Abstract
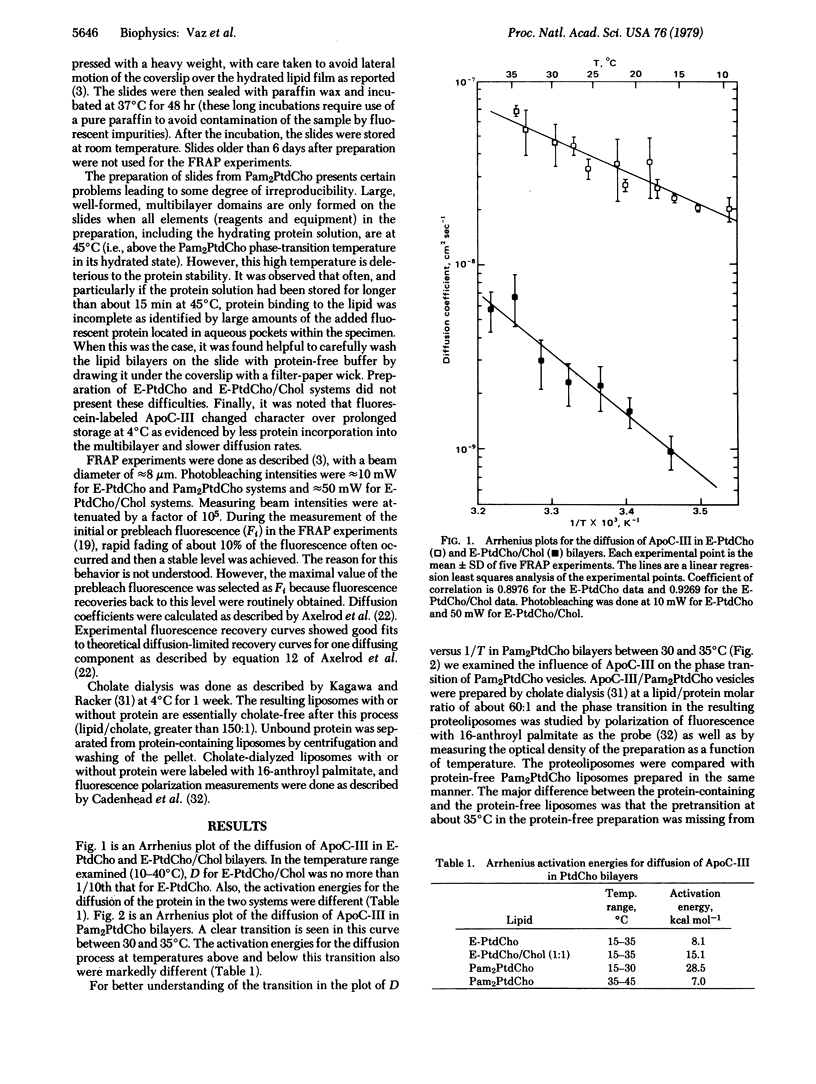
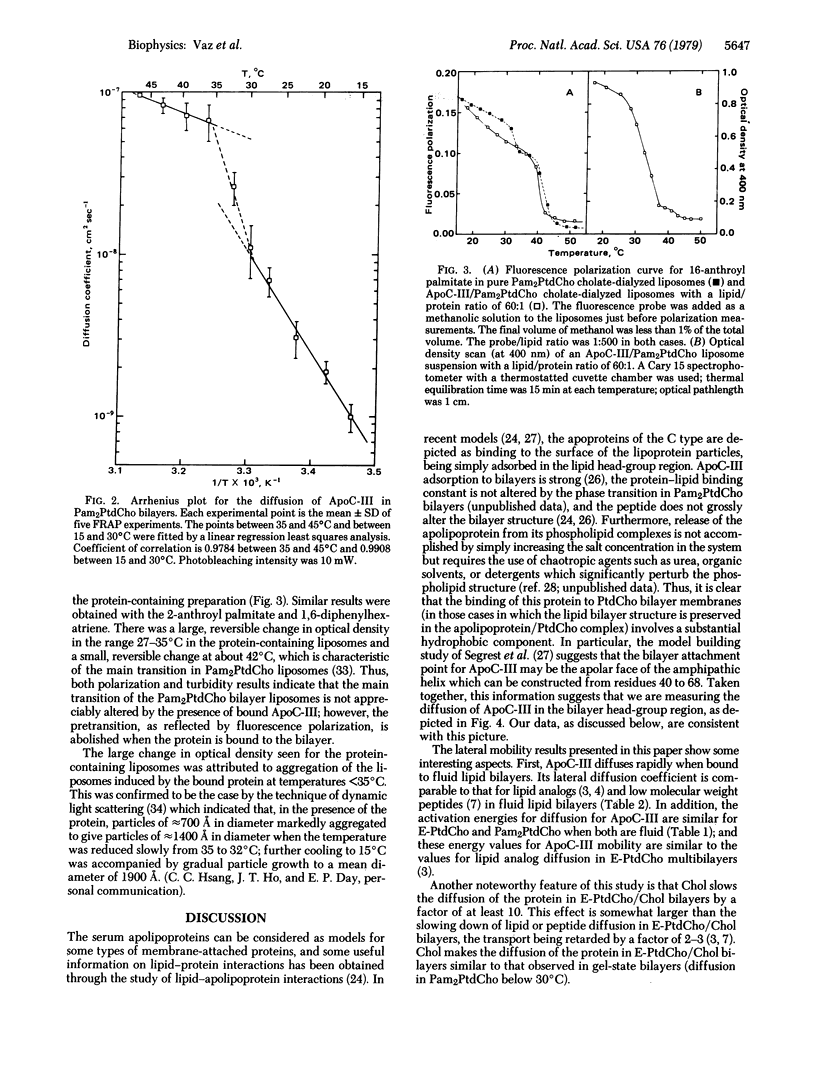
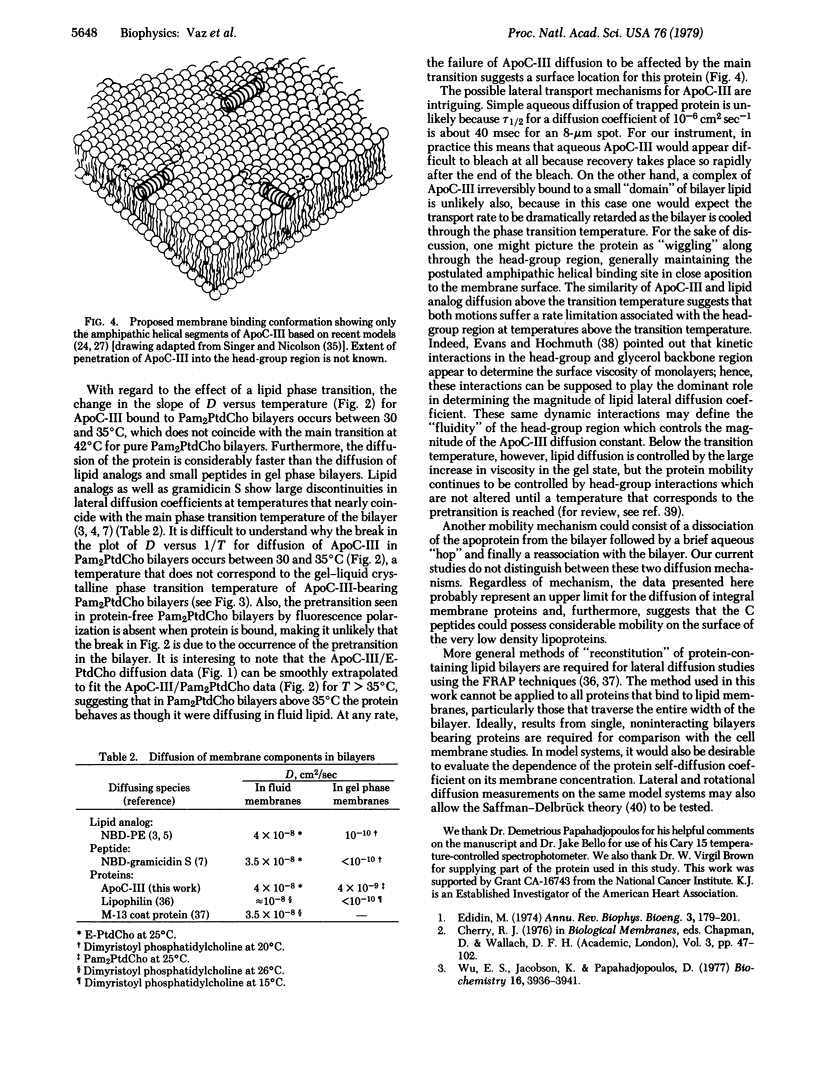
The technique of fluorescence recovery after photobleaching was used to investigate the lateral mobility of a fluorescein-labeled amphipathic apolipoprotein, ApoC-III, bound to multibilayers prepared from dipalmitoyl phosphatidylcholine, egg phosphatidylcholine, and a 1:1 (molar ratio) mixture of egg phosphatidylcholine and cholesterol. In dipalmitoyl phosphatidylcholine bilayers the lateral diffusion coefficient (D) for the protein is about 2 × 10-9 cm2 sec-1 at 20°C and about 9 × 10-8 cm2 sec-1 at 45°C. Plots of D versus temperature in this system show a transition between about 30 and 35°C. Arrhenius activation energies for the diffusion in this case between 15 and 30°C and between 35 and 45°C are 28.5 and 7.0 kcal mol-1, respectively (1 calorie = 4.18 joules). In egg phosphatidylcholine bilayers, D is about 3 × 10-8 cm2 sec-1 at 20°C and the Arrhenius activation energy for diffusion is 8.1 kcal mol-1 between 15 and 35°C in this system. In bilayers prepared from an equimolar mixture of egg phosphatidylcholine and cholesterol D at 20°C is about 1.4 × 10-9 cm2 sec-1 and the Arrhenius activation energy for the diffusion of the protein in this system between 15 and 35°C is 15.1 kcal mol-1. Light-scattering and fluorescence-polarization results indicate that binding of this protein does not affect the gel-to-liquid crystalline phase transition of bilayer membranes but does mediate a major, reversible aggregation of the vesicles at about 33°C. These results lend support to the view that ApoC-III resides in the head-group region of the bilayer and suggest that its lateral diffusion coefficient represents an upper bound for integral membrane proteins.
Keywords: membrane lateral diffusion, fluorescence recovery after photobleaching
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P. M., Podleski T. R. Control of acetylcholine receptor mobility and distribution in cultured muscle membranes. A fluorescence study. Biochim Biophys Acta. 1978 Jul 20;511(1):23–38. doi: 10.1016/0005-2736(78)90062-7. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Wight A., Webb W., Horwitz A. Influence of membrane lipids on acetylcholine receptor and lipid probe diffusion in cultured myotube membrane. Biochemistry. 1978 Aug 22;17(17):3604–3609. doi: 10.1021/bi00610a029. [DOI] [PubMed] [Google Scholar]
- Brown W. V., Levy R. I., Fredrickson D. S. Studies of the proteins in human plasma very low density lipoproteins. J Biol Chem. 1969 Oct 25;244(20):5687–5694. [PubMed] [Google Scholar]
- Cadenhead D. A., Kellner B. M., Jacobson K., Papahadjopoulos D. Fluorescent probes in model membranes I: anthroyl fatty acid derivatives in monolayers and liposomes of dipalmitoylphosphatidylcholine. Biochemistry. 1977 Nov 29;16(24):5386–5392. doi: 10.1021/bi00643a034. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Müller U. Temperature-dependent aggregation of bacteriorhodopsin in dipalmitoyl- and dimyristoylphosphatidylcholine vesicles. J Mol Biol. 1978 May 15;121(2):283–298. doi: 10.1016/s0022-2836(78)80010-2. [DOI] [PubMed] [Google Scholar]
- Cubero Robles E., van den Berg D. Synthesis of lecithins by acylation of O-(sn-glycero-3-phosphoryl) choline with fatty acid anhydrides. Biochim Biophys Acta. 1969 Dec 17;187(4):520–526. doi: 10.1016/0005-2760(69)90049-6. [DOI] [PubMed] [Google Scholar]
- Day E. P., Ho J. T., Kunze R. K., Jr, Sun S. T. Dynamic light scattering study of calcium-induced fusion in phospholipid vesicles. Biochim Biophys Acta. 1977 Nov 1;470(3):503–508. doi: 10.1016/0005-2736(77)90142-0. [DOI] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Webb W. W. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry. 1978 Jul 25;17(15):3046–3053. doi: 10.1021/bi00608a016. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- Morrisett J. D., David J. S., Pownall H. J., Gotto A. M., Jr Interaction of an apolipoprotein (apoLP-alanine) with phosphatidylcholine. Biochemistry. 1973 Mar 27;12(7):1290–1299. doi: 10.1021/bi00731a008. [DOI] [PubMed] [Google Scholar]
- Morrisett J. D., Jackson R. L., Gotto A. M., Jr Lipid-protein interactions in the plasma lipoproteins. Biochim Biophys Acta. 1977 Aug 9;472(2):93–133. doi: 10.1016/0304-4157(77)90015-6. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Miller N. Phospholipid model membranes. I. Structural characteristics of hydrated liquid crystals. Biochim Biophys Acta. 1967 Sep 9;135(4):624–638. doi: 10.1016/0005-2736(67)90094-6. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Axelrod D., Koppel D. E., Webb W. W., Elson E. L. Lateral transport of a lipid probe and labeled proteins on a cell membrane. Science. 1977 Jan 21;195(4275):307–309. doi: 10.1126/science.556653. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974 Jan 15;38(3):247–258. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Smith B. A., McConnell H. M. Lateral diffusion of M-13 coat protein in model membranes. Biochemistry. 1979 May 29;18(11):2256–2259. doi: 10.1021/bi00578a019. [DOI] [PubMed] [Google Scholar]
- Träuble H., Middelhoff G., Brown V. W. Interaction of a serum apo-lipoprotein with ordered and fluid lipid bilayers. Correlation between lipid and protein structure. FEBS Lett. 1974 Dec 15;49(2):269–275. doi: 10.1016/0014-5793(74)80528-4. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Austin R. H., Vogel H. The rotational diffusion of cytochrome b5 in lipid bilayer membranes. Influence of the lipid physical state. Biophys J. 1979 Jun;26(3):415–426. doi: 10.1016/S0006-3495(79)85262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. E., Schlessinger J., Elson E. L., Webb W. W., Blumenthal R., Henkart P. Diffusion and patching of macromolecules on planar lipid bilayer membranes. Biochemistry. 1977 Jul 26;16(15):3476–3483. doi: 10.1021/bi00634a029. [DOI] [PubMed] [Google Scholar]
- Wu E. S., Jacobson K., Szoka F., Portis A., Jr Lateral diffusion of a hydrophobic peptide, N-4-nitrobenz-2-oxa-1,3-diazole gramicidin S, in phospholipid multibilayers. Biochemistry. 1978 Dec 12;17(25):5543–5550. doi: 10.1021/bi00618a033. [DOI] [PubMed] [Google Scholar]
- Zagyansky Y., Edidin M. Lateral diffusion of concanavalin A receptors in the plasma membrane of mouse fibroblasts. Biochim Biophys Acta. 1976 Apr 16;433(1):209–214. doi: 10.1016/0005-2736(76)90188-7. [DOI] [PubMed] [Google Scholar]


