Abstract
Objectives:
The purpose of this study was to elucidate the factors that correlate with unfavorable outcomes and to develop a simple validated model for assessing risk of unfavorable outcomes in patients with minor ischemic stroke.
Methods:
The derivation cohort included 1,313 patients hospitalized within 72 hours after onset with an initial NIH Stroke Scale score of 0 to 3 enrolled in a prospective, multicenter, observational study. Unfavorable outcome was defined as dependency (modified Rankin Scale score of 3–5) or death at 90 days. The predictive values of factors related to unfavorable outcome were evaluated. External validation was performed in 879 patients from a single-center stroke registry.
Results:
In the derivation cohort, a total of 203 patients (15%) had unfavorable outcomes. On multivariable analysis, women (odds ratio [OR] 1.95, 95% confidence interval [CI] 1.30–2.94), age ≥72 years (OR 2.80, 95% CI 1.83–4.36), intra/extracranial vascular occlusive lesion (OR 2.80, 95% CI 1.82–4.28), leg weakness (OR 1.72, 95% CI 1.06–2.82), and extinction/inattention (OR 5.55, 95% CI 1.30–21.71) were independently associated with unfavorable outcome. Patients having both a vascular lesion and either leg weakness or extinction/inattention showed 4.63 (95% CI 2.23–9.33) times the risk of unfavorable outcome compared with those having neither. In the validation cohort, the risk was similar, at 3.77 (95% CI 1.64–8.37).
Conclusions:
Intra- and extracranial vascular imaging, NIH Stroke Scale items such as leg weakness and extinction/inattention, and their combination, as well as female sex and advanced age, may be useful for predicting unfavorable outcomes in patients with minor stroke.
Minor strokes account for approximately 30% of all strokes.1,2 Although the outcomes of most patients with minor symptoms, epitomized by a low NIH Stroke Scale (NIHSS) score, are favorable, a small but significant number of such patients become disabled.3–7 Thus, it is important to identify the patients at risk of unfavorable functional outcomes in the early stage in the clinical setting.
It has been reported that abnormal CT or MRI findings such as acute infarction and vascular stenosis or occlusion are associated with functional dependency at 90 days in patients with minor stroke and TIA.8–11 Although the baseline NIHSS score is also associated with functional outcome in overall patients with stroke,3 it has a significant weakness regarding capturing lesion-specific neurologic deficits, such as those with right-sided and posterior circulation lesions.12,13 There is limited information about the clinical significance of using NIHSS scores with minor stroke.
The purpose of the present study was to elucidate the factors that correlate with unfavorable outcomes and to develop a simple validated model for assessing risk of unfavorable outcomes in patients with minor stroke.
METHODS
Derivation cohort.
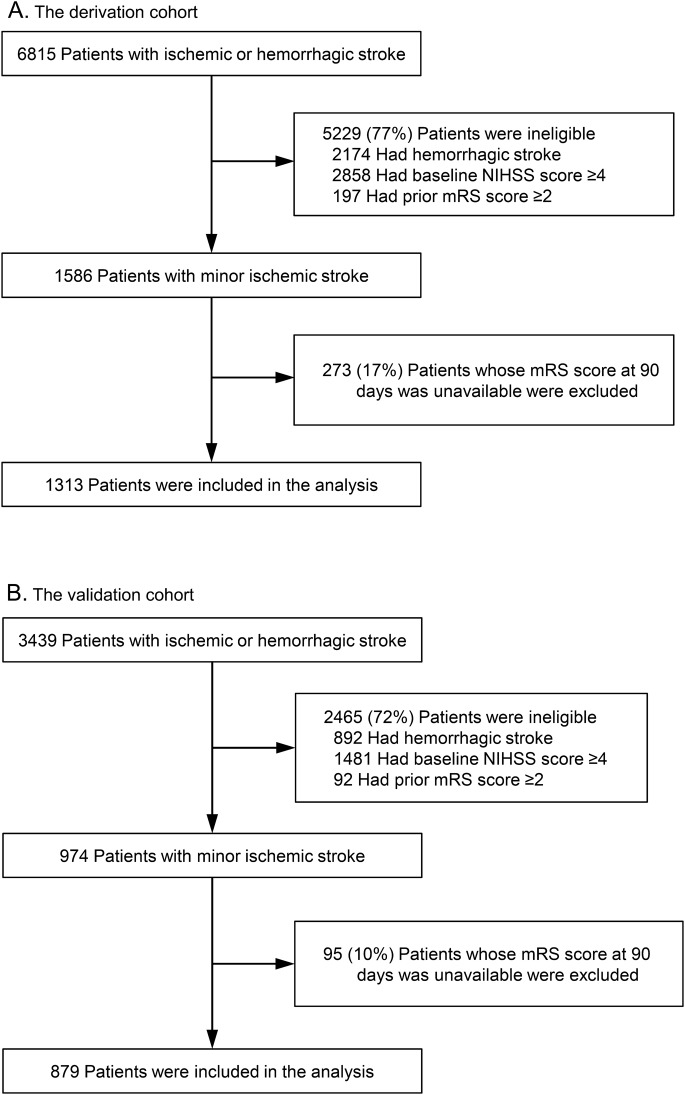
The derivation cohort was selected from the Stroke Unit Multicenter Observational (SUMO) Study, a prospective, observational, cohort study conducted between December 2004 and December 2005 in Japan.14 Its aim was to clarify diagnostic and therapeutic processes of acute stroke care that are effective for improving clinical outcomes. Eighty-four representative acute institutes participated in the SUMO Study. A total of 6,815 consecutive patients with ischemic and hemorrhagic stroke admitted within 72 hours after symptom onset were registered. Of these, patients with minor ischemic strokes, defined as a baseline total NIHSS score ≤3, were enrolled as the derivation cohort (figure 1A).
Figure 1. Flowchart of patient selection in the derivation (A) and validation (B) cohorts.
mRS = modified Rankin Scale; NIHSS = NIH Stroke Scale.
Validation cohort.
The National Cerebral and Cardiovascular Center (NCVC) Stroke Registry15,16 is a prospective database of patients with acute stroke treated in our stroke care unit. Data of 3,439 patients with ischemic and hemorrhagic stroke who were admitted within 7 days after onset between January 2006 and December 2012 were extracted from this database. Of these, patients with minor ischemic strokes, defined as a baseline total NIHSS score ≤3, were registered as the derivation cohort (figure 1B).
Clinical characteristics.
Baseline data including sex, age, onset to admission time, comorbidities, and risk factors (hypertension, diabetes, dyslipidemia, arrhythmia including atrial fibrillation, and previous all strokes/TIA) were collected from both cohorts.
An intra/extracranial vascular occlusive lesion was defined as a ≥50% narrowing in diameter or occlusion verified by imaging modalities such as magnetic resonance angiography, conventional digital subtraction angiography, or carotid duplex sonography.
Each NIHSS item was dichotomized as normal (subscore of 0) or abnormal (subscore ≥1). The sum of the right and left scores was used for motor arm and motor leg items.17
Patients who were disabled before the stroke (corresponding to a modified Rankin Scale [mRS] score ≥2) were excluded from the analysis. An unfavorable outcome was defined as an mRS score of 3 to 6 (dependency or death) at 90 days.18 The outcome corresponding to an mRS score of 2 to 6 at 90 days was also assessed.
Statistical analysis.
Statistical analysis was performed using JMP 10.0.2 (SAS Institute Inc., Cary, NC). Baseline characteristics were compared between favorable and unfavorable outcomes using χ2 tests, unpaired t tests, Fisher exact tests, or Wilcoxon/Kruskal-Wallis tests. To identify the cutoffs of variables that could be used for discriminating between favorable and unfavorable outcomes, receiver operating characteristic curves were constructed. The odds ratios (ORs) for variables associated with unfavorable outcomes were determined using multivariable logistic regression analyses by the forced entry method adjusted for variables with p < 0.05 on univariate analyses. A value of p < 0.05 was considered significant for all results.
Standard protocol approvals, registrations, and patient consents.
Each institutional Ethics and Hospital Management Committee approved the studies from the SUMO Study database. Written informed consent to participate in the study was obtained from the patient whenever possible; acceptance from a relative was obtained if patients could not consent themselves. The Regional Ethics and Hospital Management Committees of NCVC approved the studies from the NCVC Stroke Registry. The Ethics Committee waived consent because this was a registry study.
RESULTS
A total of 1,313 patients were included in the analysis as the derivation cohort. Their mean age was 69 years, and 433 (33%) were women. Of these patients, 203 (15%) had unfavorable outcomes at 90 days, and 54 (4%) had recurrent stroke (ischemic or hemorrhagic) before 28 days or discharge. Magnetic resonance angiography, digital subtraction angiography, and carotid duplex sonography were performed in 1,208 (92%), 105 (8%), and 945 (72%) patients within 7 days of hospitalization, respectively.
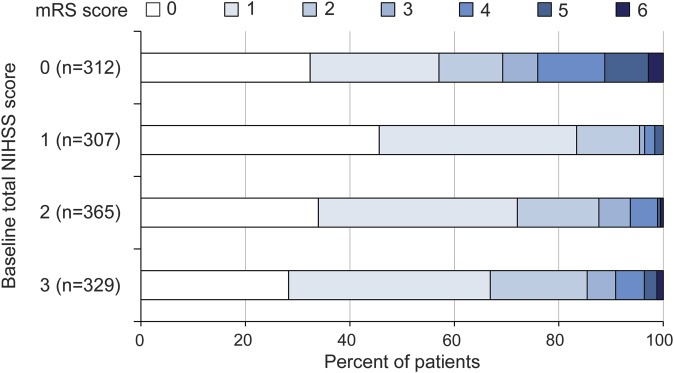
The patients' characteristics were compared between patients with and without unfavorable outcomes in the derivation cohort (table 1). Patients with unfavorable outcomes were older (p < 0.001), more frequently female (p < 0.001), had a higher percentage of onset to admission time ≤12 hours (p = 0.001), less frequently had dyslipidemia (p = 0.015), and more often had an intra/extracranial vascular occlusive lesion (p < 0.001) than those with favorable outcomes. The median total NIHSS score was 1 (interquartile range 0–2) in patients with unfavorable outcomes and 2 (interquartile range 1–3) in those with favorable outcomes (p < 0.001). Patients with unfavorable outcomes more frequently had leg weakness (p = 0.002) and extinction/inattention (p = 0.024) and less frequently had facial palsy (p = 0.013) and sensory (p = 0.027) manifestations than those with favorable outcomes. Figure 2 shows the distribution of mRS scores according to baseline total NIHSS score in the derivation cohort.
Table 1.
Patients' characteristics in the derivation cohort
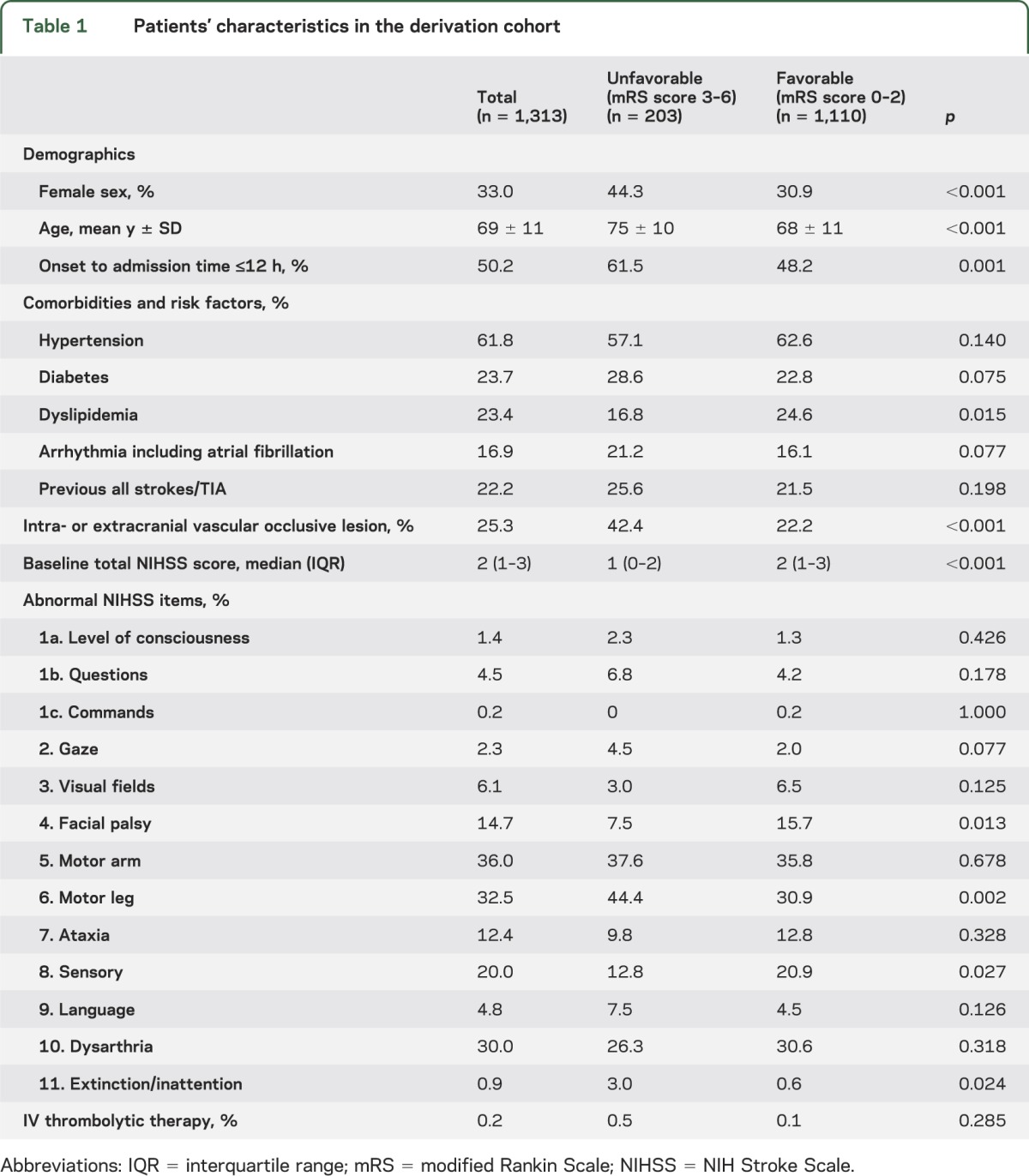
Figure 2. mRS score according to baseline total NIHSS score in the derivation cohort.
mRS = modified Rankin Scale; NIHSS = NIH Stroke Scale.
The optimal cutoff age for an unfavorable outcome was ≥72 years, with a sensitivity of 67%, a specificity of 58%, and an area under the receiver operating characteristic curve of 0.666. Table 2 shows the results of the multivariable analysis for unfavorable outcome (mRS score of 3–6) at 90 days in the derivation cohort. Female sex (OR 1.95, 95% CI 1.30–2.94), age ≥72 years (OR 2.80, 95% CI 1.83–4.36), intra/extracranial vascular occlusive lesion (OR 2.80, 95% CI 1.82–4.28), leg weakness (OR 1.72, 95% CI 1.06–2.82), and extinction/inattention (OR 5.55, 95% CI 1.30–21.71) were independently associated with unfavorable outcome after adjusting for baseline total NIHSS score. In contrast, the baseline total NIHSS score was not independently associated with unfavorable outcome.
Table 2.
Multivariable analysis for unfavorable outcome (mRS score 3–6) at 90 days in the derivation cohort
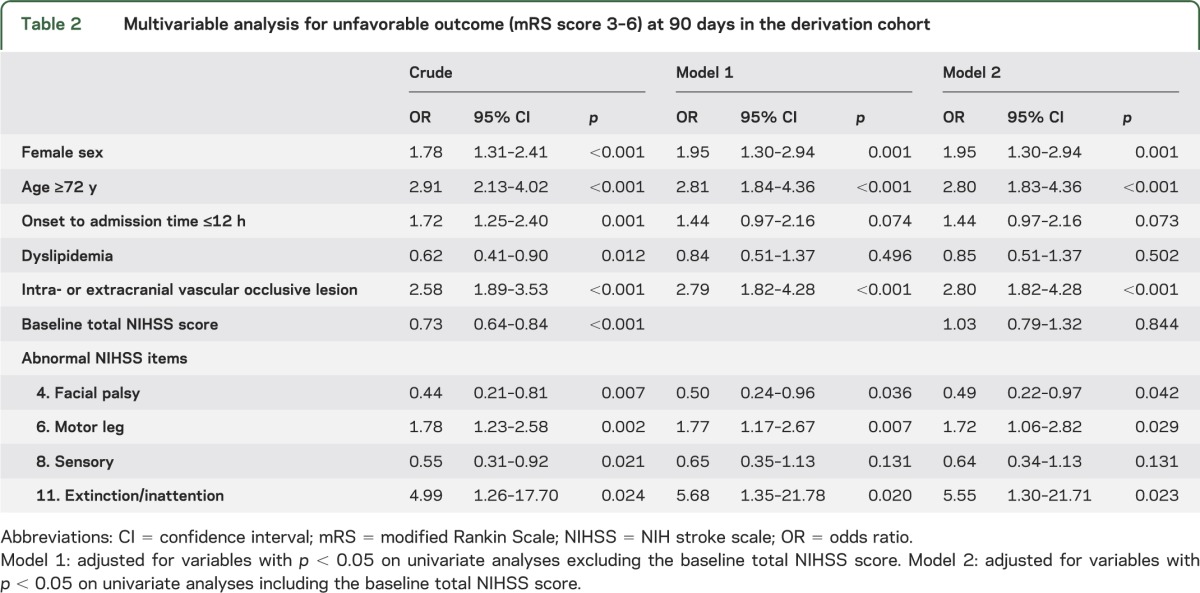
In the derivation cohort, outcomes for 17 of 65 patients (26%) having both an intra/extracranial vascular occlusive lesion and abnormal NIHSS items for either leg weakness or extinction/inattention were unfavorable. Patients with both factors had 4.63 (95% CI 2.23–9.33, p < 0.001) times the risk of an unfavorable outcome compared with those having neither after adjustment for sex, age, and other essential characteristics (figure 3A). The foregoing model was also applied to patients with baseline NIHSS scores of 0 to 4 (n = 1,941) and 0 to 5 (n = 2,233) in the SUMO Study cohort. Patients with both factors had 7.53 (95% CI 4.47–12.66, p < 0.001) and 6.96 (95% CI 4.39–11.02, p < 0.001) times the risk of an unfavorable outcome, respectively (figure e-1 on the Neurology® Web site at Neurology.org). The analysis was also performed for the outcome corresponding to mRS scores of 2 to 6. Patients with both factors had 2.72 (95% CI 1.50–4.86, p = 0.001) times the risk of having mRS scores of 2 to 6 at 90 days compared with those having neither after adjustment for the same factors. In addition, sensitivity analysis including missing follow-up patients as having an “unfavorable outcome” was performed. Patients having both factors showed 3.15 (95% CI 1.97–5.06) times the risk for an unfavorable outcome compared with those having neither.
Figure 3. Combined effects of intra/extracranial vascular occlusive lesion and leg weakness or extinction/inattention on unfavorable outcome.
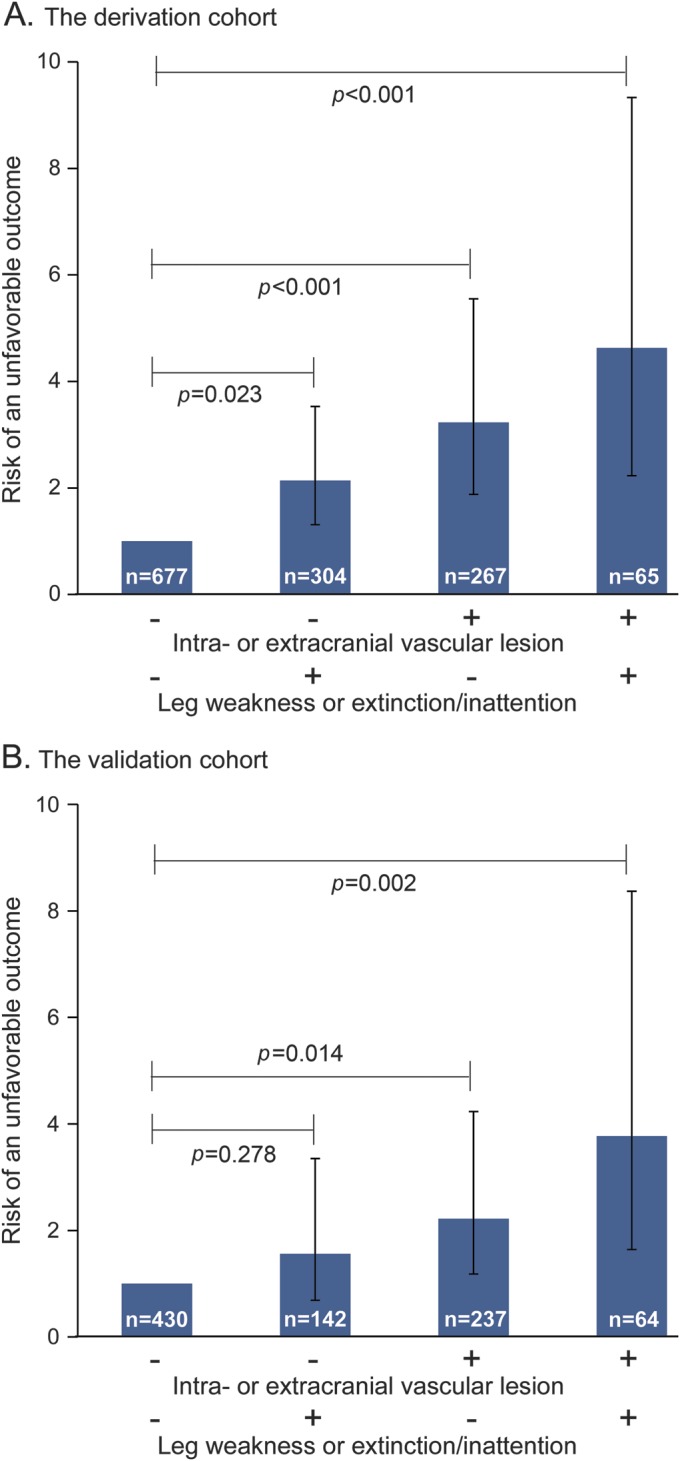
Risk of an unfavorable outcome was calculated using “patients without both” as the reference, adjusting for female sex, age ≥72 years, onset to admission time ≤12 hours, dyslipidemia, facial palsy, and sensory items of the NIH Stroke Scale in both cohorts.
The patients' characteristics included in the derivation and validation cohorts (n = 879) are shown in table e-1. In the validation cohort, the frequency of hypertension (p < 0.001), diabetes mellitus (p = 0.037), dyslipidemia (p < 0.001), previous all strokes/TIA (p = 0.040), intra- or extracranial vascular occlusive lesion (p < 0.001), and IV thrombolytic therapy (p = 0.018) were significantly higher than in the derivation cohort. Baseline total NIHSS score (p < 0.001) was also higher in the validation cohort than in the derivation cohort. The frequencies of NIHSS item abnormalities also differed between the cohorts. Level of consciousness questions (p = 0.005), motor arm (p < 0.001), motor leg (p = 0.002), and ataxia (p = 0.033) were higher in the derivation cohort. Facial palsy (p < 0.001), sensory (p < 0.001), and dysarthria (p < 0.001) were higher in the validation cohort. Fewer patients had unfavorable outcomes at 90 days in the validation cohort than in the derivation cohort (p < 0.001). Outcomes for 12 of 64 patients (19%) having both an intra/extracranial vascular occlusive lesion and abnormal NIHSS items for either leg weakness or extinction/inattention were unfavorable. Patients with both factors had 3.77 (95% CI 1.64–8.37, p = 0.002) times the risk of an unfavorable outcome compared with those having neither after adjustment for the same factors as the derivation cohort (figure 3B).
DISCUSSION
In the analysis of patients with minor stroke in the derivation cohort, it was found that (1) NIHSS leg weakness and inattention/extinction items were independently related to unfavorable outcomes; (2) advanced age, female sex, and intra/extracranial vascular occlusive lesion were independently associated with unfavorable outcomes after adjusting for NIHSS items; and (3) baseline total NIHSS score was not independently associated with outcomes in patients with minor stroke. A simple model based on the analysis of the derivation cohort was developed; the combination of intra/extracranial vascular occlusive lesion and leg weakness or extinction/inattention could be used to assess risk of unfavorable outcomes, even after adjusting for other significant factors including sex and advanced age. Predictability of this simple model was validated using our single-center cohort. Although several outcome prediction models for ischemic stroke patients, especially those receiving IV thrombolysis, have been reported,19–22 this study is unique in that an outcome prediction model for minor ischemic stroke patients was proposed.
It has been reported that advanced age,6,10 female sex,6,11 diabetes mellitus,11 and intra- or extracranial vascular occlusive lesion8,9,23 were the factors related to unfavorable outcomes in patients with minor strokes. The present results are generally in line with the previous reports. However, individual NIHSS items were not included in these reports. A recent study involving patients with minor stroke, defined as retrospectively assessed NIHSS scores ≤5, showed that patients with an NIHSS profile represented by signs of slurred speech and language deficit had the worst outcome.7 The study did not analyze each NIHSS item. In addition, the total baseline NIHSS score was reported to be inversely related to favorable outcome without significant associations between individual NIHSS items and outcome in a small study involving 194 patients with minor stroke with NIHSS scores ≤6,24 whose vascular status was not assessed.
Previous studies reported that leg weakness and extinction/inattention were major determinants of worse outcome.25–27 One possible reason for the leg weakness outcome is that the mRS, which is highly focused on walking ability, was used for outcome assessment.18,28 In a study that examined angiographic occlusion in the first hours after onset of stroke, leg weakness and extinction/inattention were related to major vessel occlusion.29 The presence of these items might indicate large hemispheric ischemia resulting only from proximal major vessel occlusion, not from peripheral vessel occlusions. In the present study, it was not possible to capture the percentage of each individual arterial occlusive lesion, because individual angiographic data were not collected in the derivation cohort. It seems paradoxical that facial palsy was inversely associated with unfavorable outcome. A potential reason for this may be that patients with facial palsy less frequently had leg weakness (34% vs 25%, p = 0.030) in the derivation cohort, because the total NIHSS score of the studied patients was limited to 3 or less.
The results suggest that a combination of NIHSS items and assessment of vascular status could help predict unfavorable outcomes in patients with minor stroke. A large-scale observational study reported that 31% of 93,517 acute stroke patients with rapidly improving or mild symptoms as the only reason for avoiding recombinant tissue-type plasminogen activator (rt-PA) were unable to be discharged home.6 Recently, it has been reported that 890 rt-PA–treated patients with mild symptoms corresponding to NIHSS scores of 0 to 5 had better outcomes at 3 months than matched controls without rt-PA.30 In these studies, onset to admission time was shorter than in the present study. In addition, NIHSS subscores and vascular status were not described. However, patients with both factors might be highly appropriate candidates for acute intensive management, such as recanalization therapy, if they appear within the therapeutic time window, regardless of age, sex, or low total NIHSS score.
One of the strengths of the present study is that it included multicenter and single-center prospective databases of minor ischemic stroke patients, excluding patients with TIA. Data such as individual NIHSS items and vascular imaging findings were systematically collected and analyzed using multivariable models. The validation cohort was from a single, highly specialized stroke center. Although there were significant differences between the cohorts in risk factors, vascular status, severity, and outcome, the model was successfully validated. That is another strength of the study.
The present study had some limitations. First, the mRS score at 90 days was not available for all patients. The follow-up rate of 83% does not preclude a possible bias. Patients' characteristics were generally similar between patients with available mRS scores at 90 days (n = 1,313) and those without (n = 273). When the mRS score at acute hospital discharge (median 17 days after onset) or at 28 days was used instead of the 90-day mRS score for these 273 patients, the results were almost identical; for example, patients having both a vascular occlusive lesion and either of the abnormal NIHSS items showed 4.43 (95% CI 2.36–8.13) times the risk of unfavorable outcome compared with those having neither. Second, the current population consisted mostly of Asian patients. It is known that there are differences in the distribution of occlusive vascular disease between Asian and other populations. Intracranial artery atherosclerosis is a common cause of ischemic stroke in Asian, African, and Hispanic patients, while extracranial carotid artery atherosclerosis develops frequently in Caucasians.31 Unfortunately, the present database did not include detailed information on the location of vascular lesions, so it was impossible to assess the distribution of occlusive vascular disease. Racial differences might affect the results and limit the generalizability. Third, the rate of rt-PA use was extremely low in the present 2 cohorts. One possible reason for this is that the use of rt-PA as a therapy for acute ischemic stroke within 3 hours of onset was approved on October 11, 2005, when registration in the SUMO Study was almost completed.14 Another reason is that the Japanese Guidelines for IV Application of rt-PA (alteplase), October 2005, stated that most patients with NIHSS scores of 4 or less were considered ineligible for rt-PA therapy.32 The model was not validated in an rt-PA–treated cohort, but this should be considered for future studies.
Several independent factors that could be assessed at the time of initial presentation were found to be associated with unfavorable outcome at 90 days in patients with minor stroke. The combination of intra- and extracranial vascular imaging and NIHSS items such as leg weakness and extinction/inattention appears to provide valuable information related to outcomes.
Supplementary Material
GLOSSARY
- CI
confidence interval
- mRS
modified Rankin Scale
- NCVC
National Cerebral and Cardiovascular Center
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- rt-PA
recombinant tissue-type plasminogen activator
- SUMO
Stroke Unit Multicenter Observational
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Sato conceived and designed the study, drafted the manuscript, and performed the statistical analyses. Dr. Uehara, Dr. Ohara, Dr. Suzuki, and Dr. Toyoda made critical revisions to the manuscript for important intellectual content. Dr. Minematsu supervised and obtained study funding.
STUDY FUNDING
Supported in part by Grants-in-Aid (H16-Junkanki-Chiho-Kossetsu-023 and H24-Junkanki-Ippan-011) from the Ministry of Health, Labor and Welfare, Japan.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ferrari J, Knoflach M, Kiechl S, et al. Early clinical worsening in patients with TIA or minor stroke: the Austrian Stroke Unit Registry. Neurology 2010;74:136–141 [DOI] [PubMed] [Google Scholar]
- 2.Ayis SA, Coker B, Rudd AG, Dennis MS, Wolfe CD. Predicting independent survival after stroke: a European study for the development and validation of standardised stroke scales and prediction models of outcome. J Neurol Neurosurg Psychiatry 2013;84:288–296 [DOI] [PubMed] [Google Scholar]
- 3.Adams HP, Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126–131 [DOI] [PubMed] [Google Scholar]
- 4.Kimura K, Kazui S, Minematsu K, Yamaguchi T. Analysis of 16,922 patients with acute ischemic stroke and transient ischemic attack in Japan: a hospital-based prospective registration study. Cerebrovasc Dis 2004;18:47–56 [DOI] [PubMed] [Google Scholar]
- 5.Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke 2005;36:2497–2499 [DOI] [PubMed] [Google Scholar]
- 6.Smith EE, Fonarow GC, Reeves MJ, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from Get With the Guidelines-Stroke. Stroke 2011;42:3110–3115 [DOI] [PubMed] [Google Scholar]
- 7.Sucharew H, Khoury J, Moomaw CJ, et al. Profiles of the National Institutes of Health Stroke Scale items as a predictor of patient outcome. Stroke 2013;44:2182–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutts SB, Simon JE, Eliasziw M, et al. Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann Neurol 2005;57:848–854 [DOI] [PubMed] [Google Scholar]
- 9.Nedeltchev K, Schwegler B, Haefeli T, et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke 2007;38:2531–2535 [DOI] [PubMed] [Google Scholar]
- 10.Coutts SB, O'Reilly C, Hill MD, et al. Computed tomography and computed tomography angiography findings predict functional impairment in patients with minor stroke and transient ischaemic attack. Int J Stroke 2009;4:448–453 [DOI] [PubMed] [Google Scholar]
- 11.Coutts SB, Modi J, Patel SK, et al. What causes disability after transient ischemic attack and minor stroke? Results from the CT and MRI in the Triage of TIA and minor Cerebrovascular Events to Identify High Risk Patients (CATCH) Study. Stroke 2012;43:3018–3022 [DOI] [PubMed] [Google Scholar]
- 12.Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke 1999;30:2355–2359 [DOI] [PubMed] [Google Scholar]
- 13.Sato S, Toyoda K, Uehara T, et al. Baseline NIH Stroke Scale score predicting outcome in anterior and posterior circulation strokes. Neurology 2008;70:2371–2377 [DOI] [PubMed] [Google Scholar]
- 14.Sato S, Uehara T, Toyoda K, et al. Impact of the approval of intravenous recombinant tissue plasminogen activator therapy on the processes of acute stroke management in Japan: the Stroke Unit Multicenter Observational (SUMO) Study. Stroke 2009;40:30–34 [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto Y, Sato S, Kuronuma Y, Nagatsuka K, Minematsu K, Toyoda K. Factors associated with proximal carotid axis occlusion in patients with acute stroke and atrial fibrillation. J Stroke Cerebrovasc Dis Epub 2013 Oct 5 [DOI] [PubMed]
- 16.Sato S, Uehara T, Hayakawa M, Nagatsuka K, Minematsu K, Toyoda K. Intra- and extracranial atherosclerotic disease in acute spontaneous intracerebral hemorrhage. J Neurol Sci 2013;332:116–120 [DOI] [PubMed] [Google Scholar]
- 17.Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994;25:2220–2226 [DOI] [PubMed] [Google Scholar]
- 18.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607 [DOI] [PubMed] [Google Scholar]
- 19.Ntaios G, Faouzi M, Ferrari J, Lang W, Vemmos K, Michel P. An integer-based score to predict functional outcome in acute ischemic stroke: the ASTRAL score. Neurology 2012;78:1916–1922 [DOI] [PubMed] [Google Scholar]
- 20.Strbian D, Meretoja A, Ahlhelm FJ, et al. Predicting outcome of IV thrombolysis-treated ischemic stroke patients: the DRAGON score. Neurology 2012;78:427–432 [DOI] [PubMed] [Google Scholar]
- 21.Kent DM, Selker HP, Ruthazer R, Bluhmki E, Hacke W. The Stroke-Thrombolytic Predictive Instrument: a predictive instrument for intravenous thrombolysis in acute ischemic stroke. Stroke 2006;37:2957–2962 [DOI] [PubMed] [Google Scholar]
- 22.Saposnik G, Raptis S, Kapral MK, et al. ; Investigators of the Registry of the Canadian Stroke Network and the Stroke Outcome Research Canada Working Group. The iScore predicts poor functional outcomes early after hospitalization for an acute ischemic stroke. Stroke 2011;42:3421–3428 [DOI] [PubMed] [Google Scholar]
- 23.Rajajee V, Kidwell C, Starkman S, et al. Early MRI and outcomes of untreated patients with mild or improving ischemic stroke. Neurology 2006;67:980–984 [DOI] [PubMed] [Google Scholar]
- 24.Leira EC, Ludwig BR, Gurol ME, Torner JC, Adams HP., Jr The types of neurological deficits might not justify withholding treatment in patients with low total National Institutes of Health Stroke Scale scores. Stroke 2012;43:782–786 [DOI] [PubMed] [Google Scholar]
- 25.Chambers BR, Norris JW, Shurvell BL, Hachinski VC. Prognosis of acute stroke. Neurology 1987;37:221–225 [DOI] [PubMed] [Google Scholar]
- 26.Paolucci S, Antonucci G, Pratesi L, Traballesi M, Lubich S, Grasso MG. Functional outcome in stroke inpatient rehabilitation: predicting no, low and high response patients. Cerebrovasc Dis 1998;8:228–234 [DOI] [PubMed] [Google Scholar]
- 27.Denes G, Semenza C, Stoppa E, Lis A. Unilateral spatial neglect and recovery from hemiplegia: a follow-up study. Brain 1982;105:543–552 [DOI] [PubMed] [Google Scholar]
- 28.Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol 2006;5:603–612 [DOI] [PubMed] [Google Scholar]
- 29.Fischer U, Arnold M, Nedeltchev K, et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke 2005;36:2121–2125 [DOI] [PubMed] [Google Scholar]
- 30.Greisenegger S, Seyfang L, Kiechl S, Lang W, Ferrari J; Austrian Stroke Unit Registry Collaborators. Thrombolysis in patients with mild stroke: results from the Austrian Stroke Unit Registry. Stroke 2014;45:765–769 [DOI] [PubMed] [Google Scholar]
- 31.Minematsu K, Bang O, Uehara T. Risk factors. In: Kim JS, Caplan LR, Wong KS, editors. Intracranial Atherosclerosis, 1st ed Philadelphia: Wiley-Blackwell; 2008:45–54 [Google Scholar]
- 32.Guideline Committee of the Japan Stroke Society for IV rt-PA (alteplase) in acute ischemic stroke. Guidelines for IV application of rt-PA (alteplase), October 2005 [in Japanese]. Jpn J Stroke 2005;27:327–354 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.