Abstract
Objective:
Describe characteristics of small strokes causing acute vestibular syndrome (AVS).
Methods:
Ambispective cross-sectional study of patients with AVS (acute vertigo or dizziness, nystagmus, nausea/vomiting, head-motion intolerance, unsteady gait) with at least one stroke risk factor from 1999 to 2011 at a single stroke referral center. Patients underwent nonquantitative HINTS “plus” examination (head impulse, nystagmus, test-of-skew plus hearing), neuroimaging to confirm diagnoses (97% by MRI), and repeat MRI in those with initially normal imaging but clinical signs of a central lesion. We identified patients with diffusion-weighted imaging (DWI) strokes ≤10 mm in axial diameter.
Results:
Of 190 high-risk AVS presentations (105 strokes), we found small strokes in 15 patients (median age 64 years, range 41–85). The most common vestibular structure infarcted was the inferior cerebellar peduncle (73%); the most common stroke location was the lateral medulla (60%). Focal neurologic signs were present in only 27%. The HINTS “plus” battery identified small strokes with greater sensitivity than early MRI-DWI (100% vs 47%, p < 0.001). False-negative initial MRIs (6–48 hours) were more common with small strokes than large strokes (53% [n = 8/15] vs 7.8% [n = 7/90], p < 0.001). Nonlacunar stroke mechanisms were responsible in 47%, including 6 vertebral artery occlusions or dissections.
Conclusions:
Small strokes affecting central vestibular projections can present with isolated AVS. The HINTS “plus” hearing battery identifies these patients with greater accuracy than early MRI-DWI, which is falsely negative in half, up to 48 hours after onset. We found nonlacunar mechanisms in half, suggesting greater risk than might otherwise be assumed for patients with such small infarctions.
Acute vestibular syndrome (AVS) is a well-defined clinical syndrome1 of continuous vertigo or dizziness with nausea or vomiting, head-motion intolerance, gait unsteadiness, and nystagmus lasting days to weeks. AVS constitutes approximately 10% to 20% of emergency department (ED) dizziness,2 so is responsible for approximately 400,000 to 800,000 US ED visits annually.2,3 AVS can be caused by peripheral or central lesions. It is estimated that perhaps 25% of cases are due to stroke.2 Most ischemic stroke patients who present with dizziness or vertigo present with AVS, but only approximately 20% have focal neurologic signs, while the remainder have isolated AVS.1,2
Despite extensive ED workups,3,4 diagnostic accuracy in cerebrovascular dizziness is low (approximately 35% of strokes missed).5 We and others have observed1,6 that very small strokes sometimes cause AVS; these are often isolated AVS presentations, potentially increasing the chance of misdiagnosis. We also noted that these patients seemed to have a higher likelihood of false-negative MRIs, further increasing that risk. There has been no systematic study of whether these smaller strokes causing AVS differ demographically, mechanistically, or in terms of prognosis.
Lesions causing central AVS may be located in the root entry zone of the eighth nerve, vestibular nucleus, nodulus, or flocculus.7 We hypothesized that small posterior fossa strokes causing AVS would mostly involve these structures and might have a higher initial false-negative MRI rate than larger strokes. We assessed the frequency, anatomical distribution, clinical manifestations, neuroimaging, and pathomechanisms of these small strokes.
METHODS
Patients with AVS who had acute vertigo or dizziness, nystagmus, nausea or vomiting, head-motion intolerance, and unsteady gait with one or more stroke risk factor were enrolled in a prospective cross-sectional study from 1999 to 2011 at a single stroke referral center. Study methods have been described in detail elsewhere.1,8,9 Briefly, patients were recruited through a combination of passive (investigators notified by emergency care providers) and active (review of admitted neurology patients) surveillance. Subjects underwent structured bedside examination, including the HINTS battery (head impulse, nystagmus, test-of-skew [table e-1 on the Neurology® Web site at Neurology.org])1 plus bedside hearing by finger rub (together referred to as HINTS “plus”).9 Links to videos demonstrating clinical findings may be found in the supplemental data. Examinations generally occurred before neuroimaging, or examiners were masked to stroke status, to minimize observer bias in the determination of clinical examination findings. All underwent neuroimaging to confirm final diagnoses. For neuroimaging, 97% of patients underwent MRI with diffusion-weighted imaging (MRI-DWI), while 3% had large infarcts with mass effect evident by CT and were not clinically stable enough for MRI. Repeat MRI-DWI was obtained in patients with normal initial imaging but clinical signs suggestive of a central lesion. Stroke was defined by a DWI-bright lesion in a compatible location without evidence of an alternative etiology by neuroimaging, as determined by a neuroradiologist. Stroke was excluded in peripheral patients by absence of focal neurologic signs (isolated AVS), negative neuroimaging, and clinical follow-up.
The sample size for this retrospective analysis was based on availability of relevant patients in our research database. We include here patients with DWI strokes ≤10 mm in axial diameter. The choice of a 10-mm cutoff was intended to focus the analysis on unequivocally “small” strokes for 2 reasons: (1) to have greater anatomical certainty about the specific vestibular structures involved; and (2) to identify lesions that might evoke the clinical notion of being somehow unimportant (e.g., presumed to have a lacunar mechanism based solely on small size, reducing the likelihood of further mechanistic workup or more aggressive stroke therapies). The axial diameter of strokes with a lacunar pathomechanism is highly variable, because the shape of lacunar strokes is often irregular,10 so the choice of 10 mm as opposed to some other specific diameter was somewhat arbitrary.
Anatomical stroke loci were confirmed by 2 experts in posterior fossa neuroimaging (A.B., S.Y.) and 2 otoneurologists (D.N.-T., J.C.K.). We show selected images representative of each anatomical region involved. We compared the rate of false-negative MRIs with the rate of false-negative bedside testing, and compared these same tests with results in those with larger strokes presenting AVS. We report similar measures for those with isolated AVS. We used Fisher exact test to compare proportions across groups and the nonparametric Mann-Whitney test to compare nonnormal distributions of time to MRI. No statistical methods were used to impute missing data, control for confounding, account for sampling strategy, or conduct sensitivity analyses. The p values <0.05 were considered statistically significant.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review board. Written informed consent was obtained from all participants. All patients reported here were included in one or more prior reports of aggregated study data.1,8,9
RESULTS
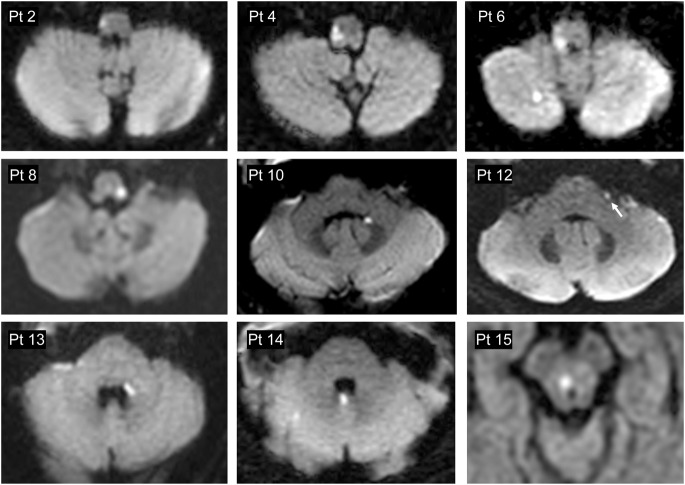
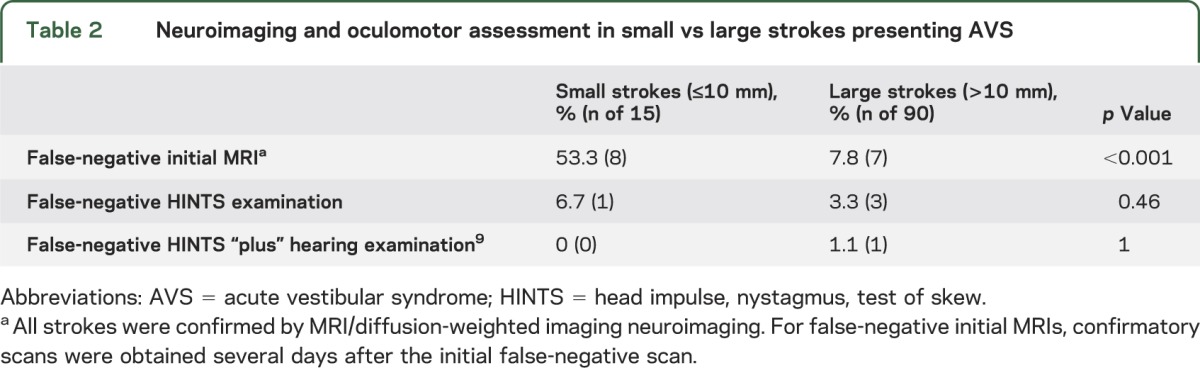
Of 190 high-risk AVS presentations, 105 were caused by stroke, all of which were reviewed for lesion size. Of these, 14% had lesions ≤10 mm (mean age 65.4 years, median 64, range 41–85, 33% female, 80% Caucasian, 20% African American). Time from onset of symptoms to MRI was similar for lesions ≤10 mm (median 12 hours, interquartile range 6–48 hours) and >10 mm (median 12 hours, interquartile range 5–24 hours) (Mann Whitney p = 0.245). Clinicoradiographic case descriptions with lesion loci are shown in table 1. A montage of representative brain MRI sections is presented in figure 1. The most frequently involved vestibular structure was the inferior cerebellar peduncle (73%). The most frequently affected location was the lateral medulla (60%); notably, however, two-thirds of these initially presented with isolated AVS and none presented the complete Wallenberg syndrome. An isolated cerebellar infarction was found in only one case.
Table 1.
Clinicoradiographic case descriptions, listed from caudal to rostral, based on anatomical lesion location
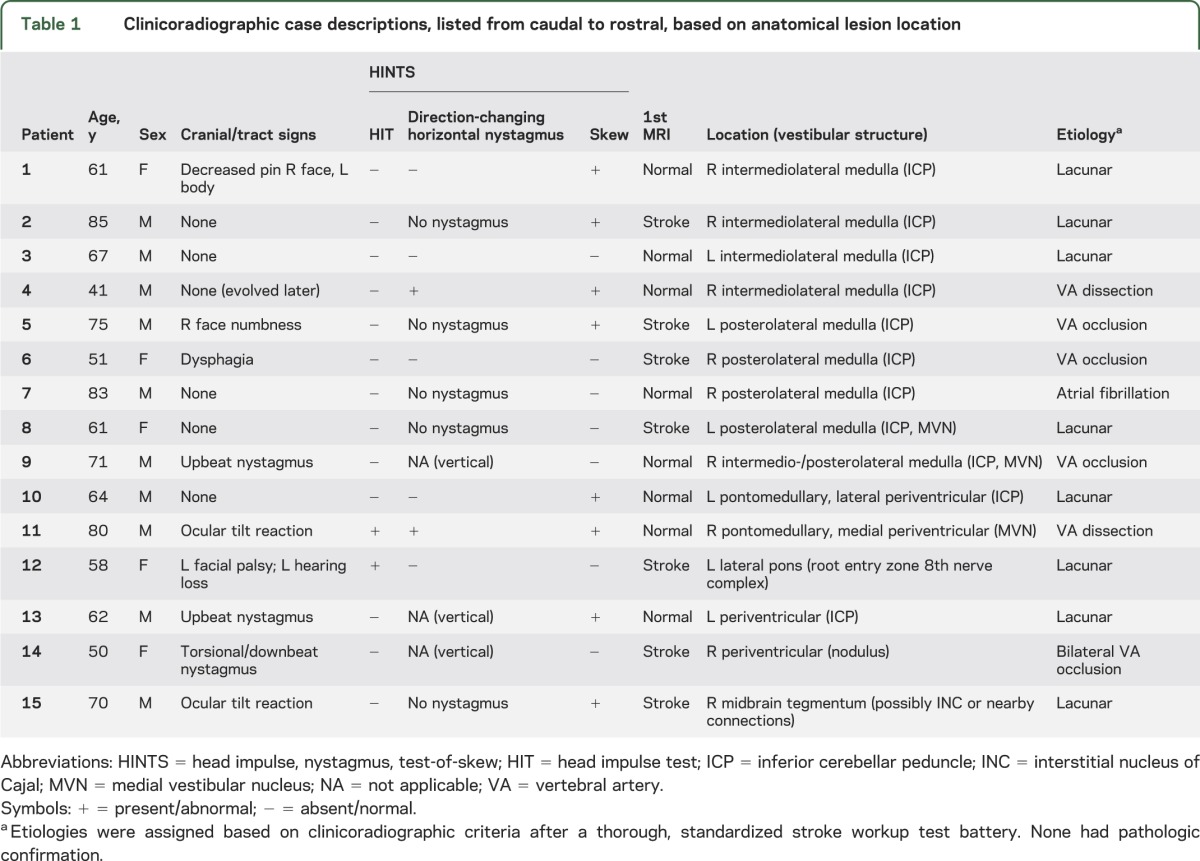
Figure 1. Montage of causal lesions in 9 of 15 patients with small strokes causing acute vestibular syndrome.
Montage of causal lesions in 9 of 15 patients with small strokes causing acute vestibular syndrome (axial MRI/diffusion-weighted imaging through the brainstem, arranged from caudal to rostral). Only selected images representative of each anatomical region involved are shown.
Nonoculomotor general neurologic signs were present in only 27% at initial presentation (table 1). The HINTS “plus” battery identified small strokes in AVS with greater sensitivity than early MRI with DWI (100% vs 47% [n = 7/15], p < 0.001), even more so in those with isolated AVS (100% vs 36% [n = 4/11], p < 0.001). False-negative MRIs with small strokes occurred 6 to 48 hours after the onset of vestibular symptoms. Anatomical neuroimaging sensitivity varied by stroke size (47% small vs 92% large), despite similar duration of symptoms before MRI, while physiology-based clinical examinations did not (100% small vs 99% large) (table 2). Head impulse testing was abnormal (erroneously suggesting a benign peripheral lesion) in 2 pontine cases (patients 11 and 12), but the presence of other signs (direction-changing nystagmus and skew in patient 11, and hearing loss and facial palsy in patient 12) correctly localized the lesion. Nonlacunar stroke mechanisms were responsible for 47% of strokes (table 1).
Table 2.
Neuroimaging and oculomotor assessment in small vs large strokes presenting AVS
DISCUSSION
Small strokes causing AVS were found throughout the brainstem, although disproportionately in the lateral medulla and affecting the inferior cerebellar peduncle; isolated cerebellar lesions were uncommon in our series. Approximately three-fourths of patients had an isolated AVS presentation, and clinical examination was far superior at detecting central lesions than early MRI neuroimaging. Approximately half were due to nonlacunar mechanisms, including 6 cases of vertebral artery dissection or occlusion. These results add to the growing body of knowledge about isolated vestibular presentations of stroke and have important implications for clinical practice.
Historically, isolated vertigo was not considered a stroke symptom,11 and, by design, this clinical presentation has been systematically excluded from most formal stroke studies.12,13 Recent research, however, makes clear that isolated vertigo, whether transient13 or persistent,1,2,8 is a common presentation of posterior circulation TIA and stroke. In fact, when cerebrovascular patients present vestibular symptoms, they are isolated much more often than nonisolated at initial presentation,1,13 and isolated vertigo or dizziness is the most common initial manifestation of vertebrobasilar ischemia.13
While isolated transient vertigo still presents substantial diagnostic challenges, ample evidence now indicates that bedside oculomotor examinations reliably distinguish central from peripheral causes in those with persistent, continuous symptoms (i.e., AVS).1,2,8,9,14 The one patient in this series missed by HINTS would have been captured by the recently described HINTS “plus” approach that identifies hearing loss as a sign of anterior inferior cerebellar artery infarction in patients with AVS.9 In the hands of subspecialists, HINTS “plus” has an estimated sensitivity of 99% and specificity of 97% for identifying central causes of AVS, whether isolated or not.9 Similar results, however, can probably be achieved by general neurologists after modest amounts of training.15
Relying on immediate MRI to exclude patients with stroke AVS is probably not sufficient. In our clinical practice, we do not accept a negative MRI <72 hours after symptom onset as definitive if oculomotor signs suggest otherwise, and routinely repeat imaging in such patients. To reduce the need for duplicative MRIs, it may sometimes be appropriate to wait until 48 to 72 hours after symptom onset before obtaining the first MRI (e.g., if the stroke etiology is known and the patient is clinically stable). Optimal, evidence-based neuroimaging protocols in AVS await further study, although we recently proposed one possible strategy (figure e-1).9
The prognosis and impact of early treatment for these specific patients remain largely unknown, but accurate diagnosis of these patients would seem quite important. The odds of stroke recurrence or death is greater with nonlacunar than lacunar mechanisms.16 Vertebrobasilar occlusions or dissections are found in more than half of patients with AVS,1 and were seen in 40% of the present series of small infarctions. Dissection is more frequent in young adults relative to unselected stroke populations,17 and youth is a risk factor for missed stroke.18 Misdiagnosed posterior circulation stroke patients appear to fare worse than those diagnosed and treated promptly.2 Dissections are often not suspected and may be difficult to detect, but the clinical outcomes of missed vertebral artery dissection in young patients can be disastrous.19 Optimal treatment for vertebrobasilar stenosis and dissection remains controversial, and clinical trials are ongoing to determine whether early thrombolysis in those with nondisabling posterior circulation strokes is beneficial. Absent clinical trials evidence, however, it is our clinical practice that isolated AVS patients with suspicious HINTS findings or new hearing loss be treated with the same urgency as other nondisabling strokes (e.g., admission and stroke risk factor workup). Accurately diagnosing these strokes by bedside HINTS could potentially save lives and decrease disability through prompt intervention. At the very least, early detection could lead to the study of new posterior circulation stroke therapies that might be applied to this understudied group of posterior fossa infarctions.
Limitations include a small sample size, imperfect case capture (some small strokes may have been missed even by second MRI or in isolated AVS patients diagnosed as peripheral by clinical follow-up rather than repeat imaging), and unknown consequences of misdiagnosis (since we report only those correctly identified). If small strokes were misclassified as peripheral (i.e., not included here), results could have overstated HINTS sensitivity; however, MRI sensitivity would then also be overstated, so it is not plausible that this limitation could have abolished the large sensitivity advantage of HINTS over MRI. Some anatomical loci known to cause AVS were not included here (e.g., no cases with hemispheric insular cortex7 lesions or dorsolateral pontine tegmental lesions with associated masseter paresis20). Theoretically, our results might not generalize to strokes occurring in these other sites.
Small strokes involving vestibular projections within the brainstem or cerebellum can produce AVS. These patients most often have lateral medullary infarctions lacking classic signs. The HINTS “plus” battery of oculomotor tests identifies these patients with greater accuracy than early MRI-DWI, which is falsely negative in more than half. We found nonlacunar mechanisms in approximately half of our patients, suggesting greater risk than might otherwise be assumed for patients with such small infarctions. Future studies should seek to determine whether early diagnosis in these patients improves clinical outcomes.
Supplementary Material
GLOSSARY
- AVS
acute vestibular syndrome
- DWI
diffusion-weighted imaging
- ED
emergency department
- HINTS
head impulse, nystagmus, test-of-skew
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Ali S. Saber Tehrani: drafted the manuscript, reviewed and critically edited the manuscript, and approved the final version. Jorge C. Kattah: assisted in data collection, reviewed and critically edited the manuscript, and approved the final version. Georgios Mantokoudis: reviewed and critically edited the manuscript, and approved the final version. John H. Pula and Deepak Nair: assisted in data collection, reviewed and critically edited the manuscript, and approved the final version. Ari Blitz and Sarah Ying: assisted in data coding, reviewed and critically edited the manuscript, and approved the final version. Daniel F. Hanley and David S. Zee: assisted in study design/conception, reviewed and critically edited the manuscript, and approved the final version. David E. Newman-Toker: designed the study and analytic plan, reviewed and critically edited the manuscript, and approved the final version.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009;40:3504–3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ 2011;183:E571–E592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saber Tehrani AS, Coughlan D, Hsieh YH, et al. Rising annual costs of dizziness presentations to US emergency departments. Acad Emerg Med 2013;20:689–696 [DOI] [PubMed] [Google Scholar]
- 4.Kim AS, Sidney S, Klingman JG, Johnston SC. Practice variation in neuroimaging to evaluate dizziness in the ED. Am J Emerg Med 2012;30:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerber KA, Brown DL, Lisabeth LD, Smith MA, Morgenstern LB. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: a population-based study. Stroke 2006;37:2484–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Lee SH, Park JH, Choi JY, Kim JS. Isolated vestibular nuclear infarction: report of two cases and review of the literature. J Neurol 2014;261:121–129 [DOI] [PubMed] [Google Scholar]
- 7.Kim HA, Lee H. Recent advances in central acute vestibular syndrome of a vascular cause. J Neurol Sci 2012;321:17–22 [DOI] [PubMed] [Google Scholar]
- 8.Newman-Toker DE, Kattah JC, Alvernia JE, Wang DZ. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology 2008;70:2378–2385 [DOI] [PubMed] [Google Scholar]
- 9.Newman-Toker D, Kerber K, Hsieh Y, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med 2013;20:986–996 [DOI] [PubMed] [Google Scholar]
- 10.Herve D, Mangin JF, Molko N, Bousser MG, Chabriat H. Shape and volume of lacunar infarcts: a 3D MRI study in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 2005;36:2384–2388 [DOI] [PubMed] [Google Scholar]
- 11.Fisher CM. Vertigo in cerebrovascular disease. Arch Otolaryngol 1967;85:529–534 [DOI] [PubMed] [Google Scholar]
- 12.A classification and outline of cerebrovascular diseases: II. Stroke 1975;6:564–616 [DOI] [PubMed] [Google Scholar]
- 13.Paul NL, Simoni M, Rothwell PM. Transient isolated brainstem symptoms preceding posterior circulation stroke: a population-based study. Lancet Neurol 2013;12:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huh YE, Koo JW, Lee H, Kim JS. Head-shaking aids in the diagnosis of acute audiovestibular loss due to anterior inferior cerebellar artery infarction. Audiol Neurootol 2013;18:114–124 [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Lee W, Chambers BR, Dewey HM. Diagnostic accuracy of acute vestibular syndrome at the bedside in a stroke unit. J Neurol 2011;258:855–861 [DOI] [PubMed] [Google Scholar]
- 16.Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain 2005;128:2507–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection: a systematic review. Neurologist 2012;18:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman-Toker DE, Moy E, Valente E, Coffey R, Hines A. Missed diagnosis of stroke in the emergency department: a cross-sectional analysis of a large population-based sample. Diagnosis 2014. Available at: http://www.degruyter.com/view/j/dx.2014.1.issue-2/dx-2013-0038/dx-2013-0038.xml. Accessed May 23, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savitz SI, Caplan LR, Edlow JA. Pitfalls in the diagnosis of cerebellar infarction. Acad Emerg Med 2007;14:63–68 [DOI] [PubMed] [Google Scholar]
- 20.Hopf HC. Vertigo and masseter paresis: a new local brainstem syndrome probably of vascular origin. J Neurol 1987;235:42–45 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.