together in alliance for the antigen presentation related to brain.
Searching the kinship among brain APCs
Research in developmental biology indicated that from later half of first trimester and early second trimester in humans and between embryonic day 10-19 in rodents microglial progenitors populate brain. Then they spread in brain parenchyma which we consider as resident ramified parenchymal microglia in normal brain.3637 Some even tried to distinguish these progenitors into two, one from myeloid mesenchymal origin and second one is transitory offshoot of the fetal macrophages.13 This distinction can be reconstructed differently if we interpret that the first population comprises of early less differentiated mesenchymal/myeloid cells that takes entry in brain, and the later one is a late differentiated myeloid cell population which already started to express monocytic/macrophagic phenotypes during their entry in brain. Another idea is that during fetal development the myeloid lineage cells or fetal macrophage take residence in brain to differentiate into microglia, which is distinct from the macrophages in adult.3839 But from the study of lineage markers and differentiation aspects it cannot be denied that all the probable progenitors of microglia are basically different early stages of myeloid monocytic lineages. Now accumulating evidence on repopulation experiments of microglia in adult brain is gradually diminishing the barriers between different distinctions of microglial progenitor subpopulations instead representing different stages of the same continuum involved in the action.
Microglial commonness with monocyte/macrophage lineage
Microglia expresses nearly all of its surface markers common with the monocytes and macrophages. It includes CD1a, CD2, CD4, CD11a, CD11b, CD16, CD18, CD31, CD32, CD45, CD45R/B220, CD45RB, CD54, CD62L, CD64, CD80, CD86, CD200R, MHC class I and II and many more.58263440 Many of them are also maturation and phagocytic markers for macrophages including CD11b, CD45, CD64, CD68, MHC class II etc, which have been identified in different subsets of microglia in pathogenic and normal brain.13 This correlation is also evident inside the cell as the transcription factors like PU.1 or MafB, required for development of macrophage and DCs from myeloid cells, are also found important for microglial differentiation.41-43
No strict barrier between microglia, macrophage and DCs
As it is also true that microglia has been identified with many DC common markers like CD2, CD4, CD11a, CD45, CD45RB, CD80, CD86 and most importantly CD11c in subsets of the cell, a perfect connection between myelo-monocyte derived DC, macrophage and microglia is clearly visible.3132 A major breakthrough was achieved when Fischer and Reichmann located some cells in brain parenchyma with DC phenotype CD11c+ that can coexpress CD11b+ and found in both perivascular as well as intraparenchymal spaces. These CD11b+/CD11c+/CD45+ cells proliferate in brain with their CD11b+/CD11c-/CD45+ counterparts. They also found that the cells are related with microglia as the microglia when exposed to GM-CSF and CD40 ligation differentiates to DC.32 When DCs isolated from GFP-transgenic mice, pulsed with OVA-antigen were injected into the mice brain parenchyma, they showed increase in population, signs of maturation, and CCR7 expression for homing. Some of these cells came out to cervical lymph node showing a migratory path of cells from brain parenchyma and also induced homing of Ag-specific T cells into brain.44 This work implies the importance on local antigen presenting cells in brain and their probable passage to the lymphatics. Simultaneously, these DCs showed functionally similar action with resident microglia. A recent study observing the role of microglia in CNS immune homeostasis interestingly found that CNS endothelial cells induce the mature DCs to differentiate into microglia like cells with similar morphology expressing high CD11b, low CD11c, MHC class II and costimulatory molecules CD80, CD86 and CD40.45 Above observations support the hypothesis that blood borne APCs (including monocytic lineage cells, macrophages and DCs) enter CNS and gradually undergo a dynamic functional transformation under the influence of CNS microenvironment into microglial forms.
Cell tracking experiments demolish myths about microglia
The series of transplantation and cell tracking experiments in the recent past provide support to the view. Bone marrow derived GFP-expressing stem cells injected in lethally irradiated mice were found to migrate into brain and populate throughout the brain. Notably, they acquire microglial morphology with expression of Iba-1, CD11c and MHC class II [Fig-1].28 An important observation is that, compared to other tissue macrophages, microglia behave more like immature myeloid cells.46 Some of these immature cells express CD34 and B220 antigens in developing brain. Blood circulating CD34+/B220+ myeloid progenitors from adult, with exposure to M-CSF and glial cell conditioned medium, differentiate to microglia in vitro.47 To define the role of blood circulating monocytes in entorhinal cortex lesion (ECL) the GFP and CFDA had been used as cell tracker and the observation demonstrated that blood derived monocytes cross BBB and infiltrate layers of anterograde axonal degeneration. In brain, the cells show transformation from round to ramified morphology at the lesion site and positivity to Iba-1, Mac-1 and F4/80.48 In other set of experiments GFP-transfected bone-marrow cells engrafted in irradiated mice showed GFP+/Iba-1+/F4/80+ cells with typical microglial morphology in the molecular layer of brain parenchyma. These cells showed enhanced MHC class II expression during acute bacterial meningitis and these donor derived microglia increased and infested brain after one month of infection, integrated into the pool of parenchymal microglia and participated in recovery process.49 Similar experiment found that DCs are also able to transmigrate through brain capillary endothelium without much changes in BBB and the process is assisted by MIP-1α chemokine and MMP-2/9.50 New evidence suggests that even microglia invites circulating monocytes to brain during peripheral infections by secreting chemokine MCP-1/CCL2 in association with TNFα.51 In all these cases, the bone marrow derived/hemopoietic stem cells or circulating monocytes used in the experiment never showed differentiation bias to any other glial cell types and always keep hemopoietic lineage fidelity.485253 These observations suggest that whether less differentiated HSC or comparatively differentiated blood monocytes or more differentiated DCs, they are all able to infest the adult brain, repopulate and mingle with parenchymal microglia according to the CNS microenvironment and situation.
Fig. 1:
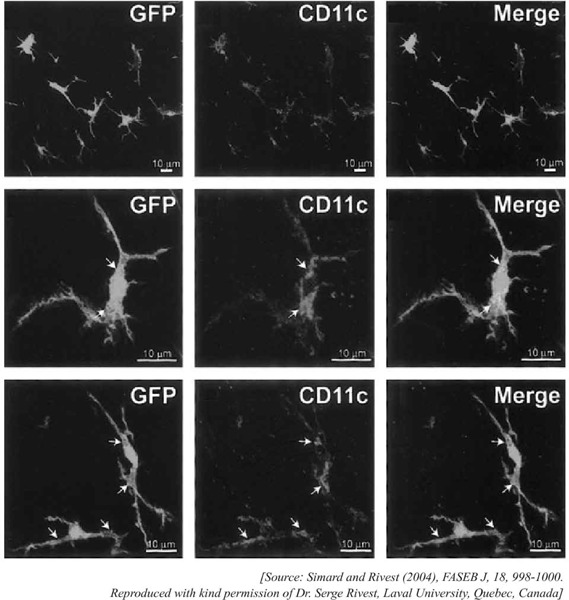
GFP-expressing bone marrow derived stem cells have been found to enter brain parenchyma in adults, populate their and express APC specific marker CD11c, which is mostly assigned for dendritic cell (DC) specificity. In the same experiment investigators found the co-expression of GFP and iba-1 (mostly specified for macrophage/microglia) with ramified morphology [Figure not reproduced here]. The observations demonstrated that even in adult, progenitor cells has the ability to repopulate in brain which transdifferentiate into microglia with both macrophage/DC phenotype and hence competent to present antigens to cells of adaptive immune systems, mostly to infiltrated lymphocytes.
Progenitors, morphs and their dynamic conversions
In glioma and other conditions microglia has shown a stable turnover and self- renewal capacity.1454 Their pre-differentiated behavior, as already mentioned, was addressed initially by Santambrogio and colleagues.55 This status was found true for neonatal and adult brain when different myeloid marker expression was analyzed. The cells had been designated as CD34+ HSC derived CD45+ uncommitted myeloid precursors. They are responsive to GM-CSF to differentiate into ‘DC-like’ phenotype with CD11c and M-CSF inducing them to ‘macrophage-like’ phenotype and functional attributes; and investigators defined the cell as ‘pro-dendritic’.4755 Microglia shows astonishing range of functional plasticity also, which varies from normal to different pathogenic conditions. With antigen presentation they express variety of co-activation receptors, cell adhesion molecules, FC, scavenger, pattern recognition, complement and death receptors; can release pro- and anti-inflammatory cytokines and chemokines, reactive oxygen and nitrogen species, neuronal growth factors; and react to varied mitogenic and non-mitogenic agents. Detailing of there is beyond the scope of this article. However, particulars of these functional aspects of microglia are available in literature.5265657 From plethora of studies on microglia and associated macrophage like cells in brain it is now evident that microglial population can adopt and act in almost every possible role of myelo-monocytic cell lineage; and in addition they can perform some CNS specific glial functions probably as local adaption in the CNS microenvironment [Fig-2].8585940 This potential for wide variability also indicates for microglial precursor state that can adapt and mature in several potential paths to reach the terminal effector state as needed in CNS.
Fig. 2:
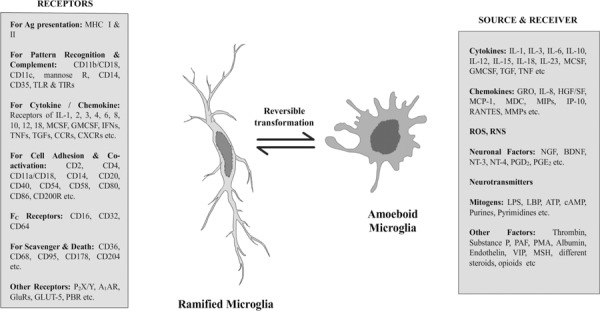
Diversity of forms and functions of microglia are enormous. The cell is designated as the hybrids between white blood cells and glial cells (Streit, 2002, Glia). Microglia shows immense immune functional potentials and can practically adopt nearly all functional forms that can possibly be adopted by mononuclear phagocytic system (MPS) cells. Additionally, they can release and respond to a variety of neuronal factors including some neurotransmitters, growth factors or trophic factors etc. At neonatal stage most microglia in brain appear with round amoeboid shape, which transform to elongated ramified form in normal adult brain. But in pathogenic or injured brain tissue they reappear in amoeboid forms. This diagram represents the functions and forms of microglia at a glance.
Human CD34+ HSC transplantation in NOD/SCID mice CNS strikingly yields microglia in vivo with ramified morphology and Iba-1.60 The fact is that microglia in adult can be generated from direct transplantation of even a single HSC and ~54-98% of transplanted cells attaining Iba1 immunolocalisation.52 From differentiation-maturation perspective it is determined that CD34+ HSC attain CD11c when GMCSF and IL-4 aid them to differentiate as DC.61 Conversely, MCSF and IL-6 guide them towards macrophage.62 In previously mentioned CD34+/B220+ myeloid progenitor differentiation, the role of MCSF in differentiation of macrophage like microglia expressing CD11b was observed.47 So, local environment profoundly control the differentiation of ‘DC like’ i.e, CD11c+ or ‘macrophage like’ i.e, CD11b+ microglial cells with varying cell morphology that mostly express Iba-1 and/or F4/80 and other monocytic phagocyte common markers. Simultaneously, it was found that IFNγ has the ability to push the monocytic progenitors or early differentiating cells with DC tendency to develop macrophage phenotype by producing autocrine MCSF and IL-6.63 The opposite was observed in a different condition found during foam cell formation in atherosclerosis. Here, monocytes entering through arterial walls, differentiate to macrophages in M-CSF or CXCL4 induced environment. But microarray data showed that in response to oxLDL, these cells transformed to DC like forms expressing MHC class II, CD83, CD206 etc.64 As in atherosclerosis many chemokines and cytokines similar to brain microenvironment are involved, comparable transformation is not improbable in brain and an exactly predicting phenotypic switch in brain was observed in Alzheimer’s disease (AD) double transgenic (APP/PS1) mice model when vaccinated with glatiramer acetate (GA). It causes CD11b+/CD11c- microglia to transform into CD11b+/CD11c+ microglia with close proximity to DC phenotype and better MHC class II expression. IL-4 induces the situation effectively with insulin-like growth factor 1 (IGF1) expression and downregulation of TGFβ in the transformed microglia.65
These observations suggest that in brain, microglia is normally present as predifferentiated monocytic cell population. According to microenvironmental cues the cells are capable of transforming into DC or macrophage like effector forms. They may interchange their characters also to a limited extent. In adult, pre-differentiated blood monocytes enter brain, can repopulate parenchymal microglia, and differentiate to its forms and functional attributes. All these cells collectively constitute microglia from perivascular space to parenchyma with dynamic differentiation potential. Their diversities can be designated as different maturation grades resulted accordingly with local atmosphere, mostly reversible except terminally differentiated.
Old ‘ramified’ vs ‘amoeboid’ microglia redefined
To describe the functional plasticity of microglia, generally short rod shaped parenchymal microglia with ramification found in normal brain had been designated as ‘resting’ phase of the cell. But in neuropathogenic condition the cells are found to cluster around the site of lesion or inflammation with more round amoeboid morphology [Fig-2]. These were designated as its ‘active’ form with effector functions including secretory properties and phagocytic abilities.614 With the abundant data of microglial functional activities this demarcation of ‘ramified/resting’ and ‘amoeboid/active’ status was gradually narrowing. But a clear blow on that idea was received with the advanced time-lapsed two-photon microscopic technique. From live CNS preparation it was documented that the so called resting microglia possesses DC like slender ramifications undulating, protruding and retracting in the tissue matrix for the surveillance of its surroundings.66 Even immediately after local injury, motility of this resting microglia to the site with active changes of processes was found. Another study in in vivo preparation of transgenic mouse brain expressing Iba1-EGFP (enhanced green fluorescent protein) in microglia/macrophage lineage cells showed that so-called resting/ramified microglia make direct contact with the synapse to monitor the functional state. In injury they move, protrude their ramification and help in remodeling the neuronal circuits disrupted by ischemia.67
These recent observations showed that the ramified forms of microglia are highly motile, always vigilant to check the integrity and function of CNS, even able to repair subclinical abnormalities.66-68 The ramified forms of microglia have striking similarities in their protrusions with dendritic cells. CNS immune- surveillance and controlled antigen presentation mostly rely on this form of cells. If we revisit the cellular morphology and receptor expression patterns from the stem cell transplantation experiments, it can be noticed that the cells with highly ramified morphology mostly express CD11c and MHC class II with Iba-1.28 But when prominent effector functions, repair and clearance in pathogenic brain is needed, microglia mostly become hypertrophic and transform into amoeboid forms. These have been found in transplantation experiments in diseased conditions with Iba1, F4/80 and CD11b+ cells having rounded morphology; and also in a huge number of functional studies on microglia.47-49 Therefore, the ramified and amoeboid forms of microglia in adult brain should not be differentiated into resting or active state. On contrary, they are different states of functional response against the prevailing circumstances in CNS.
Brain APCs/microglia are functional morphs of monocytic lineage
Microglia has long been regarded as the resident macrophage of brain and its connection with macrophage lineage is under scrutiny since its discovery. But throughout the last decade microglial connection with DC becomes more and more evident, which we have already talked about. Two simultaneous observations extended this beyond brain. Monocytic cells, just crossing the BBB, when exist adjacent to capillary endothelium and astrocytic end-feet had been assigned as perivascular microglia.2024 In the mice model of multiple sclerosis using bone marrow chimeric approach with transgenic animals, it was predicted that neither secondary lymphoid tissue nor the CNS resident microglia are essential to permit entry of autoreactive T cells into brain. Rather, DCs with CD11c+ associated with meninges, CNS blood vessels and perivascular spaces (the places for perivascular microglia) allows these T cells to recognize their cognate antigen that lead them to CNS invasion and inflammation.69 In another model of MS, the efficient presentation of endogenous antigen to activate T cells is found dependent upon F4/80-CD11c+CD45hi DCs as well as F4/80+CD45+ macrophages and F4/80+CD45lo microglia locally, but not in the cervical lymph nodes or other peripheral lymphoid organs.70 Thus the demarcation of even perivascular microglia, macrophage or DCs and also the same for the APCs in brain parenchyma is getting difficult. In every position, the brain APCs comprise several sub-populations of cells expressing any of these morpho- and immune-phenotypes with wide range of effector functions or exists at some pre-differentiated stage. Even the forms possess potential for inter-conversion between them to some extent.63-65
This issue can be viewed in the larger spectrum of monocytic cell family. The long standing efforts applied to separate macrophages from the specialized antigen presenting cells named as dendritic cells are now facing serious challenge. Hume, 2008, in his seminal article explained how DCs are just the part of mononuclear phagocytic system (MPS, they comprise the family of bone marrow precursor cells, blood circulating monocytes and tissue macrophages) and linked with macrophages.71 Basically, MPS cells encounter combinations of growth factors like MCSF/IL-34, GMCSF, IL-4, IFN-γ, Flt3L etc, all having their effects on phenotype maturation of them.72-75 Identification of common clonogenic bone marrow derived progenitors for macrophage and DC (MDP, macrophage-DC progenitor), antigen presenting capacity of both forms in vivo, CD11c non-specificity to DCs etc furnish the cell types as only the forms of heterogeneous MPS.76-78 The wide variety of monocyte subpopulation in different organs and their commonness of gene expression make it hard to distinguish these cells into distinct types.7980 Independent experiments also demonstrated that the pattern of MPS lineage phenotype during macrophage differentiation continues to shift from one cytokine environment to another and shows functional adaptability.81 In 2004, Stout and Suttles, with following others, definitely pointed out that macrophage displaying their wide functional patterns are actually representing different stages of their evolving response to the changing microenvironment with reversibility.82 Therefore, the above findings indicate that antigen presenting cell phenotype is essentially a regulated response activity, as it holds true also for phagocytic and other effector phenotypes of MPS. And brain is not the exception for the mononuclear phagocytic system (MPS) to produce its functional heterogeneity with corresponding morphological variations. That was demonstrated in vitro with Flt3+ bone-marrow progenitor cells which could commit them sequentially into different differentiation program for DC, osteoclast and macrophage; and glial cell conditioned medium (GCCM) polarize these progenitors to differentiate into microglia.83
Therefore, mounting evidence from different experimental approaches shows that the cell population termed microglia is an aggregation of different related forms, varied functional identities. Apparent inconsistencies are basically nothing but the ‘spatial’ and ‘functional’ versions of mononuclear phagocytic heterogeneity related to brain. The ‘DC like’ (mostly ramified) and ‘macrophage like’ (mostly amoeboid) appearances are derived from the ‘premature’ monocytic cells as a response behavior in that particular CNS microenvironment. From accumulated evidence a simple hypothesis about the transforming cellular nature of brain APCs designated as macrophage/microglia/DC can be redrawn. During neuropathogenesis in adult CD34+CD45+ monocytic precursors with activated PU.1 transcription factors in the CNS blood capillaries step into the perivascular space with CD11b+/CD11c+/- phenotype, which are premature DC/macrophage forms. These immature APCs entering CNS undergo phases of functional and morphological transformation under the influence of local milieu and merge with the existing mononuclear phagocytic population that colonized during embryonic and early natal days in brain from blood. This merged population forms a cooperative antigen presenting and phagocytic system; the final differentiation and effector maturation of which is still to be determined by different cytokines, chemokines and receptor ligations at the site of action [Fig-3]. These mononuclear cells, namely microglia, can move through extracellular matrix constituting projections of astrocytes to the effector site by α6β1 integrin, a receptor of laminin expressed by them.84
Fig. 3:
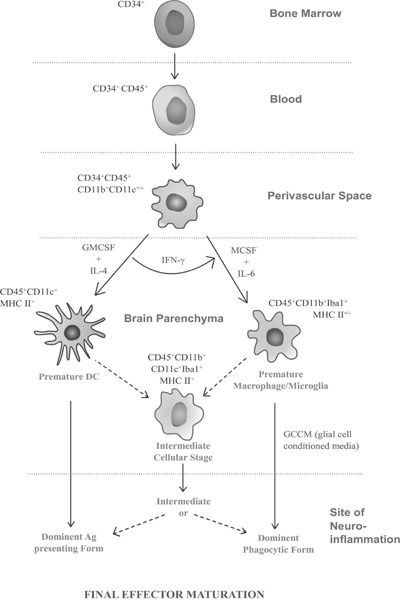
Differentiation stages and positional morphs of CD34+ hematopoietic stem cells showing that brain APCs including microglia are derivatives of the same stock of haemopoietic precursors of MPS in response to their microenvironment. It should be noted that, differentiation determining microenvironmental cues are varied and many still to identify; however, the key cytokines identified so far that are determinant for macrophage/microglia/DC like appearance is mentioned here. Finally in brain parenchyma these cells may attain the premature macrophage/microglial or Dendritic cell forms or an intermediate form, from that the cell reaches its final effector maturation stage of differentiation in the site of neuroinflammation that we can distinguish then as separate cell types. The positional and functional heterogeneity of the cell lineage is remarkable.
Conclusions
The recent disagreements on the monocytic heterogeneity yield in favor of the continuum of progeny of a common precursor.85 The brain APCs, in and around, therefore appears as a combination of different morphs of the same mononuclear phagocytic system cells. They attain different name according to their positional and functional differentiation stage. When they reside in brain parenchyma with certain morphological and immunophenotypic features, as imposed or defined extramurally, they become the ‘microglia- resident macrophage of brain’. But cumulative evidence suggest that a dynamic passage exists for the mononuclear cells, mostly from blood to brain throughout the life. The process becomes more prominent during neuropathogenesis. As these cells possess a pre-differentiated status with migratory behavior that helps them to cross the BBB, to move in brain parenchyma and gain final differentiation at the effector sites in brain, they have been planned as vechicle of gene therapy and drug delivery in brain.86 This effort is aimed to exploit its ‘pre-mature’ nature and opening up a new opportunity for treating CNS diseases. Thus brain APCs provide a new insight and possibility for regulating the brain immunity.
Acknowledgements
Financial assistance from University Grants Commission (UGC), India is acknowledged. Special thanks to Dr. Serge Rivest of Laval University in Quebec, Canada, who kindly permitted me to re-use the images of his work for this article that was previously published in FASEB J, 2004. I am grateful to my mentor Prof Swapna Chaudhuri of School of Tropical Medicine, Kolkata, India who initiated my interest on microglia. Acknowledgements are due for cooperation of my colleagues and students to continue my work.
Footnotes
Competing interests – None, Source of Funding – UGC
References
- 1.Cajal SR. Contribución al conocimiento de la neuroglia del cerebro humano. Trab del Lab de Invest Biol. 1913;11:254. [Google Scholar]
- 2.Del Rio-Hortega P. El “tercer elemento” de los centros nerviosos. I. La microglia en estado normal. II. Intervencion de la microglia en los procesos patologicos (Celulas en bastoncito y cuerpos granulo-adiposos). III. Naturaleza probable de la microglia. Bol Soc Espan Biol. 1919;9:68–120. [Google Scholar]
- 3.Lawson LJ, Perry VH, Gordon S et al. Turnover of resident microglia in the normal adult mouse brain. Neurosci. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 4.Mittelbronn M, Dietz K, Schluesener HJ et al. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001;101:249–55. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- 5.Aloisi F. Immune function of microglia. Glia. 2001;36:165–79. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 6.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–9. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 7.Nelson PT, Soma LA, Lavi E et al. Microglia in diseases of the central nervous system. Ann Med. 2002;34:491–500. doi: 10.1080/078538902321117698. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Sikri V, Sharma NK et al. Regeneration of tooth pulp and dentin: trends and advances. Annals of Neurosciences. 2010;17:31–43. [Google Scholar]
- 9.Fan R, Xu F, Previti ML et al. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27:3057–63. doi: 10.1523/JNEUROSCI.4371-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling EA, Penney D, LeBlond CP et al. Use of carbon labeling to demonstrate the role of blood monocytes as precursors of the ‘ameboid cells’ in the corpus callosum of postnatal rats. J Comp Neurol. 1980;193:631–57. doi: 10.1002/cne.901930304. [DOI] [PubMed] [Google Scholar]
- 11.Hickey WF, Vass K, Lassmann H et al. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropath Exp Neurol. 1992;51:246–56. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Imamura K, Ito M, Suzumura A et al. Generation and characterization of monoclonal antibodies against rat microglia and ontogenic distribution of positive cells. Lab Invest. 1990;63:853–61. [PubMed] [Google Scholar]
- 13.Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia—New concepts. Brain Res Rev. 2007;53:344–54. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Streit WJ, Walter SA, Pennell NA et al. Reactive microgliosis. Prog Neurobiol. 1999;57:563–81. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 15.Wu CH, Chien HF, Chang CY et al. Response of amoeboid and differentiating ramified microglia to glucocorticoids in postnatal rats: a lectin histochemical and ultrastructural study. Neurosci Res. 2001;40:235–44. doi: 10.1016/s0168-0102(01)00231-0. [DOI] [PubMed] [Google Scholar]
- 16.Streit WJ, Graeber MB. Heterogenity of microglia and perivascular cells: Insights gained from the facial nucleus paradigm. Glia. 1993;7:68–74. doi: 10.1002/glia.440070112. [DOI] [PubMed] [Google Scholar]
- 17.Kida S, Steart PV, Zhang ET et al. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol Berl. 1992;85:646–52. doi: 10.1007/BF00334675. [DOI] [PubMed] [Google Scholar]
- 18.Bechmann I, Peter S, Beyer M et al. Presence of B-7.2 (CD86) and lack of B-7.1 (CD80) on myelin-phagocytosing MHC-II positive rat microglia is associated with non-destructive immune response in vivo. FASEB J. 2001;15:1086–8. doi: 10.1096/fj.00-0563fje. [DOI] [PubMed] [Google Scholar]
- 19.Bechmann I, Priller J, Kovac A et al. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001;14:1651–8. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- 20.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow- derived and present antigen in vivo. Science. 1988;239:290–2. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 21.Lassmann H, Schmied M, Vass K et al. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993;7:19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- 22.Weller RO. Pathology of cerebrospinal fluid of the CNS: significance for Alzheimer disease, prion disorders and multiple sclerosis. J Neuropathol Exp Neurol. 1998;57:885–94. doi: 10.1097/00005072-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Ransohoff RM, Kivisäkk P, Kidd G et al. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 24.Bechmann I, Kwidzinski E, Kovac AD et al. Turnover of Rat Brain Perivascular Cells. Exp Neurol. 2001;168:242–9. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- 25.Albini TA, Wang RC, Reiser B et al. Microglial stability and repopulation in the retina. Br J Ophthalmol. 2005;89:901–3. doi: 10.1136/bjo.2004.060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–97. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 27.Sedgwick JD, Schwender S, Imrich H et al. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA. 1991;88:7438–42. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- 29.Sedgwick JD. Immune surveillance and autoantigen recognition in the central nervous system. Aust NZ J Med. 1995;25:784–92. doi: 10.1111/j.1445-5994.1995.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 30.Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–21. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- 31.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in whole mount preparations. J Comp Neurol. 1999;405:553–62. [PubMed] [Google Scholar]
- 32.Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–26. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 33.Schluesener HJ, Seid K, Kretzschmar J et al. Leucocyte chemotactic factor, a natural ligand to CD4, is expressed by lymphocytes and microglial cells of the MS plaque. J Neurosci Res. 1996;4:606–11. doi: 10.1002/(SICI)1097-4547(19960615)44:6<606::AID-JNR11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 34.Begum Z, Ghosh A, Sarkar S et al. Documentation of immune profile of microglia through cell surface marker study in glioma model primed by a novel cell surface glycopeptide T11TS/SLFA-3. Glycoconj J. 2004;20:515–23. doi: 10.1023/B:GLYC.0000043287.98081.15. [DOI] [PubMed] [Google Scholar]
- 35.Hatterer E, Davoust N, Didier-Bazes M et al. How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood. 2006;107:806–12. doi: 10.1182/blood-2005-01-0154. [DOI] [PubMed] [Google Scholar]
- 36.Corbisiero V, Hagger G, Topps S et al. Colonization of the developing mouse brain by microglial progenitors. Neuroembryol. 2003;2:181–2. [Google Scholar]
- 37.Rezaie P, Dean A, Male D et al. Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex. 2005;15:938–49. doi: 10.1093/cercor/bhh194. [DOI] [PubMed] [Google Scholar]
- 38.Lassmann H, Hickey WF. Radiation bone marrow chimeras as a tool to study microglia turnover in normal brain and inflammation. Clin Neuropathol. 1993;12:284–5. [PubMed] [Google Scholar]
- 39.Streit WJ. Microglia and macrophages in the developing CNS. Neurotoxicol. 2001;22:619–24. doi: 10.1016/s0161-813x(01)00033-x. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh A, Chaudhuri S. Microglial action in glioma: A boon turns bane. Immunol Lett. 2010;131:3–9. doi: 10.1016/j.imlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Walton MR, Gibbons H, MacGibbon GA et al. PU.1 expression in microglia. J Neuroimmunol. 2000;104:109–15. doi: 10.1016/s0165-5728(99)00262-3. [DOI] [PubMed] [Google Scholar]
- 42.Reddy VA, Iwama A, Iotzova G et al. Granulocyte inducer C/EBPα inactivates the myeloid master regulator PU.1: possible role in lineage commitment decisions. Blood. 2002;100:483–90. doi: 10.1182/blood.v100.2.483. [DOI] [PubMed] [Google Scholar]
- 43.Bakri Y, Sarrazin S, Mayer UP et al. Balance of MafB and PU.1 specifies alternative macrophage or dendritic cell fate. Blood. 2005;105:2707–16. doi: 10.1182/blood-2004-04-1448. [DOI] [PubMed] [Google Scholar]
- 44.Karman J, Ling C, Sandor M et al. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173:2353–61. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- 45.Bai B, Song W, Ji Y et al. Microglia and Microglia-Like Cell Differentiated from DC Inhibit CD4 T Cell Proliferation. PLoS One. 2009;4(e7869) doi: 10.1371/journal.pone.0007869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carson MJ, Reilly CR, Sutcliffe JG et al. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Davoust N, Vuaillat C, Cavillon G et al. Bone marrow CD34+/B220+ progenitors target the inflamed brain and display in vitro differentiation potential toward microglia. FASEB J. 2006;20:2081–92. doi: 10.1096/fj.05-5593com. [DOI] [PubMed] [Google Scholar]
- 48.Bechmann I, Goldmann J, Kovac AD et al. Circulating monocytic cells infiltrate layers of anterograde axonal degeneration where they transform into microglia. FASEB J. 2005;19:647–9. doi: 10.1096/fj.04-2599fje. [DOI] [PubMed] [Google Scholar]
- 49.Djukic M, Mildner A, Schmidt H et al. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain. 2006;129:2394–403. doi: 10.1093/brain/awl206. [DOI] [PubMed] [Google Scholar]
- 50.Zozulya AL, Reinke E, Baiu DC et al. Dendritic cell transmigration through brain microvessel endothelium is regulated by MIP-1α chemokine and matrix metalloproteinases. J Immunol. 2007;178:520–9. doi: 10.4049/jimmunol.178.1.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Mello C, Le T, Swain MG et al. Cerebral microglia recruit monocytes into the brain in response to Tumor Necrosis Factor α signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Massengale M, Wagers AJ, Vogel H et al. Hematopoietic cells maintain hematopoietic fates upon entering the brain. J Exp Med. 2005;201:1579–89. doi: 10.1084/jem.20050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wehner T, Böntert M, Eyüpoglu I et al. Bone marrow-derived cells expressing green fluorescent protein under the control of the glial fibrillary acidic protein promoter do not differentiate into astrocytes in vitro and in vivo. J Neurosci. 2003;23:5004–11. doi: 10.1523/JNEUROSCI.23-12-05004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearce SM, Ramsey MA, Miranpuri GS et al. Regulation and function of Matrix Metalloproteinases in Nervous System mjury and Neuropathic Pain. Annlas of Neurosciences. 2008;15:94–105. [Google Scholar]
- 55.Santambrogio L, Belyanskaya SL, Fischer FR et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA. 2001;98:6295–300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–55. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura Y. Regulating factors for microglial activation. Biol Pharm Bull. 2002;25:945–53. doi: 10.1248/bpb.25.945. [DOI] [PubMed] [Google Scholar]
- 58.Colton CA. Heterogeneity of Microglial Activation in the Innate Immune Response in the Brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ransohoff RM, Perry VH. Microglial Physiology: Unique Stimuli, Specialized Responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 60.Asheuer M, Pflumio F, Benhamida S et al. Human CD34+ cells differentiate into microglia and express recombinant therapeutic protein. Proc Natl Acad Sci USA. 2004;101:3557–62. doi: 10.1073/pnas.0306431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chapuis F, Rosenzwajg M, Yagello M et al. Differentiation of human dendritic cells from monocytes in vitro. Eur J Immunol. 1997;27:431, 41. doi: 10.1002/eji.1830270213. [DOI] [PubMed] [Google Scholar]
- 62.Menetrix-Caux C, Montmain G, Dieu MC et al. Inhibition of differentiation of dendritic cells from CD34+ progenitors by tumors cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–91. [PubMed] [Google Scholar]
- 63.Delneste Y, Charbonnier P, Herbault N et al. Interferon-γ switches monocyte differentiation from dendritic cells to macrophages. Blood. 2003;101:143–50. doi: 10.1182/blood-2002-04-1164. [DOI] [PubMed] [Google Scholar]
- 64.Cho HJ, Shashkin P, Gleissner CA et al. Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol Genomics. 2007;29:149–60. doi: 10.1152/physiolgenomics.00051.2006. [DOI] [PubMed] [Google Scholar]
- 65.Butovsky O, Koronyo-Hamaoui M, Kunis G et al. Glatiramer acetate fights against Alzheimer’s disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc Natl Acad Sci USA. 2006;103:11784–9. doi: 10.1073/pnas.0604681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nimmerjahn A, Kirchhoff F, Helmchen F et al. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 67.Wake H, Moorhouse AJ, Jinno S et al. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 69.Greter M, Heppner F, Lemos MP et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–34. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 70.McMohan EJ, Bailey SL, Castenada CV et al. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–9. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 71.Hume DA. Macrophages as APC and the Dendritic Cell Myth. J Immunol. 2008;181:5829–35. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 72.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 73.Ripoll VM, Irvine KM, Ravasi T et al. Gpnmb is induced in macrophages by IFN-γ and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J Immunol. 2007;178:6557–66. doi: 10.4049/jimmunol.178.10.6557. [DOI] [PubMed] [Google Scholar]
- 74.Fleetwood AJ, Lawrence T, Hamilton JA et al. Granulocyte macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178:5245–52. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 75.D’Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fogg DK, Sibon C, Miled C et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–7. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 77.Varol C, Landsman L, Fogg DK et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–80. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lindquist RL, Shakhar G, Dudziak D et al. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–50. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 79.Strauss-Ayali D, Conrad SM, Mosser DM et al. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82:244–52. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- 80.Ingersoll MA, Spanbroek R, Lottaz C et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–9. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stout RD, Jiang C, Matta B et al. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–49. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 82.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–13. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Servet-Delprat C, Arnaud S, Jurdic P et al. Flt3+ macrophage precursors commit sequentially to osteoclasts, dendritic cells and microglia. BMC Immunol. 2002;3:15. doi: 10.1186/1471-2172-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milner R, Campbell IL. Cytokines Regulate Microglial Adhesion to Laminin and Astrocyte Extracellular Matrix via Protein Kinase C-Dependent Activation of the α6β1 Integrin. J Neurosci. 2002;22:1562–72. doi: 10.1523/JNEUROSCI.22-05-01562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Geissmann F, Gordon S, Hume DA et al. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–60. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neumann H. Microglia: a cellular vehicle for CNS gene therapy. J Clin Invest. 2006;116:2857–60. doi: 10.1172/JCI30230. [DOI] [PMC free article] [PubMed] [Google Scholar]


