Abstract
Migraine is defined as recurrent attack of headache that are commonly unilateral and accompanied by gastrointestinal and visual disorders. Migraine is more prevalent in females than males with a ratio of 3:1. It is primarily a complex neurovascular disorder involving local vasodilation of intracranial, extracerebral blood vessels and simultaneous stimulation of surrounding trigeminal sensory nervous pain pathway that results in headache. The activation of ‘trigeminovascular system’ causes release of various vasodilators, especially calcitonin gene-related peptide (CGRP) that induces pain response. At the same time, decreased levels of neurotransmitter, serotonin have been observed in migraineurs. Serotonin receptors have been found on the trigeminal nerve and cranial vessels and their agonists especially triptans prove effective in migraine treatment. It has been found that triptans act on trigeminovascular system and bring the elevated serum levels of key molecules like calcitonin gene related peptide (CGRP) to normal. Currently CGRP receptor antagonists, olcegepant and telcagepant are under consideration for antimigraine therapeutics. It has been observed that varying levels of ovarian hormones especially estrogen influence serotonin neurotransmission system and CGRP levels making women more predisposed to migraine attacks. This review provides comprehensive information about the role of serotonin and CGRP in migraine, specifically the menstrual migraine.
Keywords: Migraine, Trigeminal ganglia, Serotonin, CGRP, Menstrual migraine
Introduction
Migraine is a painful neurological condition characterized by severe pain on one or both sides of the head. Although the history of headache is 9000 years old, migraine was discovered in the second century by Aretaeus of Cappadocia. Migraine is a French word but in Greek it was termed “hemicrania” meaning ‘only half the head’. It is the most debilitating chronic medical illness that hampers normal life. Globally, migraine afflicts 11% of the total adult population1 creating a significant socio-economic burden on society. The prevalance of migraine is highest (13%) in Europe and North America and 9% in Asia.2 A recent study conducted on headache disorders in India has depicted that 26% of people among different types of headache sufferers are migraineurs.3 Migraine is more prevalent in women than men with a ratio of 3:1,3 though prior to puberty, the prevalence is higher in boys than girls.2
Migraine pain is often accompanied by nausea, vomiting, fever, chills, aching, sweating and sensitivity to light (photophobia), sound (phonophobia) or movement making it different from tension-type headaches. The signs and symptoms of migraine are variable and include the four phases of prodrome, aura, pain and postdrome that are common among patients but are not necessarily experienced by all migraine sufferers. Several hours or days before the headache, patient experiences irritation, depression, excessive sleep, increased urination, muscle stiffness (especially of the neck), constipation or diarrohea etc. that constitutes the prodrome phase. Symptoms of migraine aura appear gradually for 5 to 20 minutes which can be visual, sensory (numbness) or motor in nature and generally lasts less than 60 minutes. Visual disturbances are extremely painful with bright flashing lights, black spots or partial loss of vision. Then migraine pain occurs which may be bilateral at the onset or gradually start on one side and become generalized, usually alternating sides from one attack to the next. The extremities tend to be cold and moist. The frequency of attacks is extremely variable, from a few in a lifetime to several times a week with an average of one to three headaches a month. After an attack in the postdrome phase, some people feel unusually refreshed or euphoric while others undergo depression or experience tiredness, irritablility, listlessness, impaired concentration, scalp tenderness or mood changes.
Classification of Migraine
The International Headache Society has classified the different types of migraine and described it in “International Classification of Headache Disorders, 2nd. edition (ICHD-2)”.4
Migraine without aura – This is the most common form and accounts for 80% of cases reported with migraine headache.
Migraine with aura – This constitutes second most common form with the diagnostic criteria as aura associated with fully reversible visual or sensory symptoms. However, no muscle weakness or paralysis is seen. Approximately 10% of the migraineurs experience this classical type. The subtypes of migraine with aura are -
Migraine headache with typical aura – A migraineur suffers from headache with typical aura symptoms.
Non-migraine headache with typical aura – This type is characterized by typical aura phase during headache which is non-migrainous.
Typical aura without headache – The patient experiences typical aura phase symptoms without any head pain.
Familial hemiplegic migraine (FHM) – This is the rare Mendelian dominant form of migrane which is more p revalent in monozygotic twins than dizygotic ones. Numerous genetic abnormalities, especially in the genes encoding membrane transport proteins, ion pumps and channels eg CACNA1A, ATP1A2, SCN1A are likely to be involved in this migraine type.5–7 Motor weakness and/or paralysis are the symptoms of diagnosis.
Sporadic hemiplegic migraine - This is the non-familial form of migraine associated with motor weakness where no first degree relative has identical attacks.
Basilar-type migraine – This is an uncommon type of migraine with aura wherein the symptoms are referable to brainstem and comprise bilateral blurred vision, vertigo, ataxia, incordination and nausea. This type mimics vertibrobasilar attacks and hence its name.
Childhood periodic syndromes – The syndromes occur in children and includes the following two subtypes –
Abdominal migraine – The abdominal migraine is a recurrent abdominal pain of unknown origin associated with vomiting and nausea.8
Benign paroxysmal vertigo of childhood – In benign type migraine, occasional attacks of vertigo are experienced by children.
Acephalgic migraine or complicated migraine – This is also referred to as amigrainous migraine and includes cerebral migraine, optical migraine and ocular migraine or scintillating scotoma. All the usual symptoms of migraine except headache are experienced during the attack. In ocular (retinal) migraine, the distal vessel to the bifurcation of the optic nerve head is affected and the paralytic ocular muscles recover their function in few days or weeks. However, a permanent visual field defect occurs in optical migraine which affects central retinal artery.
Menstrual migraine – Migraine headaches are influenced by changes in the ovarian hormones that occur during the menstrual cycle. Various reports suggest that menstrual migraine is more severe and disabling than nonmenstrual migraine.9 Menstrual migraine has two subtypes which are as follows -
Menstrually related migraine without aura – Migraine attacks occur during the perimenstrual time period (2 days before to 3 days after the onset of menstruation) and also occur at other times of the month.
Pure menstrual migraine without aura – Migraine headaches are strictly limited to the perimenstrual time period and donot occur at other times of the menstrual cycle.
The Trigeminal System
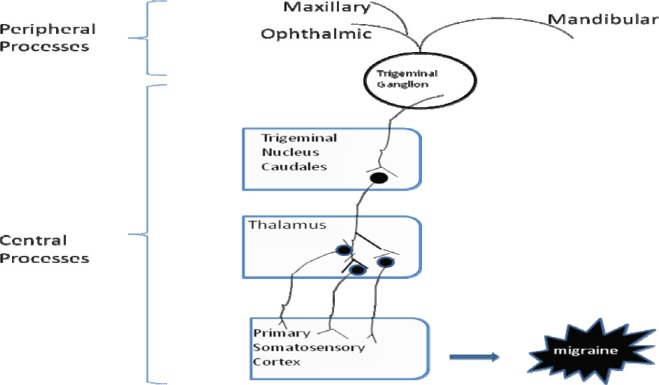
The trigeminal system represents one of the most important anatomic and functional area for the study of migraine pathophysiology. The trigeminal nerve is the largest cranial nerve, having a large sensory root and a smaller motor root. The trigeminal nerve emerges from the dorsal and median raphe nuclei located in the brain stem and bears a trigeminal or gasserion ganglion at its base. The cells in the trigeminal ganglion possess peripheral and central processes. The peripheral processes of trigeminal ganglion neurons constitute the three sensory branches - ophthalmic, maxillary and mandibular, of the trigeminal nerve. These three branches distribute to pain and temperature receptors on face, forehead, eyelids, nose, pinna, tongue, teeth, cerebral blood vessels (trigeminovascular system), dura mater (membrane that surrounds the brain) and in the posterior area of head and neck.
The central processes descend in the brain stem and comprise the spinal tract (fig. 1). Fibers of spinal tract terminate upon the spinal trigeminal nucleus and the principal trigeminal nucleus forming the region of trigeminal nucleus caudalis (TNC), the axon fibres of which further form the trigeminothalamic tract. The trigeminothalamic tract (TTT) extends deep into the higher centers in the brain, that is the somatosensory cortex via the ventral posterior medial nucleus (VPM) and posterior medial nucleus. In this way, the peripherally and centrally projecting fibres constitute the neural pathway in migraine for transmitting pain signals from the cranial vessels to the brain centers.10
Fig. 1:
Schematic pathway of the central processes of trigeminal ganglion neurons to the higher centers of brain.
Pathophysiology
The pathophysiology of migraine can be studied keeping in mind the series of clinical events occuring during an acute migraine attack. The prerequisite for migraine attack is initiation event followed by activation and transmission within sensory trigeminal neurons and finally modulation of the nociceptive trigeminal information within the central nervous system. The pain initiating events take place in the trigeminovascular system. The transmitting event is mediated by interaction between the neurons through the release of different neurotransmitters. The malfunctioning in the modulation of the pain signal in the periaquaductal grey (PAG) in midbrain was thought to be an underlying mechanism for migraine attack. Later discoveries demonstrated that the descending modulatory pathways that arise in the PAG project via medulla oblongata onto the segments of spinal cord for termination, hence dysfunction in PAG cannot generate a distinct cephalic throbbing pain.11,12 Focus on initiation phenomenon led to the postulation of different theories in the last sixty years stating migraine to be a vascular, neuronal or neurovascular disorder, but a unifying convincing mechanism for this debilitating disorder is still awaited.13 Further insight is required to understand the molecular basis for the emergence of migraine pathogenesis.
The vasogenic theory dominated the discussion of migraine pathogenesis until 1980s. Sir Harold Wolff,14 the great clinician of his time, observed three main aspects: a) extracerebral vessels dilate during the migraine attack and are agonizing; b) cranial blood vessel stimulation provokes an ipsilateral headache; c) vasoconstrictor drugs as ergot derivatives alleviate the pain, while vasodilators like nitrates may induce an attack. This led him to postulate that the vasodilation of the intracranial, extracerebral blood vessels cause throbbing head pain. The area around the dilated blood vessels becomes inflammed and irritates the nociceptive nerves. The vascular phenomenon was further explained by the studies involving arteriovenous anastomoses (shunts) which are the communication between an artery and a vein serving as back up routes for blood to flow in case of any blockage. The closure of arteriovenous anastomoses during migraine attack tend to cause dilatation of the vessels which is attributed to the decreased oxygen extraction observed in the side affected.15 Successive findings corroborate the release of vasoactive and neuroactive substances like endorphins, serotonin, histamine, adenosine, prostaglandins and nitric oxide in causing neurogenic inflammation.16–18 Various findings19,20 including non-induction of migraine headache by vasodilating substances as vasoactive intestinal peptide (VIP) could not be explained by vasogenic theory. VIP has been found to induce vasodilation of cranial vessels but failure to triggering migraine attack21 supports that vascular changes are not necessary in migraine. Further, 3T magnetic resonance angiography reports have depicted that vasodilatory nitrate derivatives, without bringing any continuing change in the cranial blood vessels, promote a delayed meningeal inflammation and induce migraine attack.22 The alternative neurogenic theory proposed migraine as a brain disorder in which vascular changes are a result of neuronal dysfunction.
Various neuroimaging techniques and genetic studies led Moskowitz and his colleagues to introduce a neurovascular theory.22–24 The studies conducted during spontaneous aura phase indicate that vasodilation takes place after the onset of headache and decreased cortical blood flow is not sufficient to cause ischemia. Attempts were made to explain the characteristic aura symptom of migraine in a hope to decipher the pathophysiology. It was found that aura is due to the spread of depression-like phenomena over an area of brain cortex which is also called as cortical spreading depression (CSD).25,26 Recent studies have shown that some genetic mutation in mice responsible for FHM is playing a role in promoting CSD-like event in hyperexcitable cortex in migraineurs.27 CSD is a slowly propagating depolarization of neuronal and glial membranes evoked when extracellular K+ concentrations ([K+]e) increase above a genetically determined threshold.28 The triggers, which may be behavioral, environmental,29 dietary, chemical or hormonal30,31 set off a chain of neurologic and biochemical events leading to the spread of CSD32 in regions of reduced blood flow. In India, the hot and humid weather for most of the year, increased noise levels, different food triggers, fasting habits in different communities, henna application, stressful school education and the stressful travelling conditions pose as potential risk factors of frequent headaches.33 It is suggested that triggering factors can influence neurons implicated in nociceptor activation causing migraine pain in a susceptible person. However, it is not the triggers but nociceptor activation which is mandatory for throbbing pain. The triggers generate a contraction at the base of the brain that closes down several arteries and reduces blood flow to the brain. This is followed by the accumulation of vasodilating substances such as adenosine, ADP, nitric oxide etc. Simultaneously, the trigeminal nerves are stimulated. This activation of the ‘trigeminovascular system’ is thought to cause the release of glutamate neurotransmitter and vasoactive sensory neuropeptides, such as substance P34 and calcitonin gene-related peptide (CGRP),1 that further dilate cerebral blood vessels and produce an inflammatory response causing pain.24 High levels of circulating CGRP have been detected during migraine.35,36 It has been observed that the expression of CGRP at peripheral synapses results in vasodilatation and at central synapses, it acts postjunctionally on second-order neurons to centrally transmit pain via the brainstem and midbrain to higher cortical regions.1 In this way the nociceptive (pain) information is conveyed to the central neurons that in turn relay the pain signals to higher centers where headache is perceived. It has been hypothesized that these central neurons may become sensitized as a migraine attack progresses, thus, worsening the headache. Further, the pathophysiological features of migraine may progress over months to years in a susceptible person leading to long-term and pervasive alterations in the brain. This leads to progression of episodic migraine to a chronic form, which is even more difficult to manage.37
A rare autosomal dominantly inherited form of migraine recognized as FHM points towards the genetic linkage of the disease and throws some light on understanding its pathogenesis. Numerous genetic abnormalities, especially in the genes encoding membrane transport proteins, ion pumps and channels eg CACNA1A, ATP1A2, SCN1A are likely to be involved in migraine. The mutations in the genes appear to enhance cerebral excitability via different mechanisms that may alter the response threshold to migraine specific triggers in the brain of a migraineur compared to a normal individual.38 It has been observed that mutations in CACNA1A gene encoding the α1 pore-forming subunit of a brain specific voltage-gated P/Q-type calcium channel enhance the probability of glutamate release in cortical cell synapses of FHM1 mutant mice.5 Another defect in the ATP1A26 gene that encodes the α2-subunit of a Na+/K+ ion pump might increase ambient [K+]e and glutamate levels in the synaptic cleft and hence disrupts resting membrane potential, cell volume and neurotransmitter uptake. A mutation that occurs in the voltage-gated sodium channel, SCN1A gene leads to accelerated channel recovery from fast inactivation which increase dendritic excitability and neuronal firing.7 Mutations in ion channels could have led towards the vasogenic path but the consequent elevated extracellular glutamate and [K+]e levels support neurogenic theory leading to reduced CSD threshold in migraine.39
Serotonergic Neurotransmitter System
Various studies have implicated serotonin in the pathogenesis of migraine.40,41 Serotonin vasoconstricts the nerve endings and blood vessels and in this way affects nociceptive pain.42 Comings43 postulated that low serotonin levels dilate blood vessels and initiate migraine. Migraine sufferers often report that the headaches stop after they have vomited. Vomiting stimulates intestinal motility and raises blood serotonin. Earlier it was suggested that probably the fluctuating serotonin levels lead to pH variations in the brain causing migraine, since serotonin also called as 5-hydroxytryptamine (5-HT), is a basic amine. In the brain, normal levels of endogenous serotonin (5-HT) prevents migraine headache. It has been found that most of the neurons present in the dorsal raphe (site of emergence of trigeminal nerve) and trigeminal ganglia are serotonergic.10,40,44 Serotonin (5-hydroxytryptamine, 5-HT) synthesis involves the rate limiting enzyme, tryptophan hydroxylase (TPH)45 and is mainly degraded by the action of monoamine oxidases.46 Tryptophan hydroxylase activity in rat brain base arteries has been suggested to be a marker of serotonergic innervations.47 Since these enzymes influence serotonin levels, any alterations at the level of their transcription, translation or post-translational modification affects serotonin neurotransmission system. There is evidence that genetic polymorphisms in TPH enzyme influence susceptibility to anxiety and depression.48 Monoamine oxidase inhibitors, which increase serotonin in the synapses, prevent migraine headaches. In addition to serotonin, about half of the rat trigeminal ganglion neurons contain nitric oxide.49 It is likely that serotonin and nitric oxide are colocalized and coreleased from the same neurons. Depletion of serotonin, a vasoconstrictor, would leave the vasodilator nitric oxide unopposed and hence pain is perceived.40 The localization of nitric oxide synthesizing enzymes throughout the migraine pain pathway suggests the involvement of nitric oxide in migraine mechanisms.50 Exogenous serotonin causes both vasoconstriction and marked arteriolar dilation of cerebral vessels. This exhibits that cerebral vessels respond to exogenous serotonin in a dramatic and complex manner.51 It has been observed that the agonists of serotonin selectively brings the elevated serum levels of the vasodilating peptide, CGRP to normal. It could be suggested that serotonin replenishment during migraine headache serves two purposes. It acts on the vasodilators by lowering the level of one (CGRP) and opposing the effect of other (nitric oxide).
The serotonin receptor system plays central role in the control of serotonergic neurotransmission and feature prominently in many behavioral and physiological functions.52 Out of the 7 receptor types, 5-HT1, 5-HT2, and 5-HT3 receptors (especially 5-HT1) have been identified as being responsible for most of the migraine activity. These receptors have been found on the trigeminal nerve endings.40,53,54 The 5-HT1 receptors are coupled to Gi/Go and mediate cellular effects through decreasing cellular levels of cAMP.55 The 5-HT2 receptors are coupled to Gq/G1156 and mediate cellular effects through increasing cellular levels of IP3 and DAG. 5-HT3 receptors are unique among the families of 5-HT receptors as these are nonselective ligand-gated Na+/K+ ion channel receptors. 5-HT3 receptors can control dopamine release and may also be involved in acetylcholine release and control of the GABAnergic system. It is suggested that the subtypes of 5-HT receptors may have different roles in migraine. Some may be involved in triggering migraines (eg. 5-HT1C)57 and others in preventing them (eg. 5-HT1D).58 Furthermore, evidence has shown that some receptors undergo desensitization when exposed to high concentrations of serotonin leading to protective effect.59 The therapeutic implication of serotonin receptors raises a need to identify target molecule in signaling cascade.
Regulation of serotonin and CGRP levels in females
There is no doubt that serotonin and CGRP levels in neuronal tissues are affected by physiological hormonal intervention specifically the estrogen making women predisposed to migraine. Migraine is a major health problem in females and needs to be tackled keeping in mind the reproductive phases of life. A drop in estrogen level during menstruation produces a decrease in serotonin by affecting its metabolism, which can bring on migraine.60 Similarly, cessation of intake of birth control pills, produce headaches due to fall in serotonin levels. Furthermore, if sudden decreases in estrogen can precipitate attacks then chronic high estrogen levels can also increase the likelihood of migraine. In fact, the varying levels of estrogen are the main culprit rather than the drop in estrogen. This has been further supported by the patient diary data studies. The headache patterns observed in ovulating women and those using oral contraceptives were found to be similar except a secondary pain peak observed in the former case during the days around ovulation i.e. at the time of exponentially changing estrogen levels.61 So, the assessment of the effects of menstrual cycle on pain sensitivity demands specific timing of measurements during the phases of the menstrual cycle. Ovariectomized mice are useful models for a hormonally dependent hyperalgesic state resembling functional pain.62 One clinical correlate of this animal model is the preliminary evidence that migraine is exacerbated by surgical menoupause in women who have undergone ovariectomy.63 So, now-a-days ovariectomized animals are used to study the effects of hormone deficit and hormone replacement on trigeminal system as well as behavioral changes of the animal that may correlate with aura symptoms and pain.64
Estrogen can act via can genomic or non-genomic mechanism to regulate the levels of neuroactive molecules. Nongenomic mechanism involve the modulation of neurotransmission unrelated to the transcription of genes and may occur within seconds to minutes following estrogen exposure. Estrogen has been found to rapidly increase nitric oxide production65 in cerebral blood vessels by increasing the phosphorylation of protein kinase B (Akt) and endothelial nitric oxide synthase (eNOS) enzyme through the PI3K/Akt/eNOS pathway. However, chronic estrogen exposure specifically increases eNOS protein levels through genomic mechanisms involving a receptor-mediated increase in transcription and translation of the eNOS gene.66 Genomic mechanism of action of estrogen consists of regulation of gene transcription via estrogen receptors (ER) having wide distribution throughout the central nervous system. Estrogen receptors may be membrane-bound or intracellular and are of two types: ERα and ERβ.67 ERα is expressed in trigeminal neurons68,69 while ERβ is within the dorsal raphe.70,71 Estrogen binds to its receptors and either causes receptor dimerizaton or activates secondary messenger system such as cAMP/protein kinase A, protein kinase C, MAP kinase/ERK etc. The estrogen-receptor complex or the secondary messengers enter the nucleus and bind to DNA regulatory regions as AP-1 sites,72 estrogen response element (ERE), cAMP response element (CRE) and serum response element (SRE) to regulate the transcription of various genes.70 Data obtained by various studies has shown up-regulation of the serotonergic system during the mid-menstrual cycle (high estrogen) while during the mid luteal and early follicular phases (low estrogen), there is down-regulation of the serotonergic system. Evidences by Gangula and coworkers73 have shown that estrogen also affect CGRP levels. Their data showed that estrogen deprivation decreases plasma CGRP concentration in ovariectomized rats which was significantly restored on subsequent treatment with 17β-estradiol. Durham and coworkers74 have investigated the control of CGRP expression by a serotonergic agonist and demonstrated that the activation of the endogenous 5-HT1 receptor is coupled to calcium signaling pathways leading to inhibition of CGRP gene transcription by repression of promoter activity through CRE and a cell-specific enhancer. Elevated calcium can inhibit CRE binding protein (CREB) activity by stimulating a CREB phosphatase75,76 and by causing an inhibitory phosphorylation of CREB.77 Further studies showed that the sustained elevation of intracellular calcium associated with administration of a selective agonist of serotonin receptors blocks the MAPK-mediated activation of CGRP gene expression.78 This finding raises a possibility that serotonin system and CGRP are linked to each other in bringing about migraine pathphysiology. Also, delineating the role of estrogen in migraine may lead to a better understanding of its cross talk with serotonin and CGRP in the trigeminal nerve paving way for rational drug development.
Treatment
Before undergoing any sort of treatment for migraine it is necessary to assess the headache burden i.e. the level upto which life of the patient is affected due to migraine. Medication overuse may worsen migraine pain or may lead to ulcers or other gastrointestinal problems. Migraine treatment either involves prevention of progressing headache or it aims at reducing the frequency and severity of migraine attack or both. However, the best way to treat migraine is to avoid trigger factors that provoke migraine.
Combination therapy - The first line of treatment of migraine includes analgesics such as paracetamol, aspirin, ibuprofen, nonsteroidal anti-inflammatory drugs (NSAIDs); sedatives such as codeine, morphine; beta blockers (eg. propanolol and atenolol); anticonvulsants (eg. valproate and topiramate); and antidepressants such as amitryptiline and selective serotonin reuptake inhibitors (commonly known as SSRIs eg. flouxetine).79
Ayurvedic therapy – Some physicians suggest a need to integrate modern medicine with ayurveda for the treatment of migraine. Bhang (Cannabis sativa), feverfew (Tanacetum parthenium), root of kudzu plant (Puereria lobata) and rhizome extract of butterbur (Petasites hybridus)80 have been used for the preparation of herbal medicines to treat migraine. The practice of yoga has proved beneficial to migraine sufferers.
Agonist/antagonist therapy – Drugs such as methysergide and other ergot derivatives as tablets, nasal sprays or injections have been used for the treatment of migraine. Ergot- derivatives such as, lysergic acid diethylamide (LSD) are fairly nonselective agents that bind at multiple populations of 5-HT receptors.81 Methysergide acts as antagonist at the 5-HT2B,82 5-HT2C and 5-HT1A receptors.83,84 It is known to have partial agonist effects on some of the other serotonin receptors as well.85 The 5-HT2B antagonist activity of methysergide appears to selectively decrease carotid blood flow by constricting arteriovenous anastomoses providing migraine prophylaxis. However, the most effective drugs include the triptans38 such as sumatriptan, frovatriptan, naratriptan, zolmitriptan and rizatriptan which causes fewer side effects than methysergide. Triptans have been thought to act as cranial vasoconstrictors in bringing relief against migraine but later it was recognized that their antimigraine action is neural. The vasoconstrictor effect of triptans is unnecessary since dilation is not a key part of migraine process. Triptans act exclusively as 5-HT1B/1D receptor agonists and mimic the role of serotonin in binding to its receptors in trigeminal nerve endings as well as the blood vessels. This leads to a decrease in the release of various pain worsening-peptides such as CGRP and substance P and cures migraine headache and its associated symptoms.86
Currently CGRP receptor antagonists, olcegepant (BIBN4096BS) and telcagepant (MK-0974) are under consideration for antimigraine therapeutics keeping in mind the recognition that targeting neuronal transmission treats migraine. These drugs have been found to selectively block the CGRP receptors without vasoconstrictor effects of triptans and rid the patient of any vascular complications.1 Further, the treatment of migraine at the signaling level might exclude the resistance effect seen due to medication overuse. The target molecule in the signaling cascade could be identified by employing bioinformatic tools and molecular biology techniques which might lead to a better understanding of the disorder and hence improve the lives of migraine patients.
Conclusions
Migraine is painful recurrence of headache due to variation in the blood flow of cerebral blood vessels. This is caused by activation of ‘trigeminovascular system’, which causes release of vasodilators eg. calcitonin gene-related peptide (CGRP) and diminution of the levels of neurotransmitter like serotonin in trigeminal nerve and cranial vessels. The interplay of the level of these peptides gives the nociceptive information to the central neurons in the brain stem that in turn relay the pain signals. The role of these neuroactive molecules can be exacerbated by physiological hormonal intervention specifically the estrogen that has been shown to regulate CGRP as well as serotonin in neuronal tissues. Understanding the relationship of estrogen in regulation of various neuropeptides can pave way to novel therapeutic targets for migraine management.
Acknowledgements
Financial support from UGC fellowship to MA, UGC major research project to VP and DST PURSE Grant is thankfully acknowledged.
Footnotes
This article complies with International Comittee of Medical Journal editor’s uniform requirements for manuscript.
Competing interests – None
Source of Funding – UGC
References
- 1.Eftekhari S, Edvinsson L. Possible sites of action of the new calcitonin gene-related peptide receptor antagonists. Ther Adv Neurol Diord. 2010;3(6):369–378. doi: 10.1177/1756285610388343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins MS, Lipton RB. The epidemiology of primary headache disorders. Semin Neurol. 2010;30(2):107–109. doi: 10.1055/s-0030-1249220. [DOI] [PubMed] [Google Scholar]
- 3.Menon R. Women more prone to headaches: study. Deccan Herald N S. 2011 Apr 25th; [Google Scholar]
- 4.Eriksen MK, Thomsen LL, Olesen J. New international classification of migraine with aura (ICHD-2) applied to 362 migraine patients. Er J Neurol. 2004;11:583–591. doi: 10.1111/j.1468-1331.2004.00890.x. [DOI] [PubMed] [Google Scholar]
- 5.Tottene A, Conti R, Fabbro A et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron. 2009;61(5):762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Stam AH, van den Maagdenberg AM, Haan J et al. Genetics of migraine: an update with special attention to genetic comorbidity. Curr Opin Neurol. 2008;21(3):288–293. doi: 10.1097/WCO.0b013e3282fd171a. [DOI] [PubMed] [Google Scholar]
- 7.Johnston D, Magee JC, Colbert CM, Cristie BR. Active properties of neuronal dendrites. Annu Rev Neurosci. 1996;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- 8.Kakisaka Y, Wakusaka K, Haginoya K et al. Abdominal migraine associated with ecchymosis of legs and buttocks: does the symptom imply an unknown mechanism of migraine? Tohoku J Exp Med. 2010;221:49–51. doi: 10.1620/tjem.221.49. [DOI] [PubMed] [Google Scholar]
- 9.Martin V, Wernke S, Mandell K et al. Defining the relationship between ovarian hormones and migraine headache. Headache. 2005;45:1190–1201. doi: 10.1111/j.1526-4610.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 10.Lambert GA. The lack of peripheral pathology in migraine headache. Headache Curr. 2010;50(5) doi: 10.1111/j.1526-4610.2010.01669.x. [DOI] [PubMed] [Google Scholar]
- 11.Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74:1742–1759. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- 12.Basbaum AI, Fields HL. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 13.Levy D. Migraine pain and nociceptor activation- where do we stand? Headache Curr. 2010:909–916. doi: 10.1111/j.1526-4610.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 14.Wolff HG. 2nd ed. New York: Oxford University Press; 1963. Headache and other head pain. [Google Scholar]
- 15.Ninck B. Migraine and epilepsy. Eur Neurol. 1970;3 doi: 10.1159/000113967. [DOI] [PubMed] [Google Scholar]
- 16.Hoskin KL, Bulmer DC, Goadsby PJ. Fos expression in the trigeminocervical complex of the cat after stimulation of the superior sagittal sinus is reduced by L-NAME. Neurosci Lett. 1999;266:173–176. doi: 10.1016/s0304-3940(99)00281-5. [DOI] [PubMed] [Google Scholar]
- 17.Sicuteri F. Migraine: a central biochemical dysnociception. Headache. 1976;16:145–159. doi: 10.1111/j.1526-4610.1976.hed1604145.x. [DOI] [PubMed] [Google Scholar]
- 18.Sicuteri F. Headache as possible expression of deficiency of brain 5-hydroxytryptamine (central denervation supersensitivity). Headache. 1972;12:69–72. doi: 10.1111/j.1526-4610.1972.hed1202069.x. [DOI] [PubMed] [Google Scholar]
- 19.Schoonman GG, van der Grond J, Kortmann C et al. Migraine headache is not associated with cerebral or meningeal vasodilatation- a 3T magnetic resonance angiography study. Brain. 2008;131(8) doi: 10.1093/brain/awn094. [DOI] [PubMed] [Google Scholar]
- 20.Kruuse C, Thomsen LL, Birk S et al. Migraine can be induced by sildenafil without changes in middle cerebral artery diameter. Brain. 2003;126(1) doi: 10.1093/brain/awg009. [DOI] [PubMed] [Google Scholar]
- 21.Rahmann A, Wienecke T, Hansen JM et al. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2007;28(3):226–236. doi: 10.1111/j.1468-2982.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 22.Reuter U, Bolay H, Jansen-Olesen I et al. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain. 2001;124:2490–2502. doi: 10.1093/brain/124.12.2490. [DOI] [PubMed] [Google Scholar]
- 23.Gargus JJ. Genetic calcium signaling abnormalities in the central nervous system: seizures, migraine and autism. Annals N Y Acad Sci. 2009:138–156. doi: 10.1111/j.1749-6632.2008.03572.x. [DOI] [PubMed] [Google Scholar]
- 24.Moskowitz MA, Macfarlane R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc Brain Metab Rev. 1993;5:159–177. [PubMed] [Google Scholar]
- 25.Leao AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7 doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 26.Lashley KS. Patterns of cerebral integration indicated by the scotomas of migraine. Arch Neurol Psychiatry. 1941;46:331–333. [Google Scholar]
- 27.Van den Maagdenberg AM, Pietrobon D, Pizzorusso T et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–710. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 28.Nozari A, Dilekoz E, Sukhotinsky I et al. Microemboli may link spreading depression migraine aura and patent foramen ovale (p NA). Ann Neurol. 2009 doi: 10.1002/ana.21871. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noseda R, Kainz V, Jakubowski M et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13(2):239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221–227. doi: 10.1007/s11916-010-0112-z. [DOI] [PubMed] [Google Scholar]
- 31.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 32.Eikermann-Haerter K, Ayata C. Cortical spreading depression and migraine. Curr Neurol Neurosci Rep. 2010;10:167–173. doi: 10.1007/s11910-010-0099-1. [DOI] [PubMed] [Google Scholar]
- 33.Ravishankar K. Barriers to headache care in India and efforts to improve the situation. Lancet. 2004;3(9):564–567. doi: 10.1016/S1474-4422(04)00855-5. [DOI] [PubMed] [Google Scholar]
- 34.Michael L, Oshinsky , Luo J. Neurochemistry of trigeminal activation in an animal model of migraine. Headache. 2006;46(Suppl 1) doi: 10.1111/j.1526-4610.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- 35.Durham PL, Vause CV. Calcitonin gene-related peptide (CGRP) receptor antagonists in the treatment of migraine. CNS Drugs. 2010;24(7):539–548. doi: 10.2165/11534920-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edvinsson L, Goadsby PJ. Neuropeptides in migraine and cluster headache. Cephalalgia. 1994;14:320–323. doi: 10.1046/j.1468-2982.1994.1405320.x. [DOI] [PubMed] [Google Scholar]
- 37.Aurora S, Kulthia A, Barrodale P M. Mechanism of chronic migraine. Curr Pain Headache Rep. 2011;15:57–63. doi: 10.1007/s11916-010-0165-z. [DOI] [PubMed] [Google Scholar]
- 38.Hargreaves RJ, Shepheard SL. Pathophysiology of migraine- new insights. Can J Neurol Sci. 1999;26(3):12–19. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- 39.Moskowitz M A, Bolay H, Dalkara T. Deciphering migraine mechanisms: clues from familial hemiplegic migraine genotypes. Ann Neurol. 2004;55(2):276–280. doi: 10.1002/ana.20035. [DOI] [PubMed] [Google Scholar]
- 40.Berman NEJ, Puri V, Chandrala S et al. Serotonin in trigeminal ganglia of female rodents: relevance to menstrual migraine. Headache. 2006;46:1230–1234. doi: 10.1111/j.1526-4610.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 41.Ferrari M D, Odink J, Tapparelli C et al. Serotonin metabolism in migraine. Neurol. 1989;39:1239–1242. doi: 10.1212/wnl.39.9.1239. [DOI] [PubMed] [Google Scholar]
- 42.Taylor B K, Basbaum A I. Neurochemical characterization of extracellular serotonin in the rostral ventromedial medulla and its modulation by noxious stimuli. J Neurochem. 1995;65(2):578–589. doi: 10.1046/j.1471-4159.1995.65020578.x. [DOI] [PubMed] [Google Scholar]
- 43.Comings DE. Serotonin: a key to migraine disorders? Nutrition Health Review, Health and Fitness Magazine. 1994 [Google Scholar]
- 44.Agranoff BW, Fisher SK, Albers RW . Siegel (Sixth, Lippincott Williams and Wilkins); 1999. Understanding the neuroanatomical organization of serotonergic cells in the brain provides insight into the functions of this neurotransmitter, in Basic Neurochemistry. [Google Scholar]
- 45.Zhang X, Beaulieu JM, Sotnikova TD et al. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305 doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- 46.Bianchi P, Kunduzova O, Masini E et al. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112(21):3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 47.Moreno MJ, de Pablo ALL, Marco EJ. Tryptophan hydroxylase activity in rat brain base arteries related to innervation originating from the dorsal raphe nucleus. Stroke. 1994;25:1046–1049. doi: 10.1161/01.str.25.5.1046. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Beaulieu JM, Gainetdinov RR et al. Functional polymorphisms of the brain serotonin synthesizing enzyme tryptophan hydroxylase-2. Cell Mol Life Sci. 2006;63(1):6–11. doi: 10.1007/s00018-005-5417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoyanova I I, Lazarov N E. Localization of nitric oxide synthase in rat trigeminal primary afferent neurons using NADPH-diaphorase histochemistry. J Mol Histol. 2005;36:187–193. doi: 10.1007/s10735-005-1694-3. [DOI] [PubMed] [Google Scholar]
- 50.Ramachandran R, Ploug K B, Schmidt A H, Olesen J, Jansen-Olesen I, Gupta S. Nitric oxide synthase (NOS) in the trigeminal vascular system and other brain structures related to pain in rats. Neurosci Lett. 2010 doi: 10.1016/j.neulet.2010.08.050. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.MacKenzie E T, Edvinsson L. (Amsterdam) 1980. Cerebral circulation and neurotransmitters, in Excerpta Medica; 163 pp. [Google Scholar]
- 52.Meneses A. Physiological, pathophysiological and therapeutic roles of 5-HT systems in learning and memory. Terapeutica Experimental Rev Neurosci. 1998;9(4):275–289. doi: 10.1515/revneuro.1998.9.4.275. [DOI] [PubMed] [Google Scholar]
- 53.Hu W P, Guan B C, Ru L Q, Chen J G et al. Potentiation of 5-HT3 receptor function by the activation of coexistent 5-HT2 receptors in trigeminal ganglion neurons of rats. Neuropharmacol. 2004;47(6):833–840. doi: 10.1016/j.neuropharm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 54.Rebeck GW, Maynardti KI, Hyman BT et al. Selective 5-HT1Da serotonin receptor gene expression in trigeminal ganglia: implications for antimigraine drug development. Proc Natl Acad Sci USA. 1994;91:3666–3669. doi: 10.1073/pnas.91.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin S L, Setya S, Johnson-Farley N N, Cowen D S. Differential coupling of 5-HT1 receptors to G proteins of the Gi family. British J Pharmacol. 2002;136:1072–1078. doi: 10.1038/sj.bjp.0704809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Apud JA, Grayson DR, De Erausquin E, Costa E. Pharmacological characterization of regulation of phosphoinositide metabolism by recombinant 5-HT2 receptors of the rat. Neuropharmacol. 1992;31:1–8. doi: 10.1016/0028-3908(92)90153-g. [DOI] [PubMed] [Google Scholar]
- 57.Brewerton TD, Murphy DL, Meuller EA et al. Induction of migraine-like headaches by the serotonin agonist, m-chlorophenylpiperazine. Clin Pharmacol Ther. 1988;43:605–608. doi: 10.1038/clpt.1988.83. [DOI] [PubMed] [Google Scholar]
- 58.Ferrari MD, Melamed E, Gawel MJ. Treatment of migraine attacks with sumatriptan. N Engl J Med. 1991;325:316–318. doi: 10.1056/NEJM199108013250504. [DOI] [PubMed] [Google Scholar]
- 59.Kagaya A, Mikuni M, Kusumi I, Yamamoto Y, Takahashi K. Serotonin-induced acute desensitization of serotonin 2 receptors in human platelets via a mechanism involving protein kinase C. J Pharmacol Exp Ther. 1990;255:305–311. [PubMed] [Google Scholar]
- 60.Sommerville B W. Estrogen-withdrawal migraine. I. Duration of exposure required and attempted prophylaxis by premenstrual estrogen administration. Neurol. 1975;25:239–244. doi: 10.1212/wnl.25.3.239. [DOI] [PubMed] [Google Scholar]
- 61.Johannes C B, Linet M S, Stewart W F, Celentano D D, Lipton R B, Szklo M. Relationship of headache to phase of the menstrual cycle among young women: a daily diary study. Neurol. 1995;45:1076–1082. doi: 10.1212/wnl.45.6.1076. [DOI] [PubMed] [Google Scholar]
- 62.Sanoja R, Cervero F. Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: a model of functional abdominal pain. Pain. 2005;118:243–253. doi: 10.1016/j.pain.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 63.Neri I, Granella F, Nappi R, Manzoni G C et al. Characteristics of headache at menopause: a clinico-epidemiologic study. Maturitas. 1993;17:31–37. doi: 10.1016/0378-5122(93)90121-w. [DOI] [PubMed] [Google Scholar]
- 64.J Mogil. Migraine Trust International Research Symposium; London UK: 2006. Personal communication. [Google Scholar]
- 65.Ebner S, Dunbar M, McKinnon R D. Distinct Roles for PI3K in proliferation and survival of oligodedrocyte progenitor cells. J Neurosci Res. 2000;62:336–345. doi: 10.1002/1097-4547(20001101)62:3<336::AID-JNR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 66.Mendelsohn M E. Nongenomic, estrogen receptor-mediated activation of endothelial nitric oxide synthase. How does it work? What does it mean? Circulation Res. 2000;87:956–960. doi: 10.1161/01.res.87.11.956. [DOI] [PubMed] [Google Scholar]
- 67.Couse J F, Lindzey J, Grandien K et al. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERα) and estrogen receptor-beta (ERβ) messenger ribonucleic acid in the wild-type and ERα –knockout mouse. Endocrin. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 68.Puri V, Cui L, Liverman C S et al. Ovarian steroids regulate neuropeptides in the trigeminal ganglion. Neuropeptides. 2005;39(4):409–417. doi: 10.1016/j.npep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Puri V, Puri S, Svojanovsky S R, Mathur S et al. Effects of oestrogen on trigeminal ganglia in culture: implications for hormonal effects on migraine. Cephalalgia. 2005;36:33–42. doi: 10.1111/j.1468-2982.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 70.McEwen B. Estrogen effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 71.Pau C Y, Pau K Y, Spies H G. Putative estrogen receptor beta and alpha mRNA expression in male and female rhesus macaques. Mol Cell Endocrinol. 1998;146:59–68. doi: 10.1016/s0303-7207(98)00197-x. [DOI] [PubMed] [Google Scholar]
- 72.Bjornstrom L, Sjoberg M. Estrogen receptor-dependent activation of AP-1 via non-genomic signaling. Nucl Recept. 2004;2 doi: 10.1186/1478-1336-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gangula P R, Zhao H, Wimalawansa S J, Supowit S C, DiPette D J, Yallampalli C. Pregnancy and steroid hormones enhance the systemic and regional hemodynamic effects of calcitonin gene-related peptide in rats. Biol Reprod. 2001;64:1776–1783. doi: 10.1095/biolreprod64.6.1776. [DOI] [PubMed] [Google Scholar]
- 74.Durham P L, Sharma R V, Russo A F. Repression of the calcitonin gene-related peptide promoter by 5-HT1 receptor activation. J Neurosci. 1997;17(24):9545–9553. doi: 10.1523/JNEUROSCI.17-24-09545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ginty D D. Calcium regulation of gene expression: isn’t that spatial? Neuron. 1997;18:183–186. doi: 10.1016/s0896-6273(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 76.Mulkey R M, Endo S, Shenolikar S, Malenka R C. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal longterm depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 77.Sun P, Enslen H, Myung P S, Maurer R A. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- 78.Durham P L. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1) doi: 10.1111/j.1526-4610.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jackson J L, Shimeall W, Sessums L Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. bmj.com. 2010. bmj.com Online First. [DOI] [PMC free article] [PubMed]
- 80.Sutherland A, Sweet B V. Butterbur: an alternative therapy for migraine prevention. Am J Health Syst Pharm. 2010;67(9):705–711. doi: 10.2146/ajhp090136. [DOI] [PubMed] [Google Scholar]
- 81.Guzman F. Serotonin (5-HT): receptors, agonists and antagonists. pharmamotion.com. 2009. pharmamotion.com
- 82.Schmuck K, Ullmer C, Kalkman H O, Probst A, Lubbert H. Activation of meningeal 5-HT2B receptors: an early step in the generation of migraine headache? Eur J Neurosci. 1996;8(5):959–967. doi: 10.1111/j.1460-9568.1996.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 83.Rang H P. Pharmacol, Edinburgh: Churchill Livingstone. 2003 [Google Scholar]
- 84.Saxena P R, Lawang A. a comparison of cardiovascular and smooth muscle effects of 5-hydroxytryptamine and 5-carboxamidotryptamine, a selective agonist of 5-HT1 receptors. Arch Int Pharmacodyn Ther. 1985;277(2):235–252. [PubMed] [Google Scholar]
- 85.Colpaert F C, Niemegeers C J, Janssen P A. In vivo evidence of partial agonist activity exerted by purpoted 5-hydroxytryptamine antagonists. Eur J Pharmacol. 1979;58(4):505–509. doi: 10.1016/0014-2999(79)90326-1. [DOI] [PubMed] [Google Scholar]
- 86.Ferrari M D, Roon K I, Lipton R B et al. Oral triptans (5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358:1668–1675. doi: 10.1016/S0140-6736(01)06711-3. [DOI] [PubMed] [Google Scholar]