Abstract
Background
Convection enhanced delivery (CED) is an emerging form of direct brain infusion therapy employed in human functional and restorative neurosurgery clinical trials delivering protein, viral vectors for gene therapy, and siRNA.
Purpose
Pressure monitoring has become a vital tool in ensuring infusion safety and success. We report details of this benchmark first trial of the use of a leading syringe infusion pump system capable of low-flow infusions.
Methods
Low-flow infusion performance of the FDA approved Alaris® System syringe pump, commonly used at our institution, was assessed during in vitro and ex vivo CED infusions. In vitro infusion cloud morphology and line pressure were analyzed utilizing a neuroinfusion catheter and delivering volumes and flow rates proposed for a human gene therapy protocol for Parkinson’s disease.
Results
Pressure monitoring results correlated with previously published in-line pressure monitoring results however the time to peak with catheter occlusion was extended due to the method of pressure monitoring with this device.
Conclusion
MRI compatible infusion pumps used for brain delivery injectables, pressure monitoring is set to be a guiding instrument for the health care professional employing this emerging form of infusion-to-brain delivery. Further development of infusion pump technology is warranted to allow for infuse/withdraw mode, infusion pressure graphical and numerical display, and pressure monitoring without the need for an inflatable reservoir pressure device. MRI safe infusion systems will need to be available and nursing staff educated to prepare infusions within the high-field environment.
Keywords: Convection enhanced delivery (CED), Gene therapy, Low-Flow infusions, Infusion therapy, Syringe pump, Pressure monitoring, Real-time MRI monitoring, Parkinson’s disease, Functional and restorative neurosurgery, Neurocatheter infusions
Introduction
Convection-enhanced delivery (CED) involves the continuous injection of a fluid containing a therapeutic agent with constant positive pressure.1 CED has the ability to distribute small volumes to targeted regions quickly, delivering protein,2 siRNA,3 and viral vector based gene therapy.4–7 CED shows promise for the treatment of disease in functional and restorative neurosurgery, yet little has been published to evaluate clinically relevant infusion parameters associated with proposed delivery strategies. Techniques for infusion validation such as pressure monitoring are poised to become a vital tool in ensuring safety and success of infusion therapy.
The syringe pump requirements for CED are very similar to requirements for pumps utilized with pediatric and neonatal patients.8 In the current study we assess low-flow infusion performance of the FDA approved Alaris® System syringe pump, commonly used at the University of Wisconsin Hospital, during in vitro and ex vivo CED infusions. Infusion cloud morphology and line pressure were analyzed while delivering volumes and flow rates proposed for a human gene therapy protocol for Parkinson’s disease. The Alaris® System is capable of microliter per minute (μL/min) low-flow infusions recently proposed for human gene therapy clinical trials with proposed infusion rates of 1.0 to 5.0 μL/min (0.06 to 0.30 mL/hr).4 We report details of this benchmark first trial of the use of a leading syringe infusion pump system capable of low-flow infusions. [Table 1, 2]
Table 1: Infusion pump characteristics of Alaris® System syringe pump used in the current study.
| Alaris® System Infusion Pump Characteristics | |||||
|---|---|---|---|---|---|
| Physical Characteristics | Infusion Monitoring | Infusion Parameters | |||
| Abbreviations: AR, accessory required; VS, via syringe | |||||
| MRI safety | Loading | Pressure Monitoring | Display | Minimum Infusion Rate | Maximum Volume at Minimum Rate |
| N/A | Vertical | Yes (AR) | Bar graph, numeric with keypress series | 0.01 mL/hr (1/6 μL/min) | 1-3 mL syringe |
Table 2: Infusion characteristics for a proposed gene therapy trial for the treatment of Parkinson’s disease with CED delivery of AAV2-GDNF using the MRI interventions SmartFlow® neurocatheter system.
| Proposed Protocol for Gene Therapy CED to the Putamen | |
| Variable | Value |
| Infusion location | Putamen |
| Incision | Cranial Vertex |
| Patient position | Semi-sitting, “beach chair” |
| Posterior Infusion site | 150 microliters |
| Anterior Infusion site | 300 microliters |
| Proposed initial infusion rate | 1.0 microliters / minute (0.06 mL/hr) |
| Proposed maximum infusion rate | 5.0 microliters / minute (0.3 mL/hr) |
Methods
Study Design and Overview
Under the test protocol in the current report, lengthy steady state infusions of bromophenol blue dye (BPB) in agarose gel were performed a) to document infusion performance without and with the Alaris® pressure sensing disc, b) to document infusion line pressures comparable to those previously published, c) to document infusion performance during lengthy steady state infusions within the proposed infusion rate range for CED in the brain, d) to document performance and ease of use in performing a proposed multi-rate stepped infusion protocol, and e) to document performance in the setting of tissue occlusion.
Test Medium and Catheter Preparation
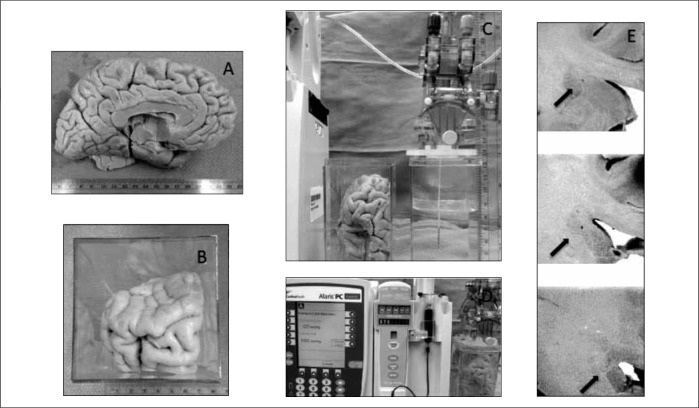
BPB infusion into agarose gel was chosen to serve as the in vitro model CED delivery to brain. BPB in concentrations of (0.017% and 4%) was prepared. Agarose gel (0.2%) was prepared in 5 cm × 5 cm × 10 cm plastic containers with the MRI interventions Clearpoint stereotactic device anchored on the container lid. The MRI Interventions Smart Flow® neurocatheter (16 Fr × 10 ft, inner diameter 0.008 inches) was cleared with degassed water, loaded with infusate (BPB), and prior to connection to a 3 mL BD syringe containing BPB with or without the Alaris® in-line pressure sensing disc. [Figures 1a, 1b, 1c]
Fig. 1:

Infusion neurocatheter and agarose gel infusion apparatus. 1A) External geometry of the MRI interventions SmartFlow® neurocatheter. 1B) The Alaris® System syringe pump was utilized to infuse indicator dye (bromophenol blue) into an agarose gel model of the brain via the MRI Interventions SmartFrame® and SmartFlow® neurocatheter system. Note the roughly 10 mm spherical infusion cloud on the left. This infusion was created without the Alaris® System pressure monitoring system in line with the infusion catheter. 1C) Detail of motion of pressure sensing plate (★) in response to pressure sensing disc (*) membrane expansion.
Infusate Delivery
The neurocatheter infusion system was assembled and primed dye was visually identified infusing into the 0.9% saline priming chamber. After a minimum of five minutes post priming and prior to insertion into the gel, the syringe pump was activated to infuse at 1.0 μL/min during insertion. Once the catheter tip reached its targeted destination, the rate was adjusted to accommodate the appropriate infusion protocol rate from 1.0 to 5.0 μL/min. [Figure 2] Details of each infusion protocol follow this paragraph. At the conclusion of each infusion, pressure monitoring was continued for a minimum of five additional minutes to allow catheter line pressure to decrease significantly from the infusion line pressure during the steady state infusion.
Fig. 2:
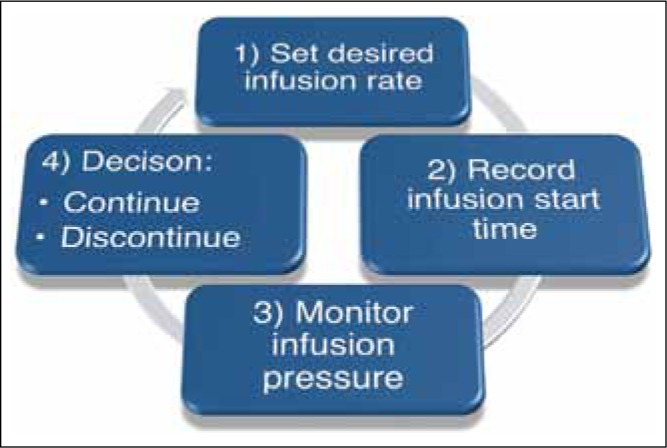
Process diagram for setting infusion rate for manual ramped infusions utilizing the five-minute ramped protocol in manual mode. Infusions with the Alaris® pump may be programmed for a volume or time. In the event a “stepped” ramp protocol is desired, manual infusion parameter changes are required according to the specified time interval
Technical details of infusion protocols
-
A)
100 μL steady state 1.0 μL/min infusion without pressure sensing disc: Alaris® System infusion of 4% BPB in 0.2% agarose gel at 1.0 μL/min for 100 minutes (100 μL total) without in-line pressure monitoring using the Alaris® pressure sensing disc. The 3 mL BD syringe was directly connected to the MRI Interventions SmartFlow® neurocatheter (10 FT × 0.008 inch inner diameter) and HD video.
-
B)
100 μL steady state 5.0 μL/min infusion after pressure stabilization at 1.0 μL/min: Alaris® System infusion of 4% BPB in 0.2% agarose gel at 1.0 μL/min for 12 minutes until pressure stabilization and then increased to 5.0 μL/min for 20 minutes (12 μL + 100 μL infusion), with in-line pressure monitoring and HD video.
-
C)
1.0 –5.0 μL/min steady state, constant duration infusions: Alaris® System infusion of 0.017% BPB in 0.2% agarose gel at 1.0, 2.0, 3.0, 4.0, and 5.0 μL/min for 30 minutes (30 – 150 μL total infusion volumes), with in-line pressure monitoring and HD video.
-
D)
100 μL infusion, stepped ramp protocol, 5.0 μL/min maximum rate: Alaris® System infusion with pressure monitoring of BPB (0.017%) in 0.2% agarose gel at 1.0 μL/min for 5 minutes, then 2.0 μL/min for 5 minutes, then 3.0 μL/min for 5 minutes, then 4.0 μL/min for 5 minutes, then 5.0 μL/min for 10 minutes (100 μL total), with in-line pressure monitoring and HD video.
-
E)
100 μL steady state 1.0 μL/min infusion, fixed brain tissue: Alaris® System infusion after manual insertion into the caudate nucleus of BPB (0.017%) in fixed human brain (formalin fixed, stored in 50% alcohol), post infusion pathological sectioning and photography. [Figure 4]
Fig. 4:
Ex-vivo infusion specimen and apparatus. 4A) External anatomy of the left hemisphere formalin fixed specimen. 4B) Specimen from 4A tailored to allow simulation of a transfrontal approach to the target. Note the olfactory bulb inferior to the letter at the picture’s top right. 4C) Priming of the neurocatheter system in fluid and measuring for planned targeting for depth of infusion. Catheter was inserted with an initial flow rate of 1.0 microliter per minute documented by indicator dye in the right-sided setup tank. 4D) Infusion catheter system at the end of the prescribed 100 microliter infusion indicating a line pressure over 500 mmHg and “infusion complete” status. 4E) Pathological sectioning showing the catheter trajectory and termination in grey matter without any evidence of indicator dye present.
Results
Primary endpoints for infusion analysis are as follows: 1) Successful delivery of infusate to the target, 2) End – infusion cloud morphology. Secondary endpoints were backflow, infusion catheter line pressure over time, time to reach 50% and 100% of the stabilization pressure, and ease of use of the pump system. [Table 3] Infusion with the Alaris® System syringe pump at low-flow rates proposed for human CED was possible with a 3 mL syringe. Expected spherical infusion cloud shape within agarose gel documented on HD video demonstrated spherical end-infusion morphology without and with the pressure sensing disc featured in Alaris® proprietary syringe sets with BPB at 1.0 μL /min (0.06 mL/hr) in agarose gel.
Table 3: Time to pressure stabilization and time to reach 50% of the stabilization pressure at the minimum and maximum infusion rates proposed for human gene therapy CED using the MRI Interventions SmartFlow® neurocatheter system.
| Infusion Rate | Pressure | Time | Time |
|---|---|---|---|
| (μL/min) | (mmHg) | (minutes) to 50% of stabilization pressure | (minutes) to maximum pressure |
| 1 | 24 | 4 | 12 |
| 5 | 60 | 5 | 7 |
Pressure Monitoring
While steady state infusion line pressures matched expected line pressures based upon flow rate and dye concentration, a significant time to stabilization was required for pressures to reach steady sate. Time to reach steady state decreased with infusion rate, however was consistently greater than 5 minutes with the tested configuration. Pressure did not consistently stabilize during performance of a 5 minute stepped ramp protocol in contrast to previously published reports with a low compliance pressure monitoring system. [Figure 3] Measured line pressure increased 5 mmHg/min during a failed infusion mimicking catheter or line occlusion. This did not however, cause the default occlusion alarm to signal an abnormality in the infusion at the end of a prescribed 100 microliter infusion. Pressure monitoring results correlated with previously published in-line pressure monitoring results however the time to peak with catheter occlusion was extended due to the method of pressure monitoring with this device.
Fig. 3:
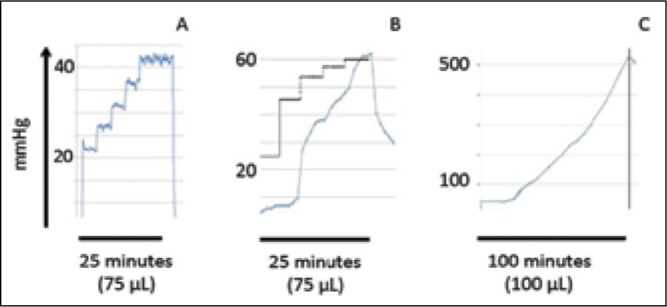
Line pressure measurements during prescribed infusions over time. 3A) In vitro step-ramp reference infusion: near prototypical infusion line pressure (in-line non-membrane pressure transducer, Harvard PHD 2000 infusion pump, previously reported). A rapid, near vertical pressure reduction is noted. 3B) In vitro step-ramp Alaris® System infusion: Curved tracing represents measured line pressure with the infusion protocol utilized in 3A. At 25 minutes and a total of 75 microliters planned infusion, the pump is inactivated leading to tapered reduction in line pressure. The stepped lines represent steady state infusion pressures at 1.0, 2.0, 3.0 (interpolated), 4.0 (interpolated), and 5.0 microliters per minute. 3C) Ex vivo occlusion demonstration: Curved tracing indicates measured line pressure increasing after 25 minutes until reaching a maximum line pressure above 500 mmHg. The desired 100 microliter infusion volume was retained internal to the infusion system as the fixed cadaveric specimen served as a complete catheter infusion at the distal catheter tip.
Study Limitations
Agarose gel as a surrogate for brain tissue
Agarose gel can model certain aspects of infusing in the human neural structures. A concentration of 2% agarose gel has been shown reliable and to reasonably represent infusion characteristics when compared to in vivo studies.
Catheter insertion technique
Three methods of gel-catheter interface creation are possible. 1) The catheter is placed and stabilized in an empty agarose gel container; agarose gel is then poured into the container and allowed to set. 2) The agarose gel is prepared and poured in the container intended for infusion; the catheter is inserted once the gel is set. 3) The catheter is inserted into agarose gel as in method 2, withdrawn and then reseated prior to beginning the infusion. Each method poses some risk of gel entering the catheter and causing an occlusion. The method of catheter insertion into gel without rotation after insertion (method 2) was chosen as it most closely represented the physical steps involved in performing an actual human insertion and infusion.
Bromophenol Blue as a Surrogate for Therapeutic Infusate
CED delivery should allow non-charged molecules of various sizes to be convected over a similar volume of tissue. BPB is chosen due to the ability for visualization within agarose gel and within tissue infusions with planned pathological sectioning. Infusion cloud size increases with dye concentration and spherical morphology is possible with both 4% and 0.017% BPB. 4% BPB allows for superior HD video imaging contrast and was used in benchmarking pressures previously. A lower concentration of dye will more efficiently correlate to infused therapy and therefore was used in the second set of experiments.
Safety and Tolerability
As observed in the graph portrayed in Figure 3c, the pressure never reached stabilization. The pressure monitoring system recorded pressures as high as 525 mmHg. Recent evidence has suggested CED infusions performed under real-time MRI monitoring in fresh ex vivo tissue may closely mimic results obtained in living tissue in in vivo models. As a first step in a protocol designed to test CED infusion performance in non-fixed human tissue we tested the setup in a fixed brain. The pump was programmed to infuse 100 μL into the fixed tissue. After the infusion was completed the tissue was sliced for further analysis. While the track left by the catheter was clearly visible, there was no trace of the 100 μL of BPB infused. The occlusion pressure setting of the Alaris® pump was 1024 mmHg (the default setting) during the infusion. Thus, the infusion was allowed to go to completion without any form of audible warning that the infusate was not actually being delivered to the targeted tissue. This exemplifies the significance of managing pump’s pressure system and performing pressure monitoring throughout the course of infusion. Without adjusting the pressure dynamics, the Alaris® pump may not be sensitive enough to sense an occlusion in the catheter with very low-flow rate infusions as specified for CED.
Line Loading and Pump Preparation
Careful study and attention to specific issues of line loading and pump preparation will be required with infusion volumes on the order of 30–300 μL. Interpretation of pressure monitoring and syringe selection is critical for CED.
Technical Challenges
Syringe motion
A 3 mL syringe may produce variability in the amount of infusate delivered over a period of time at such low-flow rates. Pressure fluctuations after pressure stabilization are seen even in the setting of an increased compliance pressure-measuring device. This could imply an inconsistency in infusate delivery that may be associated with syringe size.11 A smaller syringe may be preferred for more controlled distribution.
System compliance
The Alaris pump pressure monitoring device design requires the filling of a membrane to read the pressure in the line. This structure introduces system compliance or “slack” which may mask acute changes in catheter tip pressure or flow. During the infusion in a compliant system, pressure builds in the membrane until stabilization. Residual pressure within the compliant system could cause infusate delivery after the infusion stop command has been issued to the syringe pump. If the pump-pressure monitor-infusion catheter system is unable to maintain the pace of the protocol indicated by pressure stabilization, therapy may not be delivered as desired.
Ease-of-use Assessments
Ease of use was assessed using time-to-assemble the catheter system and presence of air bubbles after connection; mean time to syringe attachment was 2 minutes or less for each trial. There were no infusion interruptions in this series. There were no external occlusions to the infusion line. Programmable steps and ramps are not possible with this syringe pump, requiring manual increases in infusion rates to complete a clinically proposed ramped technique. Further enhancements will be required to optimize delivery via the Alaris® syringe pump.
Limitations in rCED monitoring with MRI
Not all surgical centers are equipped with intraoperative MRI scanning technology. MRI scanning alone is limited by iterative prolonged scanning periods on the order of 5 minutes optimized to detect metrics regarding volume / concentration of infusion or backflow along the injection cannula tract. Therefore the rCED is actually a “secondary” measure of the health of the infusion and pressure is a “primary” indication with a rapid and granular report. Currently, MRI scanning during CED infusions is used to detect change in backflow and alteration in the rate of increase in the volume detected. However, MRI monitoring alone is not sufficient in observing the entire infusion. In the setting of rCED, the infusion pressure can change altitude even before a noticeable change in cloud morphology or rate of volume increase. MRI scanning alone is limited by iterative prolonged scanning periods on the order of 5 minutes optimized to detect metrics regarding volume / concentration of infusion or backflow along the injection cannula tract. In addition, not all infusion pumps that may be applied for CED are MRI compatible, such as the Alaris® Systems syringe pump. This demands an alternative to the MRI monitoring of infusion clouds.
Limitations in infusion line pressure monitoring and alarms
Pressure monitoring gives the greatest insight into the behavior of the infusion. Unfortunately, to date, there are no systems available for the delivery of injectables to the brain suitable for convection enhanced delivery (CED) with line and tissue pressure monitoring. Leading systems include the Medfusion® 3500, B-Braun Perfusor® Space, Baxter, and Alaris® System. Early reports of Medfusion® 3500 use in human rCED are indicative of issues with the lack of a dedicated CED delivery system with pressure monitoring 1) Excessive line pressure can trigger occlusion alerts, interrupting the steady flow of CED due to high resistance infusion systems (personal communication Krys Bankeiwicz, Russ Lonser). Currently pressure is measured at the end of the infusion line and can exceed 120mmHg for infusion rates of 5 μL/min (0.30 mL/hr) using the MRI Interventions SmartFlow® catheter. 12 Questions arise after infusion interruption as to the best method of re-starting the infusion in the setting of stepped or ramped infusion protocols. 2) Line pressure displays lack crisp, granular display and metric capabilities. 3) Countermeasures or infusion protocol adjustments are currently not possible in an automated fashion to tailor an infusion to allow a maximum infusion rate based upon pressure rather than a pre-determined flow rate. 4) Reverse mode is not available for line loading of an injectate. 5) Occlusion mitigation algorithms are not useful as they are tailored for higher volumes to clear the occlusion and could cause damaging bolus injections in the current configuration.
Results
Primary endpoints for infusion analysis are as follows: 1) Successful delivery of infusate to the target, 2) End – infusion cloud morphology. Secondary endpoints were backflow, infusion catheter line pressure over time, time to reach 50% and 100% of the stabilization pressure, and ease of use of the pump system. [Table 3]
Infusion with the Alaris® System syringe pump at low-flow rates proposed for human CED was possible with a 3 mL syringe. Expected spherical infusion cloud shape within agarose gel documented on HD video demonstrated spherical end-infusion morphology without and with the pressure sensing disc featured in Alaris® proprietary syringe sets with BPB at 1.0 μL/min (0.06 mL/hr) in agarose gel.
Pressure Monitoring
While steady state infusion line pressures matched expected line pressures based upon flow rate and dye concentration, a significant time to stabilization was required for pressures to reach steady sate. Time to reach steady state decreased with infusion rate, however was consistently greater than 5 minutes with the tested configuration. Pressure did not consistently stabilize during performance of a 5 minute stepped ramp protocol in contrast to previously published reports with a low compliance pressure monitoring system. [Figure 3]
Measured line pressure increased 5 mmHg/min during a failed infusion mimicking catheter or line occlusion. This did not however, cause the default occlusion alarm to signal an abnormality in the infusion at the end of a prescribed 100 microliter infusion. Pressure monitoring results correlated with previously published in-line pressure monitoring results however the time to peak with catheter occlusion was extended due to the method of pressure monitoring with this device.
Discussion
CED is an emerging form of brain infusion therapy, which will involve nursing professionals extensively as therapy adoption increases. Few previous reports discuss infusion pump metrics from a nursing perspective in preparation for future nursing needs in low-flow infusion expertise. Key knowledge areas for the healthcare professional involved in pressure monitored CED infusions include: 1) proper line priming, loading and pump preparation, 2) understanding pressure monitoring to diagnose system malfunction and monitor infusions, and 3) understanding the role of real-time MRI monitoring of infusions. Key development areas for infusion technology manufacturers include: 1) MRI compatible syringe pump systems, 2) real-time pressure monitoring of infusion line pressure and pressure monitoring at the device – tissue interface, 3) line loading technologies to allow programmed phased loading of infusate and carrier.
Real-time CED with MRI Guidance
As systems are developed for CED, pressure delivery under real-time MRI guidance (rCED), a requirement for functionality within 1.5T, 3.0T, and 4.7T MRI suite environments has emerged.4,9 MRI monitoring has the ability to provide an image of the morphology of the infusion cloud. Several groups have recently published on the merits of rCED including the ability to document targeting, delivery, infusate loss, completeness of infusion, and develop countermeasures for dealing with abnormalities during the delivery process.10
Although real-time MRI monitoring has many advantages, not all infusion pumps that may be applied for CED are MRI compatible, such as the Alaris® System syringe pump.
This demands an alternative to the MRI monitoring of infusion clouds. Monitoring CED with infusion line pressure is another tool, which may be used alone as an alternative or in conjunction with real-time MRI. Pressure monitoring is achievable with the Alaris® System syringe pump approved by the FDA for delivery of injectables for patient care, but has not yet been fully utilized in infusions. Pressure monitoring gives the greatest insight into the behavior of the infusion. Unfortunately, several limitations exist with the clinical infusion systems or syringe pumps currently available in the United States. As design, testing, and the FDA approval process moves forward for rCED MRI compatible infusion pumps used for brain delivery injectables, pressure monitoring is set to be a guiding instrument for the health care professional employing this emerging form of infusion-to-brain delivery. Further work will be required to optimize design to allow pressure monitoring in the brain delivery system to 1) detect pressure at the site of delivery and, 2) relate the line or syringe pressure to the pressure at the delivery site. Such improvements will help to determine the “health” of the infusion system, 3) validate the safety and efficacy of the use of measured infusion pressure to tailor infusion protocols as infusion ramp waveforms are likely shown to allow more efficient delivery than stepped-ramp delivery paradigms. Further development of infusion pump technology is warranted to allow for infuse/withdraw mode, infusion pressure graphical and numerical display, and pressure monitoring without the need for an inflatable reservoir pressure device. MRI safe infusion systems will need to be available and nursing staff educated to prepare infusions within the high-field environment.
Conclusions
Convection Enhanced Delivery is an emerging infusion therapy to delivery neurotherapeutics of varying molecular weight and origin. Reports and recommendations include flow rates to the minimum possible with the Alaris® System syringe pump. In the absence of an ideal pressure monitoring syringe pump, rigorous testing and familiarity with currently available syringe pumps is indicated to educate nursing professionals and future infusion operators as well as to make recommendations for pump and associate system design changes for optimum therapy delivery.
Low-flow infusions are currently in use with the Alaris® System syringe pump in the pediatric population. Low-flow rate infusion therapy safety and consistency is optimized by the optimum selection of syringe size and proper line loading and pressure sensing device preparation prior to use. Minimizing infusion system compliance due to retained dead space and air bubbles may decrease the time to alarm in the setting of an Further investigation in in vitro and ex vivo infusions and modeling of CED is indicated as this field moves to human clinical trials. In addition to pressure monitoring, real-time CED technical hurdles are decreased to the extent all devices are optimized for use in and around the MRI environment.
Acknowledgments
The BADGER team and authors wish to thank Tara Anderson and Lauren Kumbier of the Department of Neurosurgery for her assistance in preparing the manuscript for publication; Paul Malischke, Nicholas Brown, Josh Meadow, and Michael Cain for their engineering support; Carol Dizack for assistance with medical illustration; and the Kinetics foundation for their gracious support in training and outfitting equipment used in these experiments.
Footnotes
The article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Conflicts of Interest: None
Source of Funding: None
References
- 1.Bobo RH. Convection-enhanced delivery of macromolecules in the brain. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelbaum MA. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery. 2007;61(5):1031–1037. doi: 10.1227/01.neu.0000303199.77370.9e. [DOI] [PubMed] [Google Scholar]
- 3.Koutsilieri E, Rethwilm A, Scheller C. The therapeutic potential of siRNA in gene therapy of neurodegenerative disorders. Journal of neural transmission. Supplementum. 2007;(72):43–49. doi: 10.1007/978-3-211-73574-9_7. [DOI] [PubMed] [Google Scholar]
- 4.Richardson RM. Interventional MRI-guided putaminal delivery of AAV2-GDNF for a planned clinical trial in Parkinson’s disease. Mol Ther. 2011;19(6):1048–1057. doi: 10.1038/mt.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks WJ. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet neurology. 2008;7(5):400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 6.LeWitt PA. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet neurology. 2011;10(4):309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 7.Christine CW. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73(20):1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass SM, Giacoia GP. Intravenous drug therapy in premature infants: practical aspects. Journal of obstetric, gynecologic, and neonatal nursing : JOGNN / NAACOG. 1987;16(5):310–318. doi: 10.1111/j.1552-6909.1987.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 9.Emborg ME. Intraoperative intracerebral MRI-guided navigation for accurate targeting in nonhuman primates. Cell transplantation. 2010;19(12):1587–1597. doi: 10.3727/096368910X514323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady ML. Minimizing Infusate Loss during Direct Delivery into the Putamen. Stereotactic and Functional Neurosurgery. 2012 doi: 10.1159/000342492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neal D, Lin JA. The effect of syringe size on reliability and safety of low-flow infusions. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2009;10(5):592–596. doi: 10.1097/PCC.0b013e3181a0e2e9. [DOI] [PubMed] [Google Scholar]
- 12.Sillay K, Dominic S, Angelica H et al. Benchmarking the ERG valve tip and MRI Interventions Smart Flow neurocatheter convection-enhanced delivery system’s performance in a gel model of the brain: employing infusion protocols proposed for gene therapy for Parkinson’s disease. Journal of neural engineering. 2012;9(2):p. 026009. doi: 10.1088/1741-2560/9/2/026009. [DOI] [PubMed] [Google Scholar]