Abstract
In the past century, physiological, molecular, and cellular-based studies have proved that the functions of the nervous system, endocrine system, and immune system are dependent upon each other and that this interaction among these systems determines the maintenance of health or susceptibility to infections. The release of neurotransmitters and neuropeptides from the brain is a response to external environmental stimuli that influences the release of hormones from the pituitary in order to regulate the functions such as metabolism and growth, reproduction, etc. In addition, there are direct sympathetic noradrenergic and peptidergic innervations of primary (bone marrow and thymus) and secondary (spleen, lymph nodes, and lymphoid tissues) lymphoid organs. The neurotransmitters and neuropeptides released in these lymphoid organs then bind to specific receptors on the cells of the immune system to modulate their functions. Another circuit in this bidirectional communication involves the products of the immune system, for e.g., cytokines that can cross the blood-brain barrier to alter the activities of the neuronal function in the central nervous system especially during fever and inflammation in infectious diseases and cancer. Dysregulation of the interactions between the neuroendocrine and immune system due to alterations in the neural activity, secretion of hormones and cytokines, and synthesis of growth factors has been demonstrated to promote the pathogenesis and progression of infectious and autoimmune diseases, cancer, and neurodegenerative diseases. It is imperative that further research is carried out to understand the mechanisms of neuroendocrine-immune interactions to facilitate development of better treatment strategies for neurodegenerative diseases.
Keywords: Neurotransmitter, Hormone, Cytokine, Brain, Lymphoid organs
Introduction
The influence of “mind” over “body” in maintaining health, susceptibility to diseases, and ability to recover from illnesses has been acknowledged in the field of medicine. The current system has focused on the reductionist approach while Eastern medicine (Ayurveda, Traditional Chinese Medicine) have attempted to understand disease pathogenesis and treat individuals as a whole including the state of mind. However, recent therapeutic strategies in modern medicine have examined the role of psychological, emotional, and spiritual factors in controlling health and disease progression to provide ‘integrated care.’
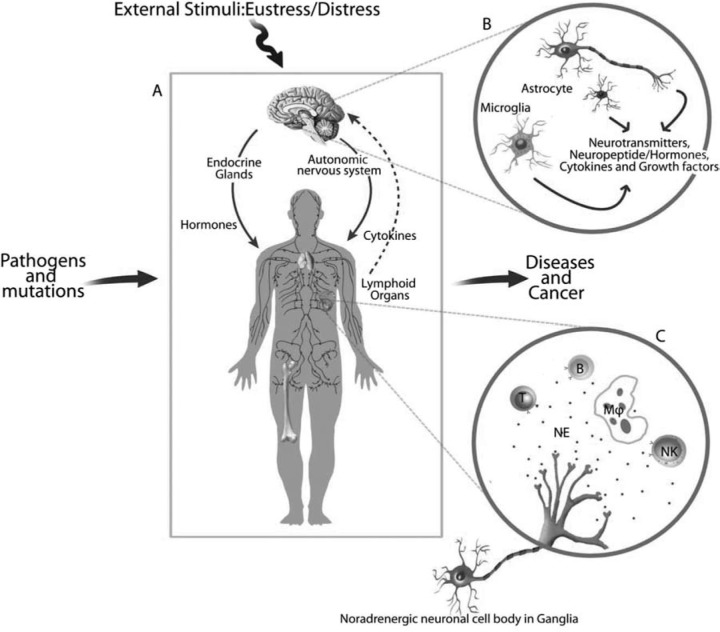
Recent studies have investigated the mechanisms through which the products of the immune system, cytokines, influence the functions of the nervous system within the brain (Figure 1). Reports on neuronal innervation of the organs of the immune system especially, sympathetic noradrenergic and peptidergic neuronal fibers that innervate the lymphoid organs, bone marrow, thymus, spleen, and lymph nodes demonstrate the network between nervous system and immune system. In these organs, neurotransmitters and neuropeptides released from the nerve endings of these neurons alter the functions of the immune system as the lymphoid cells have receptors for hormones and neurochemicals. Behavioral and pharmacological manipulation of the neuroendocrine-immune signaling has been known to alter immunity, autoimmunity, and immunosenescence. This altered neural-immune signaling may determine the course of infectious diseases and also, response of the individuals to therapeutic strategies.
Fig. 1:
Bidirectional communication between the neuroendocrine system and immune system.
- The bidirectional interaction is mainly through neurotransmitter-, hormonal-, and cytokine-specific pathways. The CNS influences the immune system via neuroendocrine outflow, and autonomic and sensory nerves that innervate lymphoid tissue. Peripherally, cytokines produced by the immune cells under the influence of neuronal activity cross the blood-brain barrier to influence CNS functions. There is a great complexity in neural-immune interactions.
- Neurotransmitter-specific and neuropeptidergic nerves are distributed in the rostral sections of the brain arising from the cell bodies located in the caudal portions of the brain. The neuroglial cells, astrocytes and microglia, regulate the neuronal survival through the release of cytokines and growth factors while the oligodendrocytes are critical to myelin formation. The neuroendocrine systems in the hypothalamus control metabolism and growth by influencing the release of hormones from the pituitary.
- Noradrenergic nerves originate from ganglia and are distributed to specific lymphoid compartments in lymphoid organs and the effects are mediated through the receptors for neurotransmitters on the cells of the immune system. The presence of β2-adrenergic receptors (AR) on the subpopulations of lymphoid cells facilitates the binding of norepinephrine (NE) to alter the release of cytokines, growth factors, and immune molecules that cross the blood-brain barrier to alter brain functions.
In this review, we describe how both the peripheral nervous system (PNS) and the central nervous system (CNS) function in response to products of immune system, and how neurotransmitter-specific innervation of lymphoid tissue impacts immune processes. We also describe neural-immune signaling under normal conditions and in disease. Immune system along with the neuroendocrine system is responsible for the maintenance of homeostasis that is critical to the health of an individual. Many argue that diseases progress due to the inability of the immune system to mount a proper response to pathogens is partly due to deficits in neural signaling at the site of antigen processing leading to breakdown of homeostasis.
Neuroendocrine-Immune interactions in the Brain
Evidence gathered over the past 40 years of research has shown that activation of the immune system is accompanied by alterations in the hypothalamic and limbic areas as well as endocrine functions, indicating that the CNS is responsive to the products of the immune system.1 Early evidence demonstrated that the administration of antigen increased neuronal firing rates in the hypothalamic ventromedial nucleus and sympathetic noradrenergic activity in the spleen, and elevated the levels of adrenocorticotropic hormone (ACTH) and corticosterone.1 Immune signaling of the CNS is achieved through release of peripheral soluble factors like cytokines, by the cells of the immune system and of non-immune origin especially the glial cells, astrocytes.2 Cytokines are low molecular weight proteins that not only facilitate communication between the lymphoid cells but also between the cells of the central nervous and endocrine systems. A number of cytokines, like IL-1, IL-2, IL-3, IL-6, IL-8, IL-12, IFN-γ and TNF-α, are reported to be expressed in the brain. Also, the peripheral administration of lipopolysaccharides (LPS) promotes synthesis of cytokines in the brain. Peripheral immunocytes and phagocytes are the major sources of cytokines and they gain entry into the brain either through poorly developed blood-brain barrier or by active transport.3
Of the various cytokines, IL-1 has been studied extensively as a possible immunotransmitter that communicates with the CNS in infectious diseases. It serves as an endogenous pyrogen and can directly stimulate the release of corticotrophin releasing hormone (CRH) from the hypothalamic paraventricular nucleus (PVN) directly by acting on PVN neurons, or indirectly by enhancing NE and dopamine release.1,4,5 Demonstration of the presence of IL-1 receptors in the hypothalamus and other regions of the brain such as the hippocampus, dorsal raphe, and pituitary provides evidence for cytokine’s actions in the brain.6
In addition to IL-1, other proinflammatory cytokines such as IL-6 and TNF-α are also expressed in the brain and pituitary where they influence neural function by modulating each other’s release.7 Effects of peripheral cytokines on CNS functions are also facilitated by binding to vascular endothelium in the brain which stimulates release of central neurochemicals. In addition, cytokines modulate peripheral afferent neurons to alter brain functions especially during ‘sickness behavior’.8 The vagus nerve is another important pathway that serves as a conduit for the cytokines produced in the periphery or cytokinemediated signals that reach the CNS areas such as nucleus of the solitary tract.9 Other pathways and mediators for transmission of peripheral immune signals to the CNS are affected by conditioning of the immune system.10
Cytokines and their related members, such as chemokines, influence the functions of CNS through a number of ways including trafficking and recruitment of leukocytes into the brain across the blood-brain barrier.11 These molecules are involved in defence against the spread of infection inside the brain and arresting the inflammatory processes in neurodegenerative diseases.12 Such defensive mechanisms include the induction of selectins, important cell adhesion molecules in inflammatory process, and chemokines which then may attract leukocytes to the site of inflammation. Infusion of IL-1 elevates the expression of P-selectin on brain endothelial cells and also, leads to disruption of cadherin/ catenin complex, tight junctions of vascular endothelial cells that facilitate the entry of leukocytes.13,14 Subsequently, the interaction between the endothelial cells and microglia results in increased stimulation of endothelial cells in a positive feedback manner and thus, promotes increased accessibility of leukocytes to the site of inflammation in the brain.
Cytokines regulate important properties of neurons such as apoptosis and survival in addition to stimulating neuronal cells to release neuropeptides and neurotransmitters. In acute (stroke and head injury) and chronic (multiple sclerosis and Alzheimer’s disease) diseases of the brain, cytokines produced by infiltrating macrophages result in inflammatory processes in the brain. A delicate balance between the proinflammatory and anti-inflammatory cytokines determine whether their effects will be neurotoxic or neuroprotective. The process of neurodegeneration is initiated when there is a shift to more proinflammatory cytokine production such as IL-1 and TNF-α while neuroprotective effects are exerted by anti-inflammatory cytokines through the inhibition of inflammatory responses. The cross-regulation between these two classes of cytokines is mediated through indirect inhibition of their synthesis. In addition to this mechanism, their actions may also be determined by receptor-receptor and cell-cell interactions. Following injury, microglial cells are the primary source of cytokines such as IL-1 and TNF-a but other glial cells, astrocytes and oligodendrocytes, also produce these cytokines and other mediators and thus, ultimately decide the course of neurotoxic or neuroprotective actions.1,2,8,9,11,12,15
In addition to its role in inflammation, injury, and the response to bacterial infections, the immune to brain communication route can also drive sickness behaviors and pain responses.8,16 From a plausible protective effect in acute cases to prevent disease progression, cytokines may also promote disease process in chronic disease states. Hopefully, improved understanding of the inflammatory processes in this neural-immune cross-talk, will lead to more effective therapies.
Neural-Immune interactions in the periphery
The vital organs of the immune system, primary and secondary lymphoid organs, are innervated by postganglionic sympathetic noradrenergic (NA) nerve fibers that are in close proximity to the cells of the immune system, lymphocytes and macrophages.17–20 In addition to sympathetic nerve fibers, there are several peptidergic (neuropeptide-Y, substance-P, vasoactive intestinal peptide, calcitonin gene-related peptide, opioid peptides, corticotrophin-releasing hormone) nerve fibers that also innervate the lymphoid organs. The neurochemicals that are released from these nerve fibers diffuse to distant areas in the lymphoid organs and bind to respective receptors on the lymphoid cells to modulate immune responses. The most widely studied neuronal innervation of lymphoid organs are the NA nerve fibers in the bone marrow, thymus, spleen, lymph nodes and other lymphoid tissues and the effect of their neurotransmitter, norepinephrine (NE), on the cells of the immune system. Initially, NA sympathetic innervation of lymphoid organs was considered to mediate its effects on vascular functions such as regulation of blood flow and to contract capsular and trabecular smooth muscles. Subsequent studies have provided evidence for the development-, age, and disease-related changes in the immune system by NA nerve fibers in the lymphoid organs. 17–21
Noradrenergic innervation of lymphoid organs
Bone Marrow: Histological studies have revealed nerve bundles of myelinated and non-myelinated nerve fibers first supply periosteum before entering the interior structures through nutrient foramina. In young rats, NA sympathetic nerve fibers travel with the appropriate spinal nerve before entering the bone through nutrient foramina.22 These fibers form dense plexuses from which numerous nerve fibers branch out into the surrounding parenchyma among hemopoietic cells in the marrow and modulate the functions of granulocyte-macrophage progenitor cells and primitive progenitor cells by binding to the adrenergic receptors on these cells.23 The functional significance of sympathetic nerve fibers in the bone marrow is yet to be defined. Multiple functions have been assigned to them such as regulating blood flow and volume within the bone marrow and serving as a source of growth factors and adhesion molecules because of close apposition between nerve terminals and stromal cells.24
Thymus: Thymus is innervated by NA nerve fibers originating from postganglionic cell bodies in the upper paravertebral ganglia of the sympathetic chain, primarily the superior cervical and stellate ganglia.25 Similar to the bone marrow, NA nerve fibers enter the thymus adjacent to large blood vessels and course into the cortical regions in close proximity with the thymoctyes. In the capsule and interlobular septa, NA nerve fibers course adjacent to mast cells, corticotrophin releasing hormone immunoreactive cells, and macrophages.18–19 In addition to these cells, NA nerve fibers are found in close apposition to thymic epithelial cells and possessing β1- and β2- adrenergic receptors. The functional significance of NA innervation of the thymus is not clear but evidence is available for its effect on maturation of thymocytes demonstrated by inhibition of proliferation and enhancement of differentiation of thymocytes in vitro through the activation of β-AR through cAMP release.18,26
Spleen: NA nerve fibers present in the spleen are derived from postganglionic cell bodies, mostly in the superior mesentericceliac ganglionic complex and to a lesser extent, the sympathetic trunk.17–21 These nerves enter the spleen as a dense plexus associated with the splenic artery and branch beneath the capsule of the spleen. After coursing along the trabeculae and its associated vasculature, NA plexuses follow the central arterioles and branch into the white pulp of the spleen. From the central arteriole, the NA nerves travel into the surrounding periarteriolar lymphatic sheath (PALS), a region that is rich in T lymphocytes of both the T-helper and T-cytotoxic/ suppressor subset. Sympathetic NA nerve fibers also course along the areas of the white pulp such as marginal sinus and marginal zone rich in macrophages and B lymphocytes. Very few nerve fibers are found in follicular areas of white pulp that are composed primarily of B lymphocytes. Electron microscopic studies have revealed that these NA nerve terminals also are found in direct contact with the lymphocytes in PALS separated by as little as 6 nm.17–21 Similar close appositions are present at the marginal sinus and marginal zone between NA nerve terminals and lymphocytes and macrophages.
Lymph Nodes: The origin of sympathetic NA enervation of lymph nodes is uncertain but believed to be regionally supplied because removal of superior cervical ganglia results in loss of NA nerve fibers in the cervical lymph nodes.27 Following entry into the lymph nodes along with the blood vessels, they travel in subcapsular plexus or continue in vascular plexuses and individual fibers in the medullary cords. After coursing adjacent to many lymphoid cell types in the medulla, NA nerve fibers continue along small vessels that course from the medulla into the paracortical regions that are rich in T lymphocytes. Linear fibers that enter into T lymphocyte compartments of the cortex and paracortex do not travel into the adjacent nodular regions and germinal centers where B lymphocytes dominate.17–18 The distribution of NA nerves in lymph node has many similarities with NA innervation in the spleen, indicating a common functional role for such innervation in secondary lymphoid organs. A major role of antigen processing is ascribed to NE as reduced primary antibody responses in spleen and lymph nodes following sympathectomy. Also, infusion of catecholamines leads to efflux of activated lymphocytes into the circulation from spleen and lymph nodes.17–21
Although NA innervation is widely investigated, its functional significance in neuroimmunomodulation is variable and multifaceted depending on the type of lymphoid organs, cell types, age, and the presence of other neurochemicals such as peptides, cytokines, and hormones.
Innervation of lymphoid organs with other Neurotransmitter and Neuropeptides
Cholinergic innervation of bone marrow is limited to passage of nerve fibers through the area without any neuroeffector junctions with hemopoietic cells while its presence in the thymus is minimal compared to that of NA innervation. Majority of splenic nerves that stain for acetylcholinesterase (AChE), a marker for cholinergic innervation, are non-neuronal in nature that may be derived from sympathetic NA nerves as well as non-NA and non-vagal nerves. There is no conclusive evidence for the localization of cholinergic nerve fibers in the lymph nodes although there are non-NA nerve fibers in the lymph nodes as observed with electron microscopy.17–21
Bone marrow is innervated by a number of neuropeptides including neuropeptides-Y (NPY), Substance-P (SP), calcitonin gene-related peptide (CGRP), and vasoactive intestinal peptide (VIP). NPY has been found to colocalize with NA nerve fibers and its concentration is also higher than the other neuropeptides. A dense innervation of NPY is found in the corticomedullary junction in the thymus that is associated closely with macrophages and mast cells. Similar to the primary lymphoid organs, NPY nerve fibers colocalize with the NA nerve fibers and are in close contact with the lymphocytes in the spleen while they extend into the various compartments of the lymph nodes forming varicose profiles among macrophages. SP and CGRP nerve fibers are present in the thymus among mast cells and are believed to play a sensory function. Spleen is densely innervated by CGRP nerve fibers than SP fibers but their roles are unclear. Lymph nodes from a number of mammalian species have been reported to possess SP and CGRP nerve fibers that travel as individual fibers in the parenchyma of the cortex and medulla among lymphocytes.17–21
Physiological significance of sympathetic innervation of Spleen and Lymph nodes
The cells of the immune system residing in secondary lymphoid organs are responsible for trapping antigens and induction of immune responses including cytokine production. Foreign substances are engulfed by the macrophages in the splenic white pulp in order for them to be phagocytozed and eliminated as metabolites. Similarly, lymph nodes filter regionally draining lymph with the sole purpose of removing pathogens and foreign materials. The elimination of foreign substances is achieved by activating the immune responses by mobilizing lymphoid cells and their mediators to sites of injury or infection. Subsequently, clonally expanded sets of lymphocytes enter the blood stream from the spleen and lymph nodes. Sympathetic signaling within lymphoid compartments modulates immune responses to antigens by affecting clonal expansion, cytokine production, and/or responsiveness of target cells by altering receptor expression.
Numerous studies have demonstrated that the adrenergic agonists act on lymphoid cells to influence induction, proliferation, and effector phases of the immune responses in vitro. T and B lymphocytes express β2-adrenoceptors (AR) while NK cells express β2-AR and α1- and α2-AR and macrophages have α2-and β2-AR.18 Stimulation of β- or β2- AR resulted in the inhibition of T cell proliferation may be mediated, in part, by its ability to inhibit IL-2 R expression and IL-2 production by activated T cells.28–30 In addition to their effects on IL-2, stimulation of β2- AR modulates IFN-γ production. Prior treatment of Th1 cells with terbutaline or agents that stimulate cAMP production before antigen challenge inhibits IFN-γ synthesis.30 Studies have demonstrated a dichotomous nature of β2-AR effects on Th1 and Th2 cells such that their activation modulates Th1 differentiation and cytokine production but does not alter Th2 effector cells.
In vivo studies have demonstrated that β-AR stimulation modulates Th1 effector cell-driven responses, including delayed-type hypersensitivity. Chemical sympathectomy-induced depletion of NE prior to hapten sensitization reduces hapten-specific cytotoxic T lymphocyte production indicating the importance of NE in mediating the Th1 cell-mediated immune responses.20 Enhancement or suppression of lipopolysaccharide (LPS)-induced B cell proliferation and antibody production has been observed following β-AR stimulation.31 This dual effect may have been due to differences in signaling pathways in B cells induced by LPS and that these pathways are modulated differently by cAMP. Depending on the presence of Th dominance (C57Bl/6 mice with a predominant Th1 antibody profile while BALB/c mice has a preference towards Th2 profile), NE depletion has been shown to differentially regulate Th1 and Th2-dependent antibody production indicating that intact NA innervation in the spleen inhibits the immune response to an antigen.32 The number of IgM-secreting cells increases following NE treatment of murine spleens cells cultured with Th cell-dependent antigen, an effect blocked by the addition of β-AR antagonist indicating that the augmentation of IgM production by NE is mediated through β-AR stimulation.28–30 These actions of β-AR on immune cells are dependent upon the timing of β-AR stimulation along with timing of antigen challenge.
Production of hormones by Lymphoid cells
The intricate nature of neuroendocrine-immune interactions is further compounded by the production of hormones by the cells of the immune system. A number of hormones (prolactin, growth hormone, luteinizing hormone, adrenocorticotropic hormone, corticotrophin releasing hormone), neuropeptides (enkephalins, endorphins) and neurotransmitters (NE, epinephrine) are released by the immunocytes to regulate the immune reactivity in the local milieu of the lymphoid organs.33 Evidence exists for the production of opioid peptides, NE, and epinephrine by the lymphocytes and macrophages.34 Human lymphocytes release growth hormone and peripheral blood monocytes secrete growth factors such as brain-derived neurotrophic factor that are up-regulated by proinflammatory cytokines, TNF and IL-6.35 These factors may regulate the functions of cells of the immune system locally in an autocrine and paracrine manner determining proliferation, production of immune mediators, trafficking of immunocytes, etc.
Relevance of Neuroendocrine-Immune interactions in aging, diseases, and Cancer
Several lines of evidence exist for the influence of the neuroendocrine system and immune system on aging, and the pathogenesis of infectious diseases, autoimmune diseases, neurodegenerative diseases, and cancer.
Age-associated decline in neuroendocrine-immune system has been well-documented in rodents and humans predisposing them to the development of infectious diseases, autoimmune diseases, and cancer.19,21 Our laboratory has preliminary evidence that estrogen may be responsible for the rapid decline in sympathetic innervation of secondary lymphoid organs in female rats that may be mediated through alterations in antioxidant enzyme activities and growth factor biosynthesis (unpublished data).
In HIV/AIDS infection, sympathetic innervation of lymphoid organs is relevant as these are the primary sites of HIV-1 pathogenesis. HIV-1 disease is marked by a shift to Th2 profile with reduced IL-12 and increased IL-10 production that promoted the viral replication by up to 11-fold in human peripheral blood mononuclear cell culture.36 The mechanism of viral replication is mediated via β-AR-cAMP-protein kinase A signaling cascade. A similar mechanism is utilized by a synthetic, immunosuppressive retroviral envelope peptide that induces cAMP production to shift the cytokine balance towards Th2 profile.37 Infection of mouse with a mixture of murine retroviruses, LPBM5, depletes splenic NE concentration due to the destruction of sympathetic innervation within 2 weeks.38,39 In this mouse model of acquired immunodeficiency syndrome (MAIDS), administration of NE uptake blocker, desipramine, does not prevent LP-BM5-induced NE depletion indicating that loss of splenic NA nerves is not mediated by NE. Enhanced pain sensation is associated with most of the diseases including HIV/AIDS and cancer and a strong therapeutic tool to deal with this persistent problem has remained elusive. Intrathecal infusion of HIV1 envelope glycoprotein, gp120, into the rat meninges increased the production of proinflammatory cytokines, TNF-α, IL-1β, and IL-6, that may serve as a source of heightened pain states.40 Neuronal and glial functions are significantly affected by HIV1 in the CNS leading to several pathological and cognitive disturbances such as neurodegeneration and HIV1-associated dementia (HAD) for which no known therapy is available.41 A number of strategies to counter this neurodegenerative process in HIV/AIDS have been tried including the use of deprenyl, a monoamine oxidase inhibitor, which has immunomodulatory and neuroregenerative properties.21 However, deprenyl’s therapeutic value in the treatment of HAD has been inconclusive that may be dependent on the dosage, route and duration of administration, and stage of disease progression in patients chosen for the study.42,43
Inflammation is a common feature in the central neurodegenerative diseases such as multiple sclerosis (MS), Alzheimer’s disease (AD), and amyotrophic lateral sclerosis (ALS). Bone marrow-derived microglia infiltration into the brain is markedly enhanced around amyloid plaques so that these cells can effectively remove the amyloid beta (Aβ) from the brain. Enhancing the levels of macrophage-colony stimulating factor (M-CSF) has been demonstrated to reverse the cognitive decline in a mouse model of AD.12 Additional factors such as brain-derived neurotrophic factor (BDNF) and estrogen influence the progression of AD. Hormone replacement therapy with estrogen has been demonstrated to reduce cognitive decline in aged women and BDNF protein expression is reduced in cortical and hippocampal areas of the AD patients.44 Basic research has revealed that estrogen enhances BDNF expression in various areas of the brain through specific neurotrophin receptor complex suggesting that the interactions between estrogen, BDNF, and immune cells and molecules can be used to develop strategies for neuroprotection in AD.
The etiology and pathogenesis of cancer is complex and is the result of combination of genetic predisposition, diet, environmental factors, and exercise status, to mention a few. These factors through a combination of physical, biochemical, and genetic damage to the involved cells, influence the process of carcinogenesis. A variety of psychological and environmental factors influence the ability of the immune system to react to various stimuli, and the magnitude of these response assist in determining the extent of carcinogenesis. Both specific and nonspecific effectors of immune responses are important in cytostatic and/or cytocidal actions on tumors. Administration of deprenyl, a monoamine oxidase inhibitor, to rats with carcinogen-induced and spontaneously developing mammary tumors suppressed tumor growth, enhanced sympathetic noradrenergic (NA) activity and cell-mediated immune reactivity in the spleen, reduced prolactin secretion indicating that restoration of sympathetic NA activity in tumor-bearing rats would reverse immunosuppression commonly observed in cancer.21 In contrast, growth of mammary tumor in sympathetically denervated ear was attenuated in comparison to sympathetically intact ear on the contralateral side and chemical sympathectomy before injection of alveolar carcinoma cells increased pulmonary metastases.45,46 Preliminary data from our laboratory has shown that terbutaline (selective β2-AR agonist) induces cellular proliferation of estrogen receptor-positive T47D breast cancer cells which can be inhibited by β-AR blocker, propranolol, suggesting the modulatory role of NE in breast cancer. Ben-Eliyahu and his coworkers have demonstrated that sympathetic ganglionic blockade, adrenal demedullation, or administration of a non-selective β-AR blocker either meliorated or attenuated swim stress-induced increases in MADB106 tumor metastases compared with non-stressed animals.47 Similarly, adrenal medullation and β-AR blocker prevented the tumor-promoting effects of social confrontation and hypothermic stress suggesting that adrenal catecholamines promote stress-induced metastases through β-ARmediated mechanism.
Brain is a key mediator of reaction to and recovery from stress through a network of neural, endocrine, immune, and behavioral systems.48 Chronic stress with increased allostatic load interferes with this network altering the coping strategies to stress and thus, compromising health status of an individual. This becomes more severe with advancing age especially in people with dementia, caregivers of patients with dementia and infectious diseases, stressful situations such as divorce, loss of spouse, etc.49 Psychosocial stress observed in people with depression and taking care of dementia patients resulted in enhanced production of proinflammatory cytokine, IL-6 and another proinflammatory marker, C-reactive protein. On the contrary, social integration with friends, family, and society reduced the levels of these proinflammatory markers suggesting that managing psychosocial stress can be tool to regulate inflammatory process that is common to infectious diseases, neurodegenerative diseases, and cancer49.
Summary
Neuroendocrine-immune interactions are dynamic processes that require a composite investigation of neurotransmitter/ neuropeptides, hormonal, and cytokine/ immune molecules effects on a number of target cells. The anatomical associations between nerves and immunocytes in the lymphoid tissue provide the basis for direct neural modulation of the immune system. The presence of neurotransmitter-defined receptors on cells of the immune system and functional alterations following receptor activation support this route of neural-immune communication. Conversely, the products of immune system cross the brain and influence the functions of the neurons that form the basis for behavioral changes and maintenance of homeostasis in response to infections. Although the distribution of sympathetic noradrenergic nerves in the lymphoid organs and its role in immune reactivity is well described, the mechanism(s) of action(s) are not clear yet. Published and preliminary results from our laboratory suggest that several compensatory factors such as antioxidant enzymes and growth factors influence the neuroendocrine-immune interactions in female reproductive aging and mammary tumorigenesis. Further research is essential to understand the role of bidirectional communication between the neuroendocrine and immune systems to develop effective therapeutic strategies against various diseases including infectious and autoimmune diseases, neurodegenerative diseases, and cancer.
Acknowledgements
Partial support from the Department of Science & Technology (F. NO. SR/SO/ HS-46/2007) and Department of Biotechnology (BT/PR9199/Med/30/12/2007), New Delhi
Footnotes
The article complies with International Committee of Medical Journal Editor’s uniform requirements for the manuscripts.
Competing interests – None
Source of Funding – DST and DBT
References
- 1.Besedovsky HO, del Rey A. Physiological implications of the immuno-neuro-endocrine network. Psychoneuroimmunology. In: Ader R, Cohen N, Felten DL, editors. 2nd ed. New York: Academic Press; 1991. pp. 589–608. [Google Scholar]
- 2.Dinarello CA. IL-1: discoveries, controversies and future directions. Eur J Immunol. 2010;40(3):599–606. doi: 10.1002/eji.201040319. [DOI] [PubMed] [Google Scholar]
- 3.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2(4):241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 4.Dunn A. Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism paralleling the increased plasma corticosterone. Life Science. 1988;43:429–435. doi: 10.1016/0024-3205(88)90522-x. [DOI] [PubMed] [Google Scholar]
- 5.Mohan Kumar PS, ThyagaRajan S, Quadri SK. Interleukin-1 stimulates the release of dopamine and dihydroxyphenylacetic acid from the hypothalamus in vivo. Life Science. 1991;48:925–930. doi: 10.1016/0024-3205(91)90040-i. [DOI] [PubMed] [Google Scholar]
- 6.Breder CD, Dinarello CA, Saper CB. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988;240(4850):321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- 7.Spangelo BL, Gorospe WC. Role of the cytokines in the neuroendocrine-immune system axis. Front Neuroendocrinol. 1995;16(1):1–22. doi: 10.1006/frne.1995.1001. [DOI] [PubMed] [Google Scholar]
- 8.Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29(2):247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thayer JF, Sternberg EM. Neural aspects of immunomodulation: focus on the vagus nerve. Brain Behav Immun. 2010;24(8):1223–1228. doi: 10.1016/j.bbi.2010.07.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ader R. Conditioned immunomodulation: Research needs and directions. Brain Behav Immun. 2003;17:S51–S57. doi: 10.1016/s0889-1591(02)00067-3. [DOI] [PubMed] [Google Scholar]
- 11.Quan N, Herkenham M. Connecting cytokines and brain: a review of current issues. Histol Histopathol. 2002;17(1):273–288. doi: 10.14670/HH-17.273. [DOI] [PubMed] [Google Scholar]
- 12.Yong VW, Rivest S. Taking advantage of the systemic immune system to cure brain diseases. Neuron. 2009;64(1):55–60. doi: 10.1016/j.neuron.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Bernardes-Silva M, Anthony DC, Issekutz AC et al. Recruitment of neutrophils across the blood-brain barrier: the role of E- and P-selectins. J Cereb Blood Flow Metab. 2001;21(9):1115–1124. doi: 10.1097/00004647-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Del Maschio A, Zanetti A, Corada M et al. Polymorphonuclear leukocyte adhesion triggers the disorganization of endothelial cell-to-cell adherens junctions. J Cell Biol. 1996;135(2):497–510. doi: 10.1083/jcb.135.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denes A, Thornton P, Rothwell NJ et al. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun. 2010;24(5):708–723. doi: 10.1016/j.bbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felten DL, Felten SY, Carlson SL et al. Noradrenergic and peptidergic innervation of lymphoid tissue. Journal of Immunology. 1985;135:755s–765s. [PubMed] [Google Scholar]
- 18.Bellinger DL, Lorton D, Lubahn C . Innervation of lymphoid organs—Association of nerves with cells of the immune system and their implications in disease. Psychoneuroimmunology. In: Ader R, Felten DL, Cohen N, editors. Vol. 1. San Diego: Academic Press; 2001. pp. 5–111. [Google Scholar]
- 19.Bellinger DL, Madden KS, Lorton D . Age-related alterations in neural-immune interactions and neural strategies in immunosenescence. Psychoneuroimmunology. In: Ader R, Felten DL, Cohen N, editors. Vol. 1. Vol. 2001. San Diego: Academic Press; pp. 241–288. [Google Scholar]
- 20.Madden KS. Catecholamines, sympathetic innervation, and immunity. Brain Behav Immun. 2003;17(Suppl 1):S5–S10. doi: 10.1016/s0889-1591(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 21.Thyagarajan S, Felten DL. Modulation of neuroendocrine–immune signaling by Ldeprenyl and L-desmethyldeprenyl in aging and mammary cancer. Mechanisms of Ageing and Development. 2002;123:1065–1079. doi: 10.1016/s0047-6374(01)00390-6. [DOI] [PubMed] [Google Scholar]
- 22.Tabarowski Z, Gibson-Berry K, Felten SY. Noradrenergic and peptidergic innervation of the mouse femur bone marrow, Acta Histochemica. 1996;98:453–457. doi: 10.1016/S0065-1281(96)80013-4. [DOI] [PubMed] [Google Scholar]
- 23.Maestroni GJ, Conti A. Modulation of hematopoiesis via alpha 1-adrenergic receptors on bone marrow cells, Experimental Hematology. 1994;22:313–320. [PubMed] [Google Scholar]
- 24.Yamazaki K, Allen TD. Ultrastructural morphometric study of efferent nerve terminals on murine bone marrow stromal cells, and the recognition of a novel anatomical unit: the ‘‘neuro-reticular complex.”. American Journal of Anatomy. 1990;187:261–276. doi: 10.1002/aja.1001870306. [DOI] [PubMed] [Google Scholar]
- 25.Nance DM, Hopkins DA, Bieger D. Re-investigation of the innervation of the thymus gland in mice and rats. Brain Behav Immun. 1987;1:134–147. doi: 10.1016/0889-1591(87)90016-x. [DOI] [PubMed] [Google Scholar]
- 26.Singh U, Owen JJT. Studies on the maturation of thymus stem cells. The effects of catecholamines, histamine, and peptide hormones on the expression of T allo-antigens. European Journal of Immunology. 1976;6:59–62. doi: 10.1002/eji.1830060113. [DOI] [PubMed] [Google Scholar]
- 27.Giron LTJ, Crutcher KA, Davis JN. Lymph nodes-a possible site for sympathetic neuronal regulation of immune responses. Annals of Neurology. 1980;8:520–525. doi: 10.1002/ana.410080509. [DOI] [PubMed] [Google Scholar]
- 28.Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79(6):1093–1104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- 29.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav Immun. 2007;21(6):736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders VM. Epigenetic regulation of Th1 and Th2 cell development. Brain Behav Immun. 2006;20(4):317–324. doi: 10.1016/j.bbi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Kouassi E, Li YS, Boukhris W et al. Opposite effects of the catecholamines dopamine and norepinephrine on murine polyclonal B-cell activation. Immunopharmacology. 1988;16:125–137. doi: 10.1016/0162-3109(88)90001-x. [DOI] [PubMed] [Google Scholar]
- 32.Kruszewska B, Felten SY, Moynihan JA. Alterations in cytokine and antibody production following chemical sympathectomy in two strains of mice. Journal of Immunology. 1995;155:4613–4620. [PubMed] [Google Scholar]
- 33.Blalock JE. Shared ligands and receptors as a molecular mechanism for communication between the immune and neuroendocrine systems. Annals of New York Academcy of Sciences. 1994;741:292–298. doi: 10.1111/j.1749-6632.1994.tb23112.x. [DOI] [PubMed] [Google Scholar]
- 34.Engler KL, Rudd ML, Ryan JJ et al. Autocrine actions of macrophage-derived catecholamines on interleukin-1 beta. J Neuroimmunol. 2005;160(1-2):87–91. doi: 10.1016/j.jneuroim.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Schulte-Herbru¨ggen O, Nassenstein C, Lommatzsch M et al. Tumor necrosis factor-a and interleukin-6 regulates secretion of brain-derived neurotrophic factor in human monocytes. J Neuroimmunol. 2005;160:204–209. doi: 10.1016/j.jneuroim.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 36.Cole SW, Korin YD, Fahey JL et al. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J Neuroimmunol. 1998;161:610–616. [PubMed] [Google Scholar]
- 37.Haraguchi S, Good RA, James-Yarish M et al. Induction of intracellular cAMP by a synthetic retroviral circulation following envelope peptide: a possible mechanism of immunopathogenesis in retroviral infections, Proceedings of National Academy of Sciences USA. 1995;92:5568–5571. doi: 10.1073/pnas.92.12.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelley SP, Moynihan JA, Stevens SY et al. Sympathetic nerve destruction in spleen in murine AIDS. Brain Behav Immun. 2003;17(2):94–109. doi: 10.1016/s0889-1591(02)00101-0. [DOI] [PubMed] [Google Scholar]
- 39.Kelley SP, Moynihan JA, Stevens SY et al. Chemical sympathectomy has no effect on the severity of murine AIDS: murine AIDS alone depletes norepinephrine levels in infected spleen. Brain Behav Immun. 2002;16(2):118–139. doi: 10.1006/brbi.2001.0627. [DOI] [PubMed] [Google Scholar]
- 40.Wieseler-Frank J, Jekich BM, Mahoney JH et al. A novel immune-to-CNS communication pathway: cells of the meninges surrounding the spinal cord CSF space produce proinflammatory cytokines in response to an inflammatory stimulus. Brain Behav Immun. 2007;21(5):711–718. doi: 10.1016/j.bbi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- 42.Evans SR, Yeh TM, Sacktor N et al. Selegiline transdermal system (STS) for HIV-associated cognitive impairment: open-label report of ACTG 5090. HIV Clinical Trials. 2007;8:437–446. doi: 10.1310/hct0806-437. [DOI] [PubMed] [Google Scholar]
- 43.Schifitto G, Zhang J, Evans SR et al. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology. 2007;69:1314–1321. doi: 10.1212/01.wnl.0000268487.78753.0f. [DOI] [PubMed] [Google Scholar]
- 44.Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27(4):404–414. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romeo HE, Colombo LL, Esquifino AI et al. Slower growth of tumours in sympathetically denervated murine skin. J Auton Nerv Syst. 1991;32(2):159–164. doi: 10.1016/0165-1838(91)90066-c. [DOI] [PubMed] [Google Scholar]
- 46.Brenner GJ, Felten SY, Felten DL et al. Sympathetic nervous system modulation of tumor metastases and host defense mechanisms. J Neuroimmunol. 1992;37(3):191–201. doi: 10.1016/0165-5728(92)90003-4. [DOI] [PubMed] [Google Scholar]
- 47.Ben-Eliyahu S, Page GG, Schleifer SJ. Stress, NK cells, and cancer: Still a promissory note. Brain Behav Immun. 2007;21(7):881–887. doi: 10.1016/j.bbi.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 48.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neurosci Biobehav Rev. 2010;35(1):33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]