Abstract
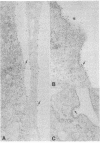
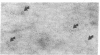
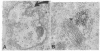
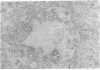
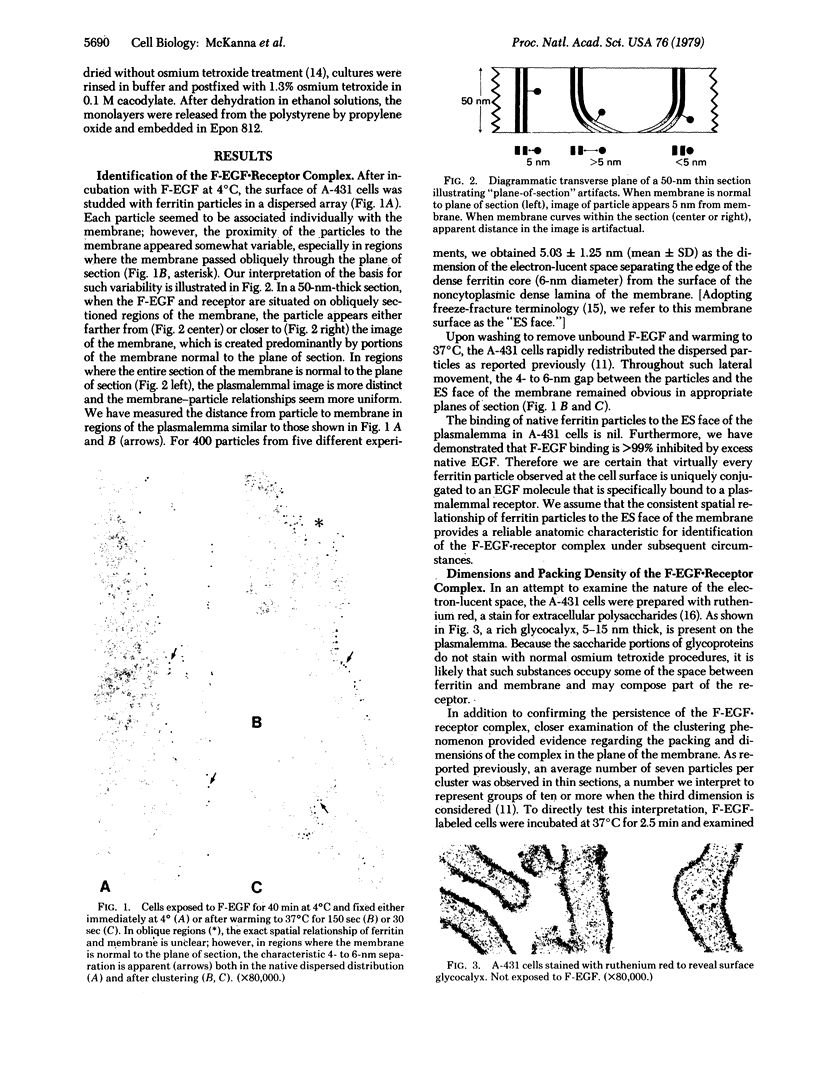
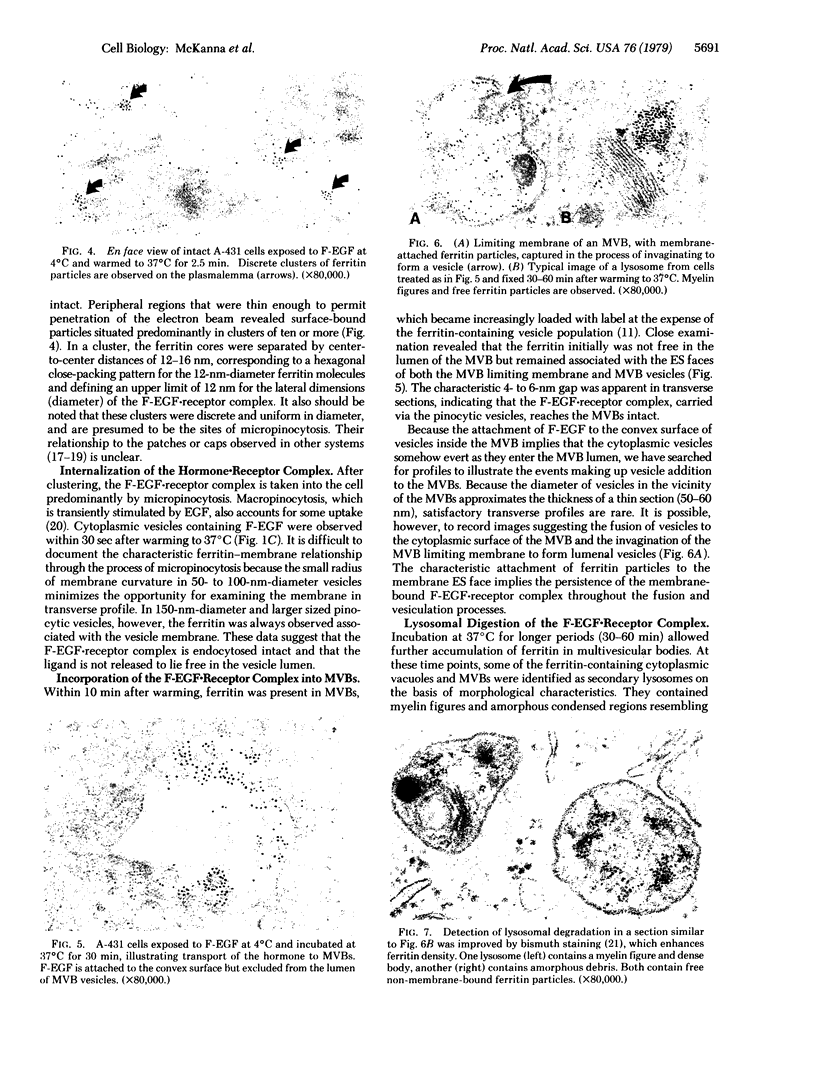
Previous studies using a biologically active 1:1 conjugate of EGF and ferritin (F-EGF) have traced the binding and internalization of the hormone molecules. In the present report, we develop ultrastructural criteria for identification of the F-EGF·receptor complex, and, thereby, enable utilization of the F-EGF as an indirect marker to localize the receptor for this peptide hormone. The ferritin cores of bound F-EGF are situated 4-6 nm from the extracellular surface of the membrane. When cells were incubated for up to 30 min at 37°C, this characteristic spatial relationship was observed in all uptake stages (surface clustering, endocytosis, and incorporation into multivesicular bodies), indicating that the hormone·receptor complex remains intact through these steps. However, when incubation was continued for periods sufficient to allow hormone degradation (30-60 min), pools of free ferritin were observed in lysosomes. In the presence of various amine inhibitors of hormone degradation, internalization and multivesicular body incorporation proceeded, but hormone·receptor degradation was blocked as evidenced by preservation of the ferritin—membrane relationship; i.e., no pools of free ferritin were seen after 60 min. These data provide morphological support for the hypothesis that down-regulation of surface receptors involves internalization of intact hormone·receptor complexes. In addition, we have developed a method for viewing the surface of intact cells en face, allowing closer scrutiny of the clustering of F-EGF·receptor complexes in the plane of the membrane prior to internalization. The particles in the F-EGF clusters observed by this method are spaced at 12 nm center-to-center, serving to set upper limits on the packing dimensions of the EGF·receptor complex.
Keywords: endocytosis, lysosomes, lysosome inhibitors, multivesicular bodies
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonov A., Pruss R. M., Herschman H. R. Epidermal growth factor. Relationship between receptor regulation and mitogenesis in 3T3 cells. J Biol Chem. 1978 Jun 10;253(11):3970–3977. [PubMed] [Google Scholar]
- Ainsworth S. K., Karnovsky M. J. An ultrastructural staining method for enhancing the size and electron opacity of ferritin in thin sections. J Histochem Cytochem. 1972 Mar;20(3):225–229. doi: 10.1177/20.3.225. [DOI] [PubMed] [Google Scholar]
- Baker J. B., Simmer R. L., Glenn K. C., Cunningham D. D. Thrombin and epidermal growth factor become linked to cell surface receptors during mitogenic stimulation. Nature. 1979 Apr 19;278(5706):743–745. doi: 10.1038/278743a0. [DOI] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D. S. Cytochemical staining of multivesicular body and golgi vesicles. J Cell Biol. 1969 Apr;41(1):269–279. doi: 10.1083/jcb.41.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Cohen S., Orci L. Epidermal growth factor: morphological demonstration of binding, internalization, and lysosomal association in human fibroblasts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5025–5029. doi: 10.1073/pnas.75.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Rapid stimulation of pinocytosis in human carcinoma cells A-431 by epidermal growth factor. J Cell Biol. 1979 Oct;83(1):82–90. doi: 10.1083/jcb.83.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H., Ash J. F., Singer S. J., Cohen S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3317–3321. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Blifeld C., Wrann M., Fox C. F. Direct linkage of epidermal growth factor to its receptor. Nature. 1979 Apr 19;278(5706):745–748. doi: 10.1038/278745a0. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., Schlessinger J., Shechter Y., Pastan I., Willingham M. C. Collection of insulin, EGF and alpha2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978 Aug;14(4):805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., Willingham M. C., Davies P. J., Pastan I. Amines inhibit the clustering of alpha2-macroglobulin and EGF on the fibroblast cell surface. Nature. 1979 Feb 22;277(5698):661–663. doi: 10.1038/277661a0. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Schlessinger J., Jacobs S., Chang K. J., Cuatrecasas P. Fluorescent labeling of hormone receptors in viable cells: preparation and properties of highly fluorescent derivatives of epidermal growth factor and insulin. Proc Natl Acad Sci U S A. 1978 May;75(5):2135–2139. doi: 10.1073/pnas.75.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Brown K. D., Gospodarowicz D. A comparison of the binding of epidermal growth factor to cultured granulosa and luteal cells. J Biol Chem. 1978 May 25;253(10):3744–3750. [PubMed] [Google Scholar]