Abstract
Objective:
We performed a longitudinal cohort study of infants with tuberous sclerosis complex (TSC), with the overarching goal of defining early clinical, behavioral, and biological markers of autism spectrum disorder (ASD) in this high-risk population.
Methods:
Infants with TSC and typically developing controls were recruited as early as 3 months of age and followed longitudinally until 36 months of age. Data gathered at each time point included detailed seizure history, developmental testing using the Mullen Scales of Early Learning, and social-communication assessments using the Autism Observation Scale for Infants. At 18 to 36 months, a diagnostic evaluation for ASD was performed using the Autism Diagnostic Observation Schedule.
Results:
Infants with TSC demonstrated delays confined to nonverbal abilities, particularly in the visual domain, which then generalized to more global delays by age 9 months. Twenty-two of 40 infants with TSC were diagnosed with ASD. Both 12-month cognitive ability and developmental trajectories over the second and third years of life differentiated the groups. By 12 months of age, the ASD group demonstrated significantly greater cognitive delays and a significant decline in nonverbal IQ from 12 to 36 months.
Conclusions:
This prospective study characterizes early developmental markers of ASD in infants with TSC. The early delay in visual reception and fine motor ability in the TSC group as a whole, coupled with the decline in nonverbal ability in infants diagnosed with ASD, suggests a domain-specific pathway to ASD that can inform more targeted interventions for these high-risk infants.
Tuberous sclerosis complex (TSC) confers a high risk of autism spectrum disorders (ASDs) and intellectual disability,1–3 with rates of ASD ranging from 25% to 60%, much higher than the 1% to 2% reported in the general population.4–7 Despite hypotheses about the role of epilepsy, cortical pathology, and co-occurring genetic mutations in the development of ASD in TSC,8–10 no single clinical factor has been identified as a consistent predictor of atypical neurodevelopment in this disorder.
The fact that TSC is diagnosed early in development, often prenatally,11 facilitates the prospective investigation of developmental trajectories and early markers of ASD in this population. Early detection research in ASD has mostly focused on infant siblings of children with ASD, in whom the recurrence risk is approximately 18%.12 Emerging evidence from these studies supports behavioral and neurobiological differences based on risk status in the first year of life.13,14 However, because of the genetic heterogeneity within these infant sibling cohorts, the measures used to identify early markers must cover a range of possible mechanisms and pathways to ASD. In contrast, infants with TSC can be studied with targeted measures that quantify clinical, behavioral, and biological variables likely to contribute to the development of ASD in TSC, such as epilepsy severity, and early cognitive development.
Drawing on the model of prospective studies of infant siblings, we performed a longitudinal investigation of infants with TSC, with the overarching goal of defining early clinical, behavioral, and biological markers of ASD in this high-risk population.
METHODS
Procedures.
These data represent one portion of a multisite, longitudinal study that includes behavioral and electrophysiologic assessments in children with and without TSC, aged 3 to 36 months. Recruitment and testing were performed at the University of California, Los Angeles (UCLA) Center for Autism Research and Treatment and the Boston Children's Hospital Laboratories of Cognitive Neuroscience from June 2011 to June 2013. Families were compensated for transportation and lodging. Any significant concerns about the infant's development were relayed to the primary neurologist or pediatrician by the principal investigators of the study.
Standard protocol approvals, registrations, and patient consents.
Institutional review board (IRB) approval was obtained at each of the 2 sites (UCLA IRB no. 11-002349; Boston Children's Hospital IRB no. P00001144). All families gave informed consent before participation.
Participants.
Infants with TSC were recruited through TSC specialty clinics, newborn nurseries, pediatrician offices, and the Tuberous Sclerosis Alliance. Control infants were recruited through IRB-approved infant databases in the greater Los Angeles and Boston areas. Control exclusion criteria included prematurity (<37 weeks' gestational age), birth trauma, developmental concerns, or immediate family history of ASD or intellectual disability. Children were followed longitudinally at 3, 6, 9, 12, 18, 24, and 36 months. Because of the rarity of TSC, all children with TSC younger than 24 months could be enrolled in the study. Children enrolled at an age not represented by a study time point were assessed at the next appropriate time point. For instance, a 15-month-old child was first studied at age 18 months. While control recruitment targeted the 3-month time point, controls also were recruited on an individual basis to match the age of each TSC case. There was no matching on demographics or IQ. The research team maintained close phone and e-mail contact with the families to minimize attrition.
Clinical information.
At each time point, the research team gathered clinical data regarding seizures, medications, operations, and other medical issues through a standardized medical questionnaire. Variables of interest included presence of infantile spasms, presence of seizures, age of seizure onset, seizure duration, proportion of life with active seizures (defined as seizure duration in months/age of assessment), and number of antiepileptics.
Behavioral testing.
Although participant information was deidentified, the clinical presentation of infants with TSC (developmental delay, epilepsy, cutaneous lesions) precluded blinding of examiners to participant diagnosis. At each age, the Mullen Scales of Early Learning (MSEL) was performed. The MSEL is a standardized cognitive measure for children 0 to 69 months of age, testing 5 developmental domains: gross motor (GM), fine motor (FM), visual reception (VR), receptive language (RL), and expressive language (EL).15 Raw scores are converted to age-standardized t scores, which facilitates the distinction between normative and nonnormative development.
At ages 6, 9, 12, and 18 months, the Autism Observation Scale for Infants (AOSI) was performed. The AOSI is a 20-minute play-based instrument that operationalizes and detects behavioral risk markers for ASD. Developed to study infants at high risk (infant siblings of children with ASD), the AOSI has high interrater and test-retest reliability.16 Several items of the AOSI have high predictive value of ASD, including transitions, motor control, and reactivity.17 We chose the AOSI because it quantifies infant behaviors relevant to social communication function not specifically targeted in the MSEL, such as temperament, attention, and overall social engagement.
At ages 18, 24, and 36 months, the Autism Diagnostic Observation Schedule (ADOS) was performed for ASD diagnosis. The ADOS is a semistructured, play-based interview with standardized probes and scoring for social interaction, communication, repetitive behaviors, and play.18 Trained research assistants administered and scored the assessments, with interrater reliability established to that of ADOS-trained staff members. The ADOS has been demonstrated to have excellent interrater reliability among formally trained examiners.18 Severity scores were calculated according to the revised algorithm.19,20 Diagnoses were based on the convergence of ADOS scores and the clinical judgment of a board-certified pediatric neurologist (S.S.J.). For children tested with the ADOS at multiple time points (ages 18–36 months), the last ADOS score was used for diagnosis and severity calculation. In the 4 controls and 9 children with TSC who were administered the ADOS at multiple time points, clinical diagnosis remained consistent across time points.
Statistical methods.
MSEL raw scores were converted to t scores for each developmental domain. Per MSEL protocol, all but the GM scores were used to generate a developmental quotient (DQ). FM and VR were used to calculate a nonverbal IQ (NVIQ), and RL and EL were used to calculate a verbal IQ (VIQ). AOSI raw scores were used to calculate a total score, with higher scores signifying a higher risk of ASD. ADOS scoring was based on the new algorithm, with subscores for social affect, repetitive behaviors, and restricted interests.19
TSC vs controls.
At each age, independent samples t tests were performed to compare MSEL and AOSI scores between groups. Because no infants with TSC were recruited at 3 months of age, analyses at 6, 9, 12, and 18 months were performed.
ASD vs no ASD.
Cross-sectional analysis.
Based on ADOS scores at 18, 24, and/or 36 months, children with TSC fell into 2 diagnostic groups: ASD or no ASD. Independent samples t tests were performed at each age to compare clinical variables, MSEL subscores, and AOSI raw scores between groups. Focus was placed on the 12-month MSEL in order to identify developmental delays in the infants who went on to have an ASD diagnosis.
Developmental trajectories.
To control for the effects of seizures on development, repeated measures on DQ, VIQ, and NVIQ scores were modeled with linear mixed-effects models using group (TSC/no-ASD, TSC/ASD), time (from 12 to 36 months), group by time interaction, seizure percentage, and group by seizure percentage interaction as predictors (in SAS PROC MIXED) with an autoregressive correlation structure of order one. Linear mixed-effects models are flexible modeling tools for longitudinal data where subjects are independent variables, but the time points within each subject are highly correlated, with measurements farther apart in time being less correlated. Unlike a repeated-measures analysis of variance, a linear mixed model does not require that the data are balanced (i.e., same number of subjects observed at each time).
RESULTS
Sample size.
Sample size at each age varied because of the flexible enrollment of infants with TSC. Only infants with at least 2 study time points before a diagnostic assessment at 18, 24, or 36 months were included in the ASD analysis (n = 40). For the characterization of developmental trajectories by ASD diagnosis, only children with a history of epilepsy and seizure duration data were included (n = 34).
TSC vs controls.
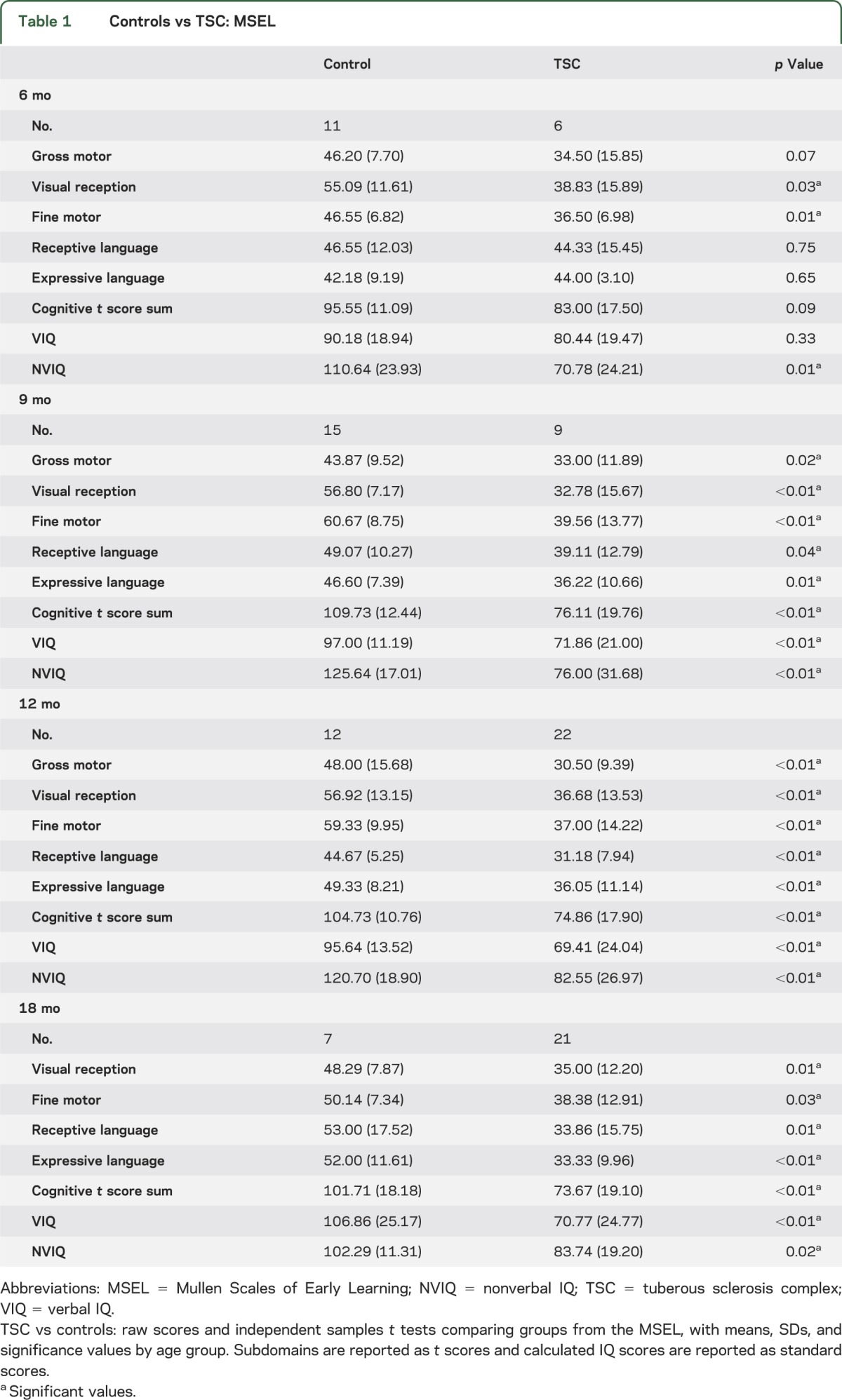
At the whole group level, we were most interested in the timing of developmental delay in infants with TSC and whether there were particular domains more significantly affected. Based on the MSEL, infants with TSC did demonstrate developmental delays in all age groups. However, there was evidence of domain specificity at the first analyzed time point of age 6 months, with the TSC group showing delays in NVIQ (both FM and VR domains). By age 9 months, infants with TSC exhibited delays in all developmental domains of the MSEL (table 1).
Table 1.
Controls vs TSC: MSEL
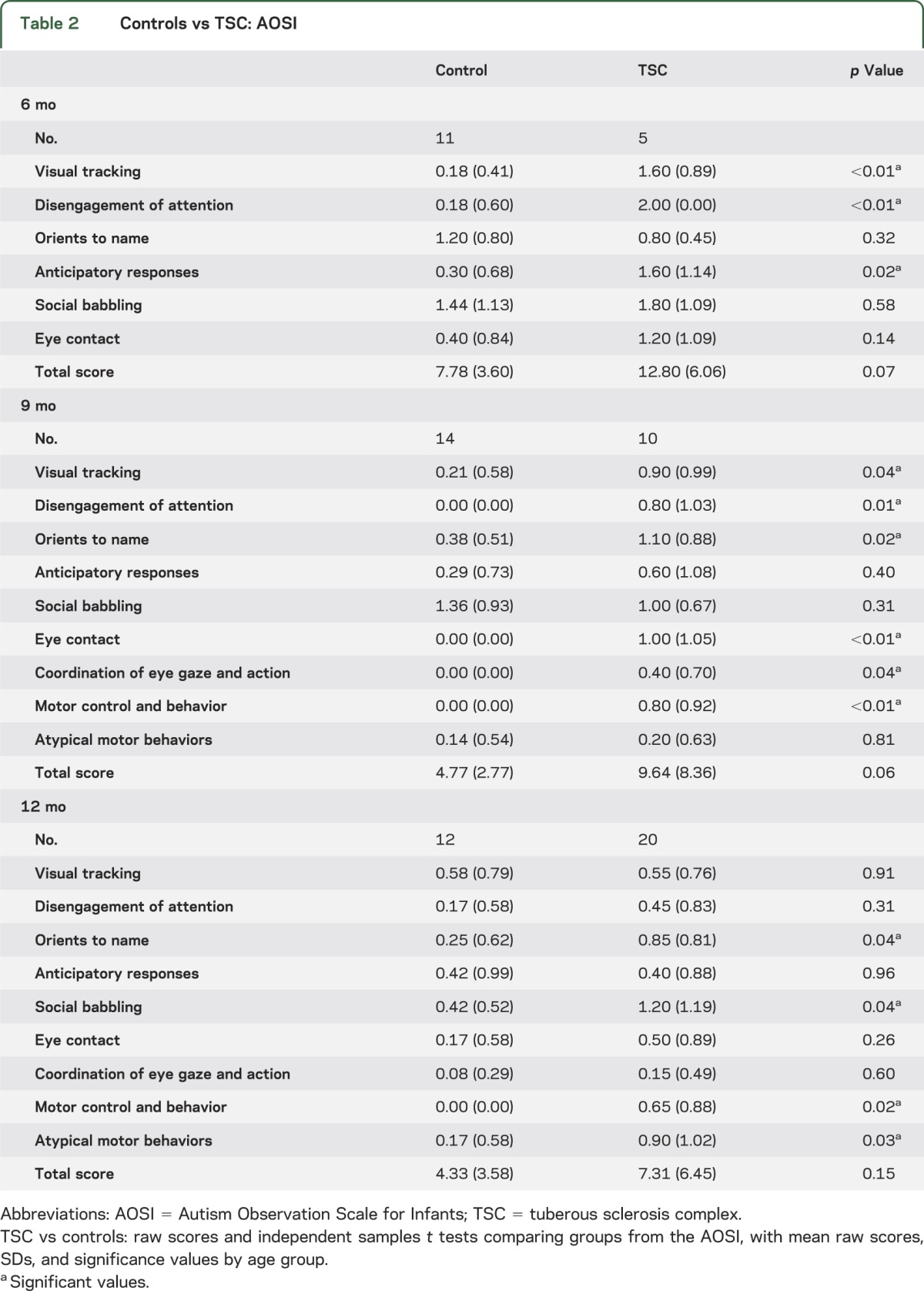
On the AOSI, the TSC group also demonstrated more atypical social behaviors by 6 months of age in visual tracking, disengagement of attention, and anticipatory responses. At age 9 and 12 months, atypicalities were also observed in eye contact, orienting to name, and motor control and behavior (table 2).
Table 2.
Controls vs TSC: AOSI
ASD vs non-ASD.
Clinical variables.
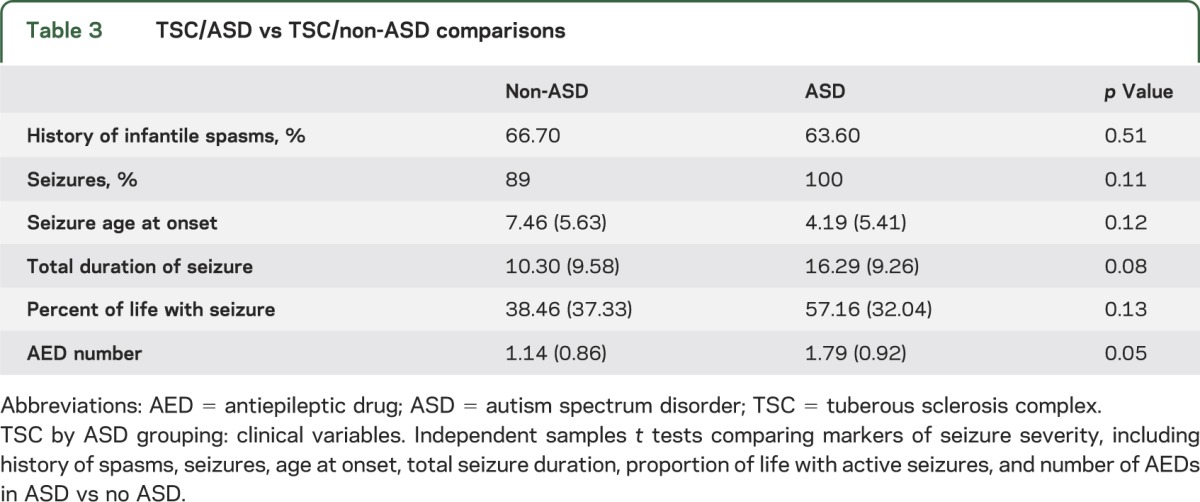
Twenty-two of 40 children with TSC met criteria for ASD. Average autism severity for the ASD group was 5.4 (SD 2.0) and for the non-ASD group was 2.2 (SD 1.4). Therefore, as expected from the AOSI differences in early infancy, there were social communication deficits noted even in the non-ASD group.
All but 4 children with TSC had epilepsy, and none of the 4 without epilepsy had an ASD diagnosis. There was a trend toward greater seizure severity in the ASD group, as indicated by younger age of seizure onset and greater proportion of life with active seizures, but these differences did not reach statistical significance. Children with ASD were treated with a significantly greater number of antiepileptics at their final assessment compared with the non-ASD group, a variable that also may serve as a proxy for seizure frequency or disease course (table 3).
Table 3.
TSC/ASD vs TSC/non-ASD comparisons
Cognitive ability at 12 months.
Given the paucity of data on cognitive precursors of ASD in infants with TSC, we performed a cross-sectional analysis of MSEL scores by ASD diagnosis at 12 months of age. Twelve-month-old infants later diagnosed with ASD demonstrated significantly lower MSEL scores in the domains of VR, FM, EL, and RL, with resulting significant differences in their DQ, NVIQ, and VIQ (figure 1). Only the GM score did not significantly differ between diagnostic groups.
Figure 1. TSC by ASD grouping: 12 months MSEL.
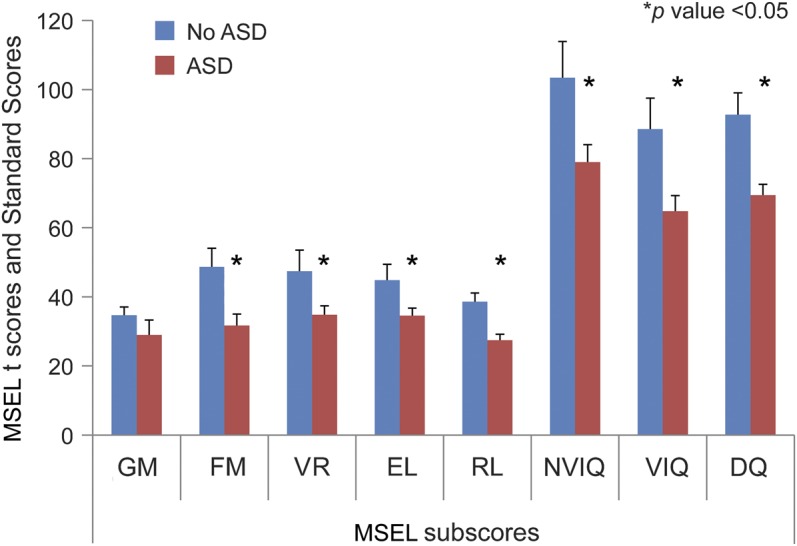
Independent samples t tests comparing MSEL t scores and standard scores by ASD grouping. Means and standard error bars are provided; asterisk represents p < 0.05. ASD = autism spectrum disorder; DQ = developmental quotient; EL = expressive language; FM = fine motor; GM = gross motor; MSEL = Mullen Scales of Early Learning; NVIQ = nonverbal IQ; RL = receptive language; TSC = tuberous sclerosis complex; VR = visual reception; VIQ = verbal IQ.
Developmental trajectories 12 to 36 months.
Appreciating the dynamic nature of development, particularly in the context of epilepsy, we investigated the developmental trajectories of infants based on ASD diagnosis. Controlling for seizure duration, we found significant differences in trajectories of DQ, NVIQ, and VIQ in years 2 and 3 of life (figure 2). The ASD group made no change in full DQ (slope estimate −0.22, p = 0.51) or VIQ (slope estimate −0.22, p = 0.66), but they experienced a significant decline in NVIQ between ages 12 and 36 months (slope estimate −0.80, p = 0.01). While there was heterogeneity in the individual trajectories for DQ, VIQ, and NVIQ, a more refined downward trend in time emerged for NVIQ. The non-ASD group showed significant developmental gain in DQ (slope estimate 1.03, p = 0.02) and NVIQ (slope estimate 0.42, p = 0.01) but no significant change over the second 2 years of life in VIQ (slope estimate 0.82, p = 0.19). Furthermore, in the ASD group, there was a significant negative interaction between seizure duration and NVIQ (estimate −0.25, p = 0.03), such that having greater proportion of life with seizures was associated with a lower NVIQ across ages.
Figure 2. TSC/ASD vs TSC/no-ASD developmental trajectories of full DQ, NVIQ, and VIQ from MSEL.
(A) Mean population DQ, NVIQ, and VIQ trajectories (raw data) from 12 to 36 months. (B) Regression statistics. Statistics from linear mixed-effects models using group, time, group by time interaction, seizure duration, and group by seizure duration interaction as predictors. ASD = autism spectrum disorder; DQ = developmental quotient; MSEL = Mullen Scales of Early Learning; NVIQ = nonverbal IQ; sz = seizure; TSC = tuberous sclerosis complex; VIQ = verbal IQ.
DISCUSSION
In this prospective, longitudinal study, we sought to define early cognitive and behavioral markers of ASD in TSC. Infants with TSC demonstrated early developmental delays, with relative specificity to the visual domain at age 6 months that generalized to all developmental domains by age 9 months. We also identified early signs of atypical social communication function by age 6 months, particularly in visual behaviors. Infants later diagnosed with ASD demonstrated significantly more delays in all cognitive domains by age 12 months. Developmental trajectories also differed based on ASD diagnosis, with the ASD group demonstrating a significant decline in NVIQ between 12 and 36 months while the non-ASD group showed gains in both full DQ and VIQ between 12 and 36 months.
At age 6 months, the delay in the visual domain translated to deficits in visually mediated social behaviors, such as visual tracking and disengagement of attention, as opposed to more language-based behaviors such as social babbling or orienting to name. Such domain specificity might be explained by the TSC mouse model, where there is evidence of aberrant structural connectivity in visual projections, which appear more diffuse and less organized.21 Furthermore, a recent electrophysiologic study also demonstrated atypical face processing in children with TSC compared with typically developing children.22 It is possible that, in TSC, disorganization of visual pathways leads to impairment in early visual perception, which, in turn, places these infants at higher risk of deficits in social function. Because of our small sample size at 6 months of age, we could not analyze VR based on ASD grouping, but continuing studies will characterize visual perception both behaviorally and electrophysiologically in infants with TSC.
While high, the rate of ASD diagnosis in 55% of our infants does lie within the range reported in the literature. Our sample represents a more clinically affected population, because most infants had the clinical sequelae of TSC that prompted an early diagnosis. Ninety-five percent of our sample had epilepsy, with 65% reporting infantile spasms. While there were trends toward greater epilepsy severity in our ASD sample, only the number of antiepileptics significantly differentiated the groups, with the ASD group taking more antiepileptics than the non-ASD group. These data suggest that clinical variables alone cannot predict the development of ASD, despite the strong association between ASD and epilepsy reported in children with TSC.5,23–28
Trajectories of cognitive function may serve as a promising predictor of ASD in TSC. A prospective study of infants at high risk of ASD (infant siblings of children with ASD) found that development in the first 2 years of life, as measured by the MSEL, improved in all 3 outcome groups—ASD, language delayed, and non-ASD—but that the rate of developmental gain was slowest in the ASD group.29 The same group also quantified MSEL trajectories to investigate clusters within the high-risk group using latent class analysis.30 Although none of the clusters defined in their data exhibited declines in NVIQ, one cluster was identified as the “developmental slowing” group, where children increasingly diverged from age-based norms over time in all domains. Our sample similarly shows a slowing of development in the VR and FM domains, because infants with TSC/ASD fail to acquire age-appropriate skills in the second and third years of life. This phenomenon is distinct from a regression, because we did not identify a loss of skills in infants at any age. A future analysis of individual raw scores and their relation to seizure severity could be clinically informative, because regressions in the setting of epilepsy have been reported in young children with TSC.8,31,32
The fact that the decline is specific to NVIQ in the TSC/ASD group warrants further consideration, because one might expect the language domain to be most affected in infants at risk of ASD. However, in TSC, deficits in VR and FM function may undermine the development of nonverbal communication, which, in turn, compromises the development of typical social behavior. The decline in nonverbal ability may represent a TSC-specific pathway to ASD, rooted in early deficits in VR and FM skills seen at 6 months of age.
In the ASD group, children with greater seizure duration had lower NVIQ. In infants who are particularly vulnerable to aberrant development, epilepsy may further impair cognitive and social function. The fact that some children with ASD showed stable development or even developmental gains from years 1 to 3 supports the contention that there is likely a dynamic association among developmental delay, seizures, and ASD. However, whether epilepsy is causal or epiphenomenonal of aberrant development warrants further investigation. Large prospective studies of epileptogenesis and autism in infants with TSC are currently addressing such questions (clinicaltrials.gov: NCT01780441; NCT01767779).
To our knowledge, despite the high rate of ASD, no early behavioral intervention studies have targeted infants with TSC. Case reports of early intervention have documented an improvement in cognition and behavior in toddlers and young children with other neurogenetic syndromes, such as Down syndrome and fragile X.33,34 Thus, the fact that infants with TSC have a clear genetic and neurobiological basis for their delayed development may not preclude the potential efficacy of early intervention, particularly as we identify specific developmental domains associated with ASD. By age 12 months, nonverbal ability can place children with TSC in risk categories for ASD and, at that time, one could argue that interventions targeting not only social communication function, but also nonverbal cognitive function, particularly in the area of VR, may improve developmental trajectories. These data support the need for intervention research focused on these high-risk infants.
One limitation of our study was the small sample size in the first year of life, thereby limiting our ability to investigate earlier behavioral predictors of ASD. In addition, we did not collect detailed early intervention data from our participants, partly because the quality and access to services varied greatly in our national sample. While unlikely given their young age, existing interventions could have an impact on developmental trajectories. Finally, because of the variable age of enrollment, our longitudinal data are cross-sectional at each time point. With a larger sample size, we can investigate individual differences in developmental trajectories that may shed more light on the dynamic interplay of clinical, biological, and behavioral factors that lead to an ASD diagnosis in infants with TSC.
In this prospective study, we have characterized developmental markers of ASD in infants with TSC. The early delay in VR and FM ability in the TSC group, coupled with the decline in nonverbal ability in infants diagnosed with ASD, suggests a domain-specific pathway to ASD that can inform more targeted interventions for these high-risk infants.
ACKNOWLEDGMENT
The authors acknowledge the efforts of Tessa Clarkson, Geneva Degregorio, Angela Martinez, Danielle Escobedo, and Elizabeth Baker in recruitment, data collection, and processing.
GLOSSARY
- ADOS
Autism Diagnostic Observation Schedule
- AOSI
Autism Observation Scale for Infants
- ASD
autism spectrum disorder
- DQ
developmental quotient
- EL
expressive language
- FM
fine motor
- GM
gross motor
- IRB
institutional review board
- MSEL
Mullen Scales of Early Learning
- NVIQ
nonverbal IQ
- RL
receptive language
- TSC
tuberous sclerosis complex
- UCLA
University of California, Los Angeles
- VIQ
verbal IQ
- VR
visual reception
AUTHOR CONTRIBUTIONS
Dr. Shafali Jeste was responsible for study design, supervision of data acquisition, behavioral and clinical data analysis, and manuscript preparation. Dr. Joyce Wu was actively involved in study design, analysis of the clinical data, manuscript preparation, and revisions. Dr. Damla Senturk was primarily responsible for statistical analysis of data and contributed to manuscript preparation. Dr. Kandice Varcin was involved in processing and analysis of clinical data and manuscript revisions. Jordan Ko was primarily responsible for data collection and preparation of figures for the manuscript. Brigid McCarthy was involved with data collection and analysis of behavioral data, as well as manuscript review. Christina Shimizu was involved with data collection and processing of behavioral data, as well as manuscript review. Kira Dies was involved with study implementation and data collection, as well as manuscript review. Vanessa Vogel-Farley was involved with study design, study implementation and data processing, as well as manuscript review. Dr. Mustafa Sahin was involved with study design and manuscript preparation. Dr. Charles A. Nelson III was involved with study design, study implementation, and manuscript preparation and revisions.
STUDY FUNDING
Supported by the Department of Defense (DOD CDMRP TSCRP: 2011–2014) and UCLA CTSI (UL1RR033176).
DISCLOSURE
S. Jeste has served on the professional advisory board for the Tuberous Sclerosis Alliance. She receives research support from the Department of Defense (DOD CDMRP TSCRP) and the NIH (NIMH P30 MH089901-1, NIMH 1K34MH094517, NIH/NIMH RO1 MH0028, NICHD 2P50HD055784-06). She has also received support from the Child Neurology Foundation, the American Academy of Neurology, and Autism Speaks. J. Wu has served on the professional advisory board for the Tuberous Sclerosis Alliance and has received honoraria from and has served on the scientific advisory board and speakers bureau for Novartis Pharmaceutical Inc. and Lundbeck. She has received research support from the Tuberous Sclerosis Alliance, Today's and Tomorrow's Children Fund, Novartis Pharmaceuticals Inc., Department of Defense/Congressionally Directed Medical Research Program, and the NIH (K23 NS051637, P20 NS080199, U01 NS082320, R34 MH089299, and R01 NS082649). D. Senturk, K. Varcin, J. Ko, B. McCarthy, T. Shimizu, K. Dies, and V. Vogel-Farley report no disclosures relevant to the manuscript. M. Sahin has served on the scientific advisory board for the Tuberous Sclerosis Alliance, and has received research support from Children's Hospital Boston Translational Research Program, Autism Speaks, the Tuberous Sclerosis Alliance, Novartis Pharmaceuticals Inc., Hoffmann-La Roche, Shire, Department of Defense/Congressionally Directed Medical Research Program, the Nancy Lurie Marks Foundation, and the NIH (NIH U01 NS082320 and NIH/NICHD P30 HD018655-31). C. Nelson III has received and receives funding from Autism Speaks, the Department of Defense, the Simons Foundation, the Thrasher Foundation, the MacArthur Foundation, and the NIH. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 2006;355:1345–1356 [DOI] [PubMed] [Google Scholar]
- 2.Orlova KA, Crino PB. The tuberous sclerosis complex. Ann NY Acad Sci 2010;1184:87–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann NY Acad Sci 1991;615:125–127 [DOI] [PubMed] [Google Scholar]
- 4.Smalley SL. Autism and tuberous sclerosis. J Autism Dev Disord 1998;28:407–414 [DOI] [PubMed] [Google Scholar]
- 5.Curatolo P, Napolioni V, Moavero R. Autism spectrum disorders in tuberous sclerosis: pathogenetic pathways and implications for treatment. J Child Neurol 2010;25:873–880 [DOI] [PubMed] [Google Scholar]
- 6.Wiznitzer M. Autism and tuberous sclerosis. J Child Neurol 2004;19:675–679 [DOI] [PubMed] [Google Scholar]
- 7.Jeste SS, Sahin M, Bolton P, Ploubidis GB, Humphrey A. Characterization of autism in young children with tuberous sclerosis complex. J Child Neurol 2008;23:520–525 [DOI] [PubMed] [Google Scholar]
- 8.Humphrey A, Neville BG, Clarke A, Bolton PF. Autistic regression associated with seizure onset in an infant with tuberous sclerosis. Dev Med Child Neurol 2006;48:609–611 [DOI] [PubMed] [Google Scholar]
- 9.Deonna T, Roulet-Perez E, Chappuis H, Ziegler AL. Autistic regression associated with seizure onset in an infant with tuberous sclerosis. Dev Med Child Neurol 2007;49:320. [PubMed] [Google Scholar]
- 10.Bombardieri R, Pinci M, Moavero R, Cerminara C, Curatolo P. Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol 2010;14:146–149 [DOI] [PubMed] [Google Scholar]
- 11.Datta AN, Hahn CD, Sahin M. Clinical presentation and diagnosis of tuberous sclerosis complex in infancy. J Child Neurol 2008;23:268–273 [DOI] [PubMed] [Google Scholar]
- 12.Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics 2011;128:e488–e495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwaigenbaum L. Advances in the early detection of autism. Curr Opin Neurol 2010;23:97–102 [DOI] [PubMed] [Google Scholar]
- 14.Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature 2013;504:427–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullen EM. Mullen Scales of Early Learning: AGS Edition. Circle Pines, MN: American Guidance Service; 1995 [Google Scholar]
- 16.Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The Autism Observation Scale for Infants: scale development and reliability data. J Autism Dev Disord 2008;38:731–738 [DOI] [PubMed] [Google Scholar]
- 17.Brian J, Bryson SE, Garon N, et al. Clinical assessment of autism in high-risk 18-month-olds. Autism 2008;12:433–456 [DOI] [PubMed] [Google Scholar]
- 18.Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205–223 [PubMed] [Google Scholar]
- 19.Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord 2007;37:613–627 [DOI] [PubMed] [Google Scholar]
- 20.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord 2009;39:693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nie D, Di Nardo A, Han JM, et al. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci 2010;13:163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeste SS, Hirsch S, Vogel-Farley V, et al. Atypical face processing in children with tuberous sclerosis complex. J Child Neurol 2012;28:1569–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolton PF, Park RJ, Higgins JN, Griffiths PD, Pickles A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain 2002;125:1247–1255 [DOI] [PubMed] [Google Scholar]
- 24.Jambaque I, Cusmai R, Curatolo P, Cortesi F, Perrot C, Dulac O. Neuropsychological aspects of tuberous sclerosis in relation to epilepsy and MRI findings. Dev Med Child Neurol 1991;33:698–705 [DOI] [PubMed] [Google Scholar]
- 25.Mettin RR, Merkenschlager A, Bernhard MK, et al. Wide spectrum of clinical manifestations in children with tuberous sclerosis complex: follow-up of 20 children. Brain Dev 2014;36:306–314 [DOI] [PubMed] [Google Scholar]
- 26.de Vries PJ, Hunt A, Bolton PF. The psychopathologies of children and adolescents with tuberous sclerosis complex (TSC): a postal survey of UK families. Eur Child Adolesc Psychiatry 2007;16:16–24 [DOI] [PubMed] [Google Scholar]
- 27.de Vries PJ, Prather PA. The tuberous sclerosis complex. N Engl J Med 2007;356:92. [DOI] [PubMed] [Google Scholar]
- 28.Cusmai R, Moavero R, Bombardieri R, Vigevano F, Curatolo P. Long-term neurological outcome in children with early-onset epilepsy associated with tuberous sclerosis. Epilepsy Behav 2011;22:735–739 [DOI] [PubMed] [Google Scholar]
- 29.Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry 2006;47:629–638 [DOI] [PubMed] [Google Scholar]
- 30.Landa RJ, Gross AL, Stuart EA, Bauman M. Latent class analysis of early developmental trajectory in baby siblings of children with autism. J Child Psychol Psychiatry 2012;53:986–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Callaghan FJ, Harris T, Joinson C, et al. The relation of infantile spasms, tubers, and intelligence in tuberous sclerosis complex. Arch Dis Child 2004;89:530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korman B, Krsek P, Duchowny M, Maton B, Pacheco-Jacome E, Rey G. Early seizure onset and dysplastic lesion extent independently disrupt cognitive networks. Neurology 2013;81:745–751 [DOI] [PubMed] [Google Scholar]
- 33.Wright CA, Kaiser AP, Reikowsky DI, Roberts MY. Effects of a naturalistic sign intervention on expressive language of toddlers with Down syndrome. J Speech Lang Hear Res 2013;56:994–1008 [DOI] [PubMed] [Google Scholar]
- 34.Winarni TI, Schneider A, Borodyanskara M, Hagerman RJ. Early intervention combined with targeted treatment promotes cognitive and behavioral improvements in young children with fragile X syndrome. Case Rep Genet 2012;2012:280813. [DOI] [PMC free article] [PubMed] [Google Scholar]